Study of the Phase-Change Thermal-Storage Characteristics of a Solar Collector
Abstract
:1. Introduction
2. Materials and Methods
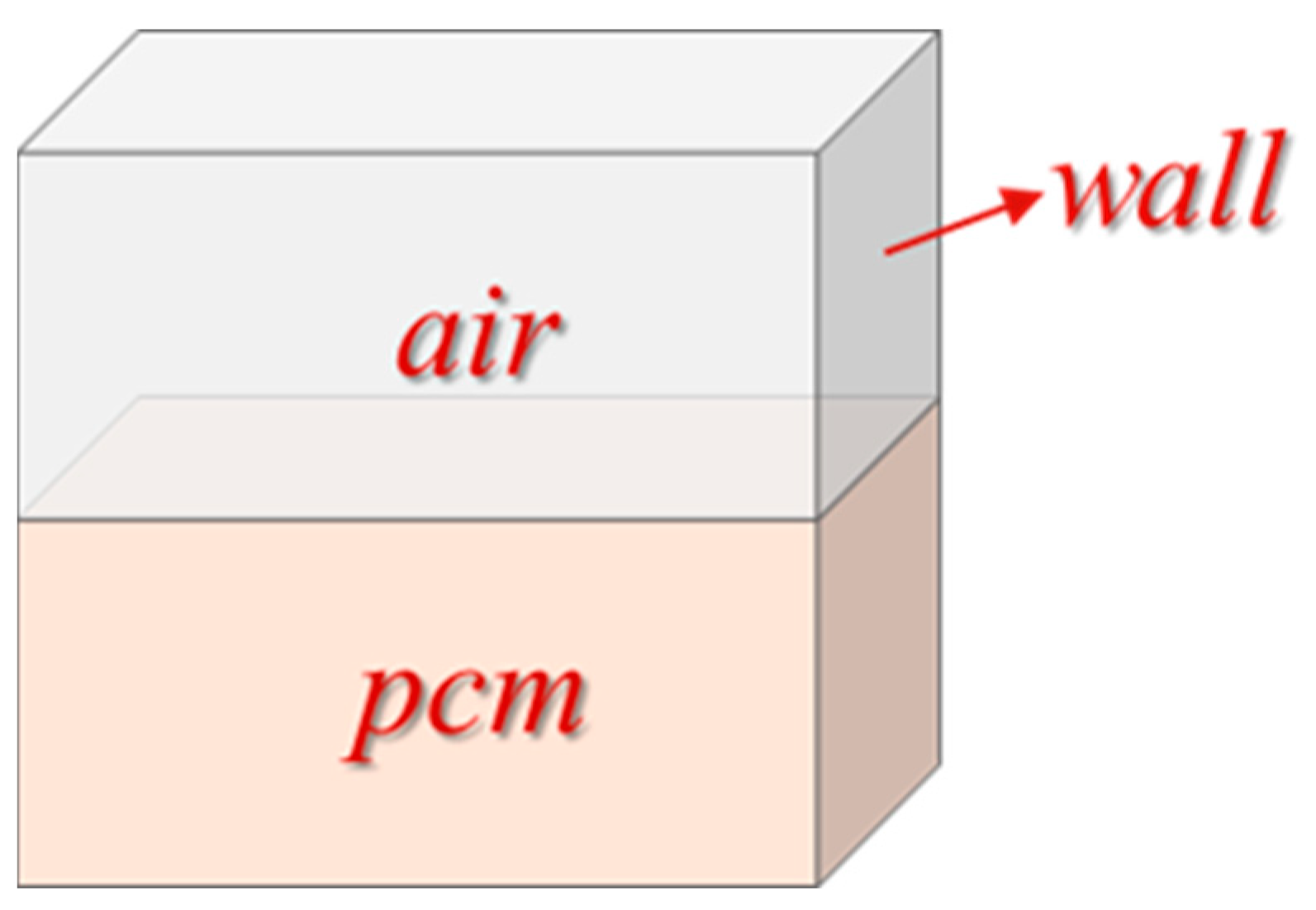
2.1. Physical Model
2.2. Governing Equation
2.3. Materials
2.4. Numerical Simulation
2.4.1. Grid Independence
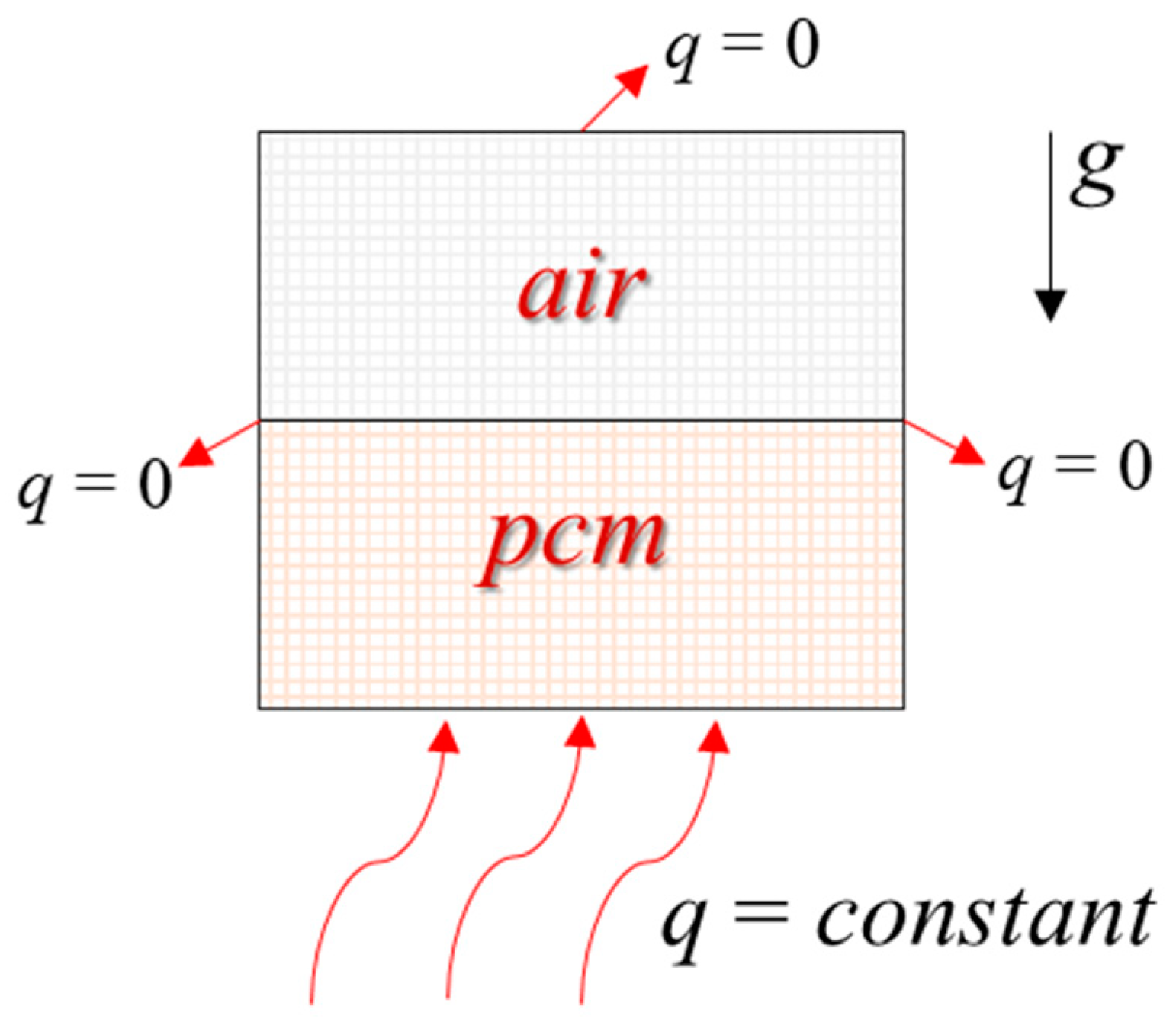
2.4.2. Boundary Conditions and Setting
2.5. Experiment Test
3. Results and Discussion
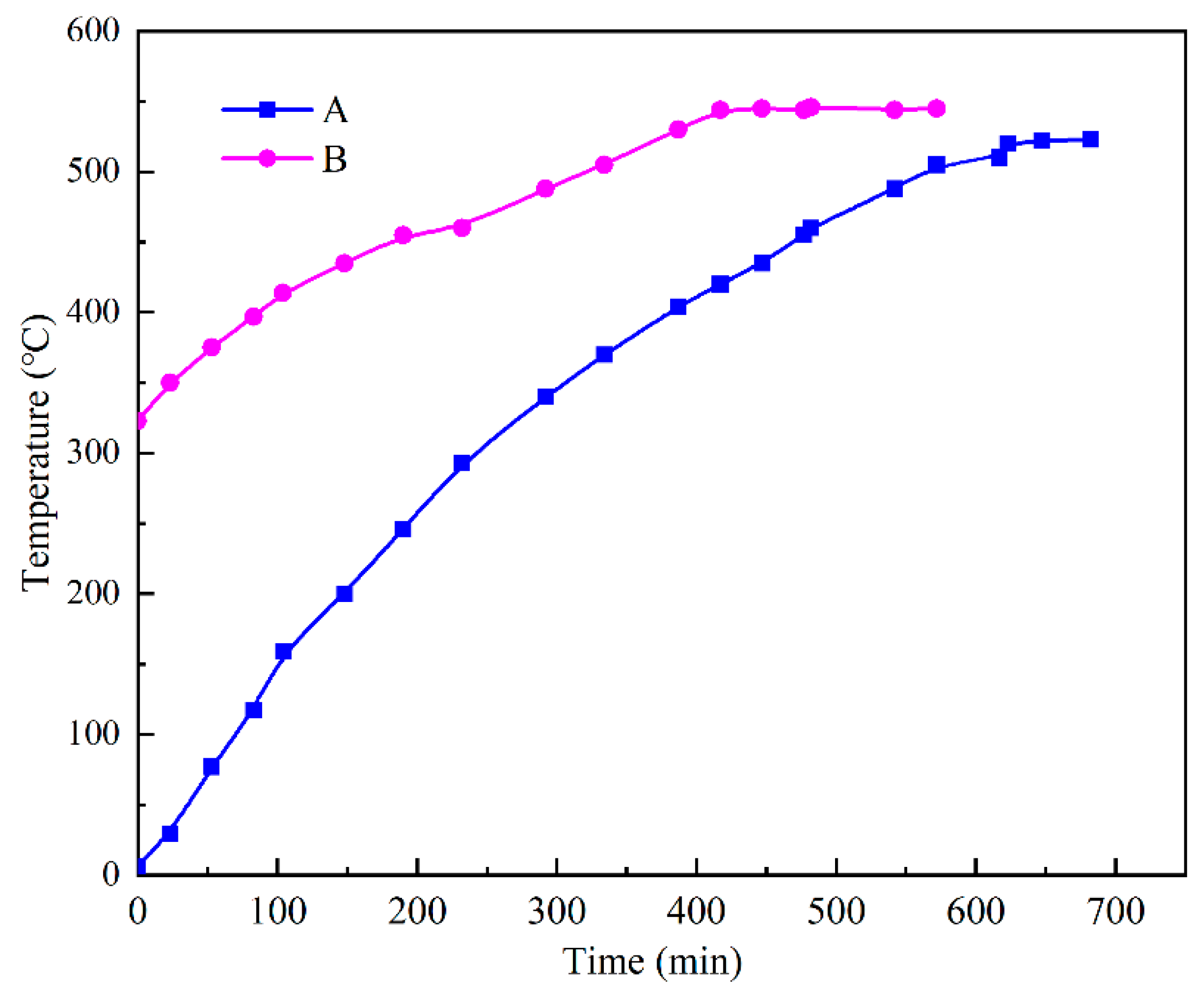
3.1. Model Validation
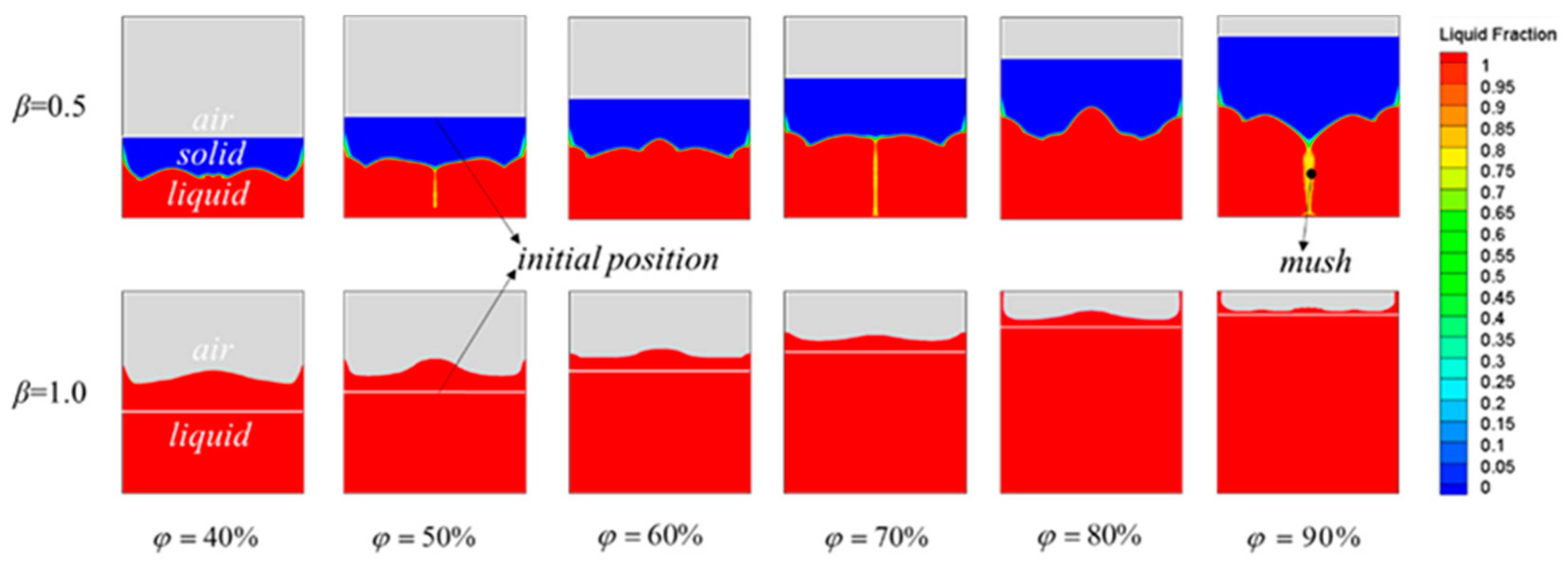
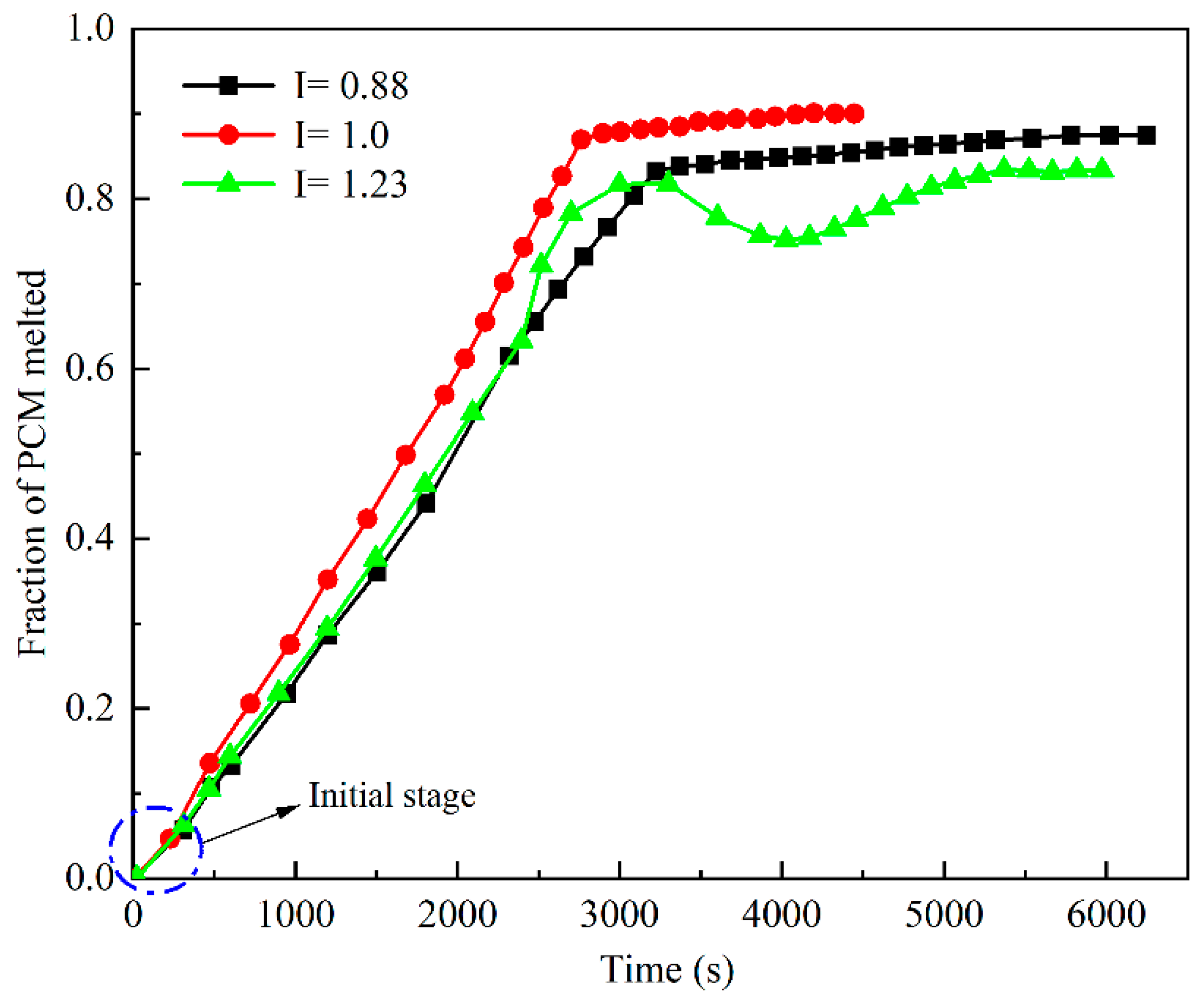
3.2. Effect of the PCM Volume Fraction on the Thermal-Storage Process
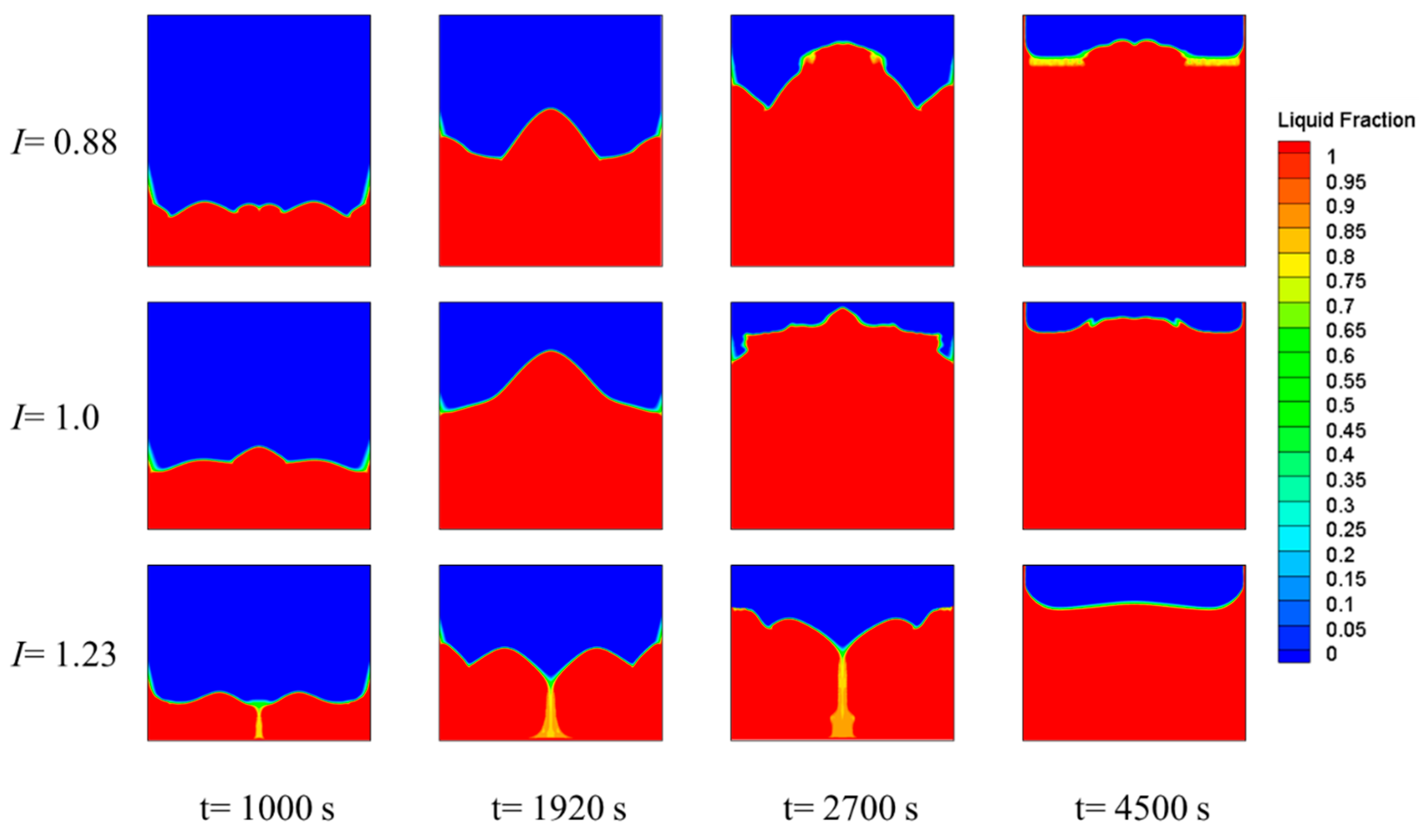
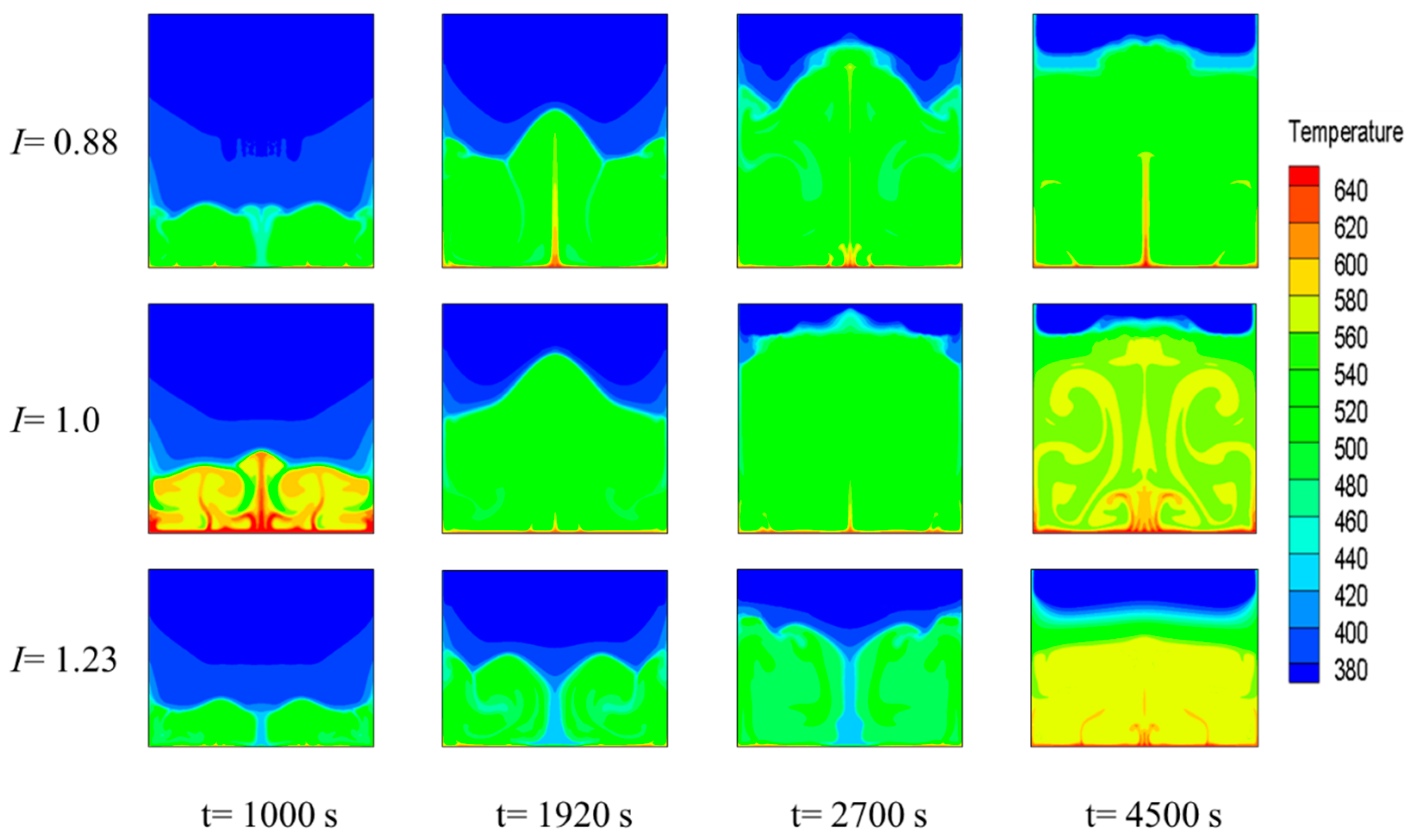
3.3. Effect of the Square Cavity Structure on the Heat-Storage Process
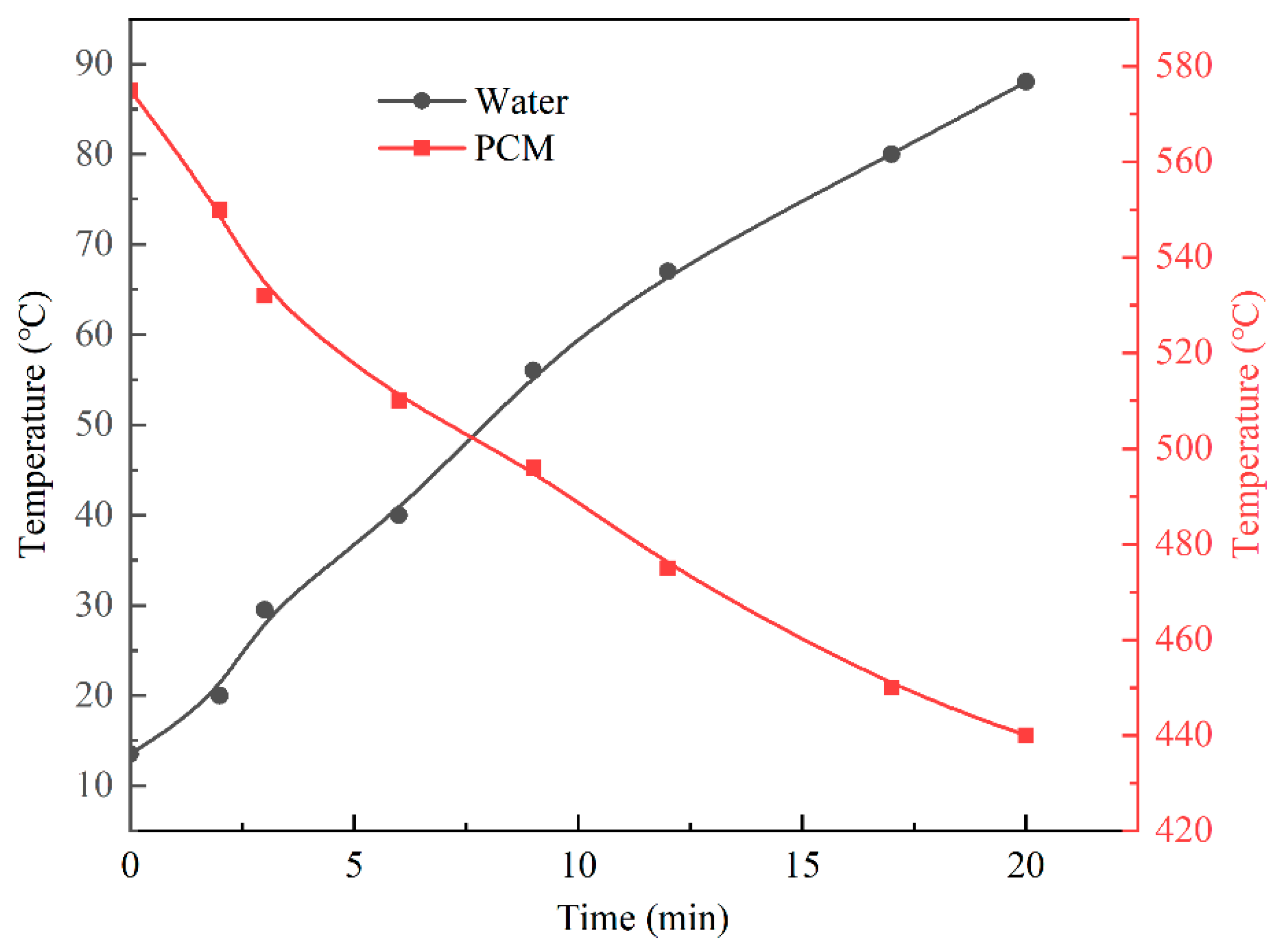
3.4. Experimental Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guney, M.S. Solar power and application methods. Renew. Sustain. Energy Rev. 2016, 57, 776–785. [Google Scholar] [CrossRef]
- Li, X.; Li, A.D.; Li, B.; Zheng, F. Economic analysis of Dish/Stirling solar power system. Proc.-Chin. Soc. Electr. Eng. 2005, 25, 108–111. (In Chinese) [Google Scholar]
- Katsaprakakis, D.A. Computational Simulation and Dimensioning of Solar-Combi Systems for Large-Size Sports Facilities: A Case Study for the Pancretan Stadium, Crete, Greece. Energies 2020, 13, 2285. [Google Scholar] [CrossRef]
- Katsaprakakis, D.A.; Zidianakis, G. Optimized dimensioning and operation automation for a solar-combi system for indoor space heating. A case study for a school building in Crete. Energies 2019, 12, 177. [Google Scholar] [CrossRef] [Green Version]
- Hafez, A.Z.; Soliman, A.; El-Metwally, K.A.; Ismail, I.M. Design analysis factors and specifications of solar dish technologies for different systems and applications. Renew. Sustain. Energy Rev. 2017, 67, 1019–1036. [Google Scholar] [CrossRef]
- Reddy, K.S.; Natarajan, S.K.; Veershetty, G. Experimental performance investigation of modified cavity receiver with fuzzy focal solar dish concentrator. Renew. Energy 2015, 74, 148–157. [Google Scholar] [CrossRef]
- Xia, X.L.; Dai, G.L.; Shuai, Y. Experimental and numerical investigation on solar concentrating characteristics of a sixteen-dish concentrator. Int. J. Hydrogen Energy 2012, 37, 18694–18703. [Google Scholar] [CrossRef]
- Giannuzzi, A.; Diolaiti, E.; Lombini, M.; De Rosa, A.; Marano, B.; Bregoli, G.; Cosentino, G.; Foppiani, I.; Schreiber, L. Enhancing the efficiency of solar concentrators by controlled optical aberrations: Method and photovoltaic application. Appl. Energy 2015, 145, 211–222. [Google Scholar] [CrossRef]
- Cheng, Q.; Chai, J.L.; Zhou, Z.; Song, J.L.; Su, Y. Tailored non-imaging secondary reflectors designed for solar concentration systems. Sol. Energy 2014, 110, 160–167. [Google Scholar] [CrossRef]
- Kalogirou, S.A. Solar thermal collectors and applications. Prog. Energy Combust. Sci. 2004, 30, 231–295. [Google Scholar] [CrossRef]
- Arnaoutakis, G.E.; Katsaprakakis, D.A. Concentrating Solar Power Advances in Geometric Optics, Materials and System Integration. Energies 2021, 14, 6229. [Google Scholar] [CrossRef]
- Arnaoutakis, G.E.; Katsaprakakis, D.A.; Christakis, D.G. Dynamic modeling of combined concentrating solar tower and parabolic trough for increased day-to-day performance. Appl. Energy 2022, 323, 119450. [Google Scholar] [CrossRef]
- Balzar, A.; Stumpf, P.; Eckhoff, S.; Ackermann, H.; Grupp, M. A solar cooker using vacuum-tube collectors with integrated heat pipes. Sol. Energy 1996, 58, 63–68. [Google Scholar] [CrossRef]
- Raturi, M.P.; Deolal, M.H.; Grover, J.; Kumar, A. Parabolic dish type solar cooking system with gravity based solar tracking system. IOSR J. Mech. Civ. Eng. 2014, 11, 34–36. [Google Scholar] [CrossRef]
- Grupp, M.; Balmer, M.; Beall, B.; Bergler, H.; Cieslok, J.; Hancock, D.; Schröder, G. On-line recording of solar cooker use rate by a novel metering device: Prototype description and experimental verification of output data. Sol. Energy 2009, 83, 276–279. [Google Scholar] [CrossRef]
- Lokeswaran, S.; Eswaramoorthy, M. Experimental studies on solar parabolic dish cooker with porous medium. Appl. Sol. Energy 2012, 48, 169–174. [Google Scholar] [CrossRef]
- Badran, A.A.; Yousef, I.A.; Joudeh, N.K.; Hamad, R.A.; Halawa, H.; Hassouneh, H.K. Portable solar cooker and water heater. Energy Convers. Manag. 2010, 51, 1605–1609. [Google Scholar] [CrossRef]
- Aidan, J. Perform eval a parabol sol dish cooker Yola. Niger IOSRJAP 2014, 6, 46–50. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Experimental investigation of energy and exergyefficiencies of domestic size parabolic dish solar cooker. J. Renew. Sustain. Energy 2012, 4, 023111. [Google Scholar] [CrossRef]
- Mohammed, I.L. Design and development of a parabolic dish solar water heater. Int. J. Eng. Res. Appl. 2012, 2, 822–830. [Google Scholar]
- Sakhare, V.; Kapatkar, V.N. Experimental Analysis of Parabolic Solar Dish with Copper Helical coil Receiver. International. J. Innov. Res. Adv. Eng. 2014, 1, 199–204. [Google Scholar]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.; Ghafoor, A. A review of performance enhancement of PCM based latent heat storage system within the context of materials, thermal stability and compatibility. Energy Convers. Manag. 2016, 115, 132–158. [Google Scholar] [CrossRef]
- Wang, P.L.; Yao, H.; Lan, Z.P.; Peng, Z.J.; Huang, Y.; Ding, Y.L. Numerical investigation of PCM melting process in sleeve tube with internal fins. Energy Convers. Manag. 2016, 110, 428–435. [Google Scholar] [CrossRef]
- Wang, Z.C.; Zhang, Z.Q.; Jia, L.; Yang, L.X. Paraffin and paraffin/aluminum foam composite phase change material heat storage experimental study based on thermal management of Li-ion battery. Appl. Therm. Eng. 2015, 78, 428–436. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Lu, W.; Tian, Y. Heat transfer enhancement for thermal energy storage using metal foams embedded within phase change materials (PCMs). Sol. Energy 2010, 84, 1402–1412. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.Q.; He, X.D. Three-dimensional numerical study on solid-liquid phase change within open-celled aluminum foam with porosity gradient. Appl. Therm. Eng. 2017, 113, 298–308. [Google Scholar] [CrossRef]
- Sundarram, S.S.; Li, W. The effect of pore size and porosity on thermal management performance of phase change material infiltrated microcellular metal foams. Appl. Therm. Eng. 2014, 64, 147–154. [Google Scholar] [CrossRef]
- Li, X.R.; Li, Z.G.; Zhu, B.S.; Wang, W.J. Effect of Tip Clearance Size on Tubular Turbine Leakage Characteristics. Processes 2021, 9, 1481. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Y.; Zheng, Z.J. Study on Melting Process of Latent Heat Thermal Energy Storage Unit Strengthened by Two-dimensional Graded Metal Foam. J. Eng. Thermophys. 2021, 42, 2946–2953. (In Chinese) [Google Scholar]
- Rolka, P.; Przybylinski, T.; Kwidzinski, R.; Lackowski, M. The heat capacity of low-temperature phase change materials (PCM) applied in thermal energy storage systems. Renew. Energy 2021, 172, 541–550. [Google Scholar] [CrossRef]
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Sari, A.; Kaygusuz, K. Thermal energy storage system using some fatty acids as latent heat energy storage materials. Energy Sources 2001, 23, 275–285. [Google Scholar] [CrossRef]
- Ye, C.M.; Shentu, B.Q.; Weng, Z.X. Thermal conductivity of high density polyethylene filled with graphite. J. Appl. Polym. Sci. 2006, 101, 3806–3810. [Google Scholar] [CrossRef]





| Number | Material | Phase-Change Temperature (°C) | Latent Heat of Phase Change (kJ/kg) | Thermal Conductivity (W/m·K) | Density (kg/m3) |
|---|---|---|---|---|---|
| 1 | Fatty acid | 70.0 | 186.5 | 0.172 | 848 |
| 2 | Barium hydroxide octahydrate | 78.0 | 265.7 | 0.653 | 1660 |
| 3 | Naphthalene | 80.0 | 147.7 | 0.132 | 976 |
| 4 | Magnesium chloride hexahydrate | 117 | 168.0 | 0.570 | 1450 |
| 5 | Erythritol | 118 | 339.0 | 0.326 | 1330 |
| Working Condition | W/mm | H/mm | I |
|---|---|---|---|
| 1 | 150.0 | 170.0 | 0.88 |
| 2 | 156.4 | 156.4 | 1.00 |
| 3 | 180.0 | 145.8 | 1.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Xu, J.; Li, Y.; Zhang, Y.; Kuang, C. Study of the Phase-Change Thermal-Storage Characteristics of a Solar Collector. Materials 2022, 15, 7497. https://doi.org/10.3390/ma15217497
Deng Y, Xu J, Li Y, Zhang Y, Kuang C. Study of the Phase-Change Thermal-Storage Characteristics of a Solar Collector. Materials. 2022; 15(21):7497. https://doi.org/10.3390/ma15217497
Chicago/Turabian StyleDeng, Yuxuan, Jing Xu, Yanna Li, Yanli Zhang, and Chunyan Kuang. 2022. "Study of the Phase-Change Thermal-Storage Characteristics of a Solar Collector" Materials 15, no. 21: 7497. https://doi.org/10.3390/ma15217497




