Effect of Rolling Temperature on Microstructure and Properties of Al-Mg-Li Alloy
Abstract
:1. Introduction
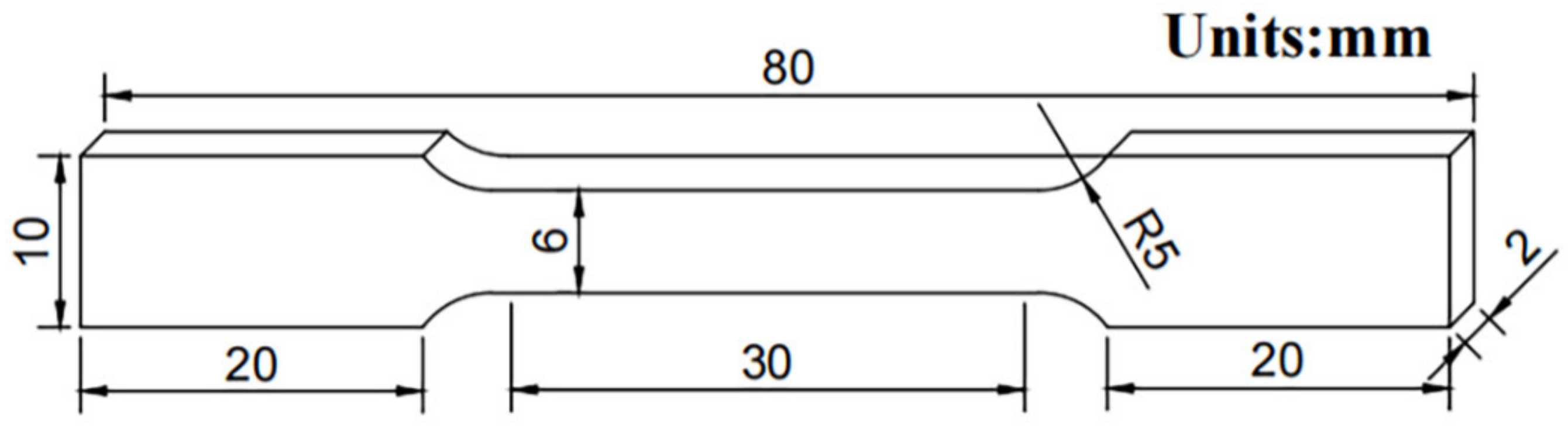
2. Experimental Procedures
Experimental Methods
3. Results
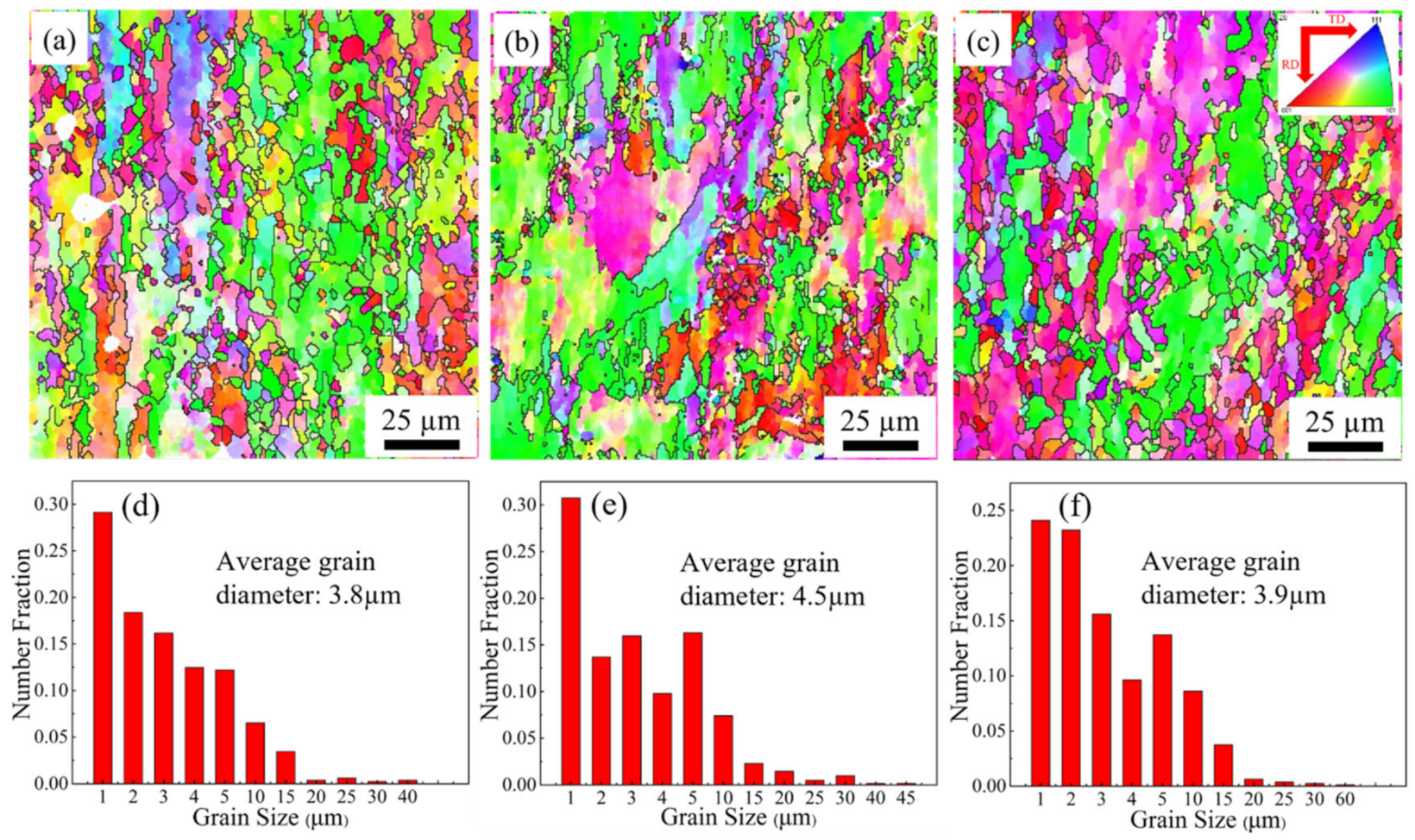
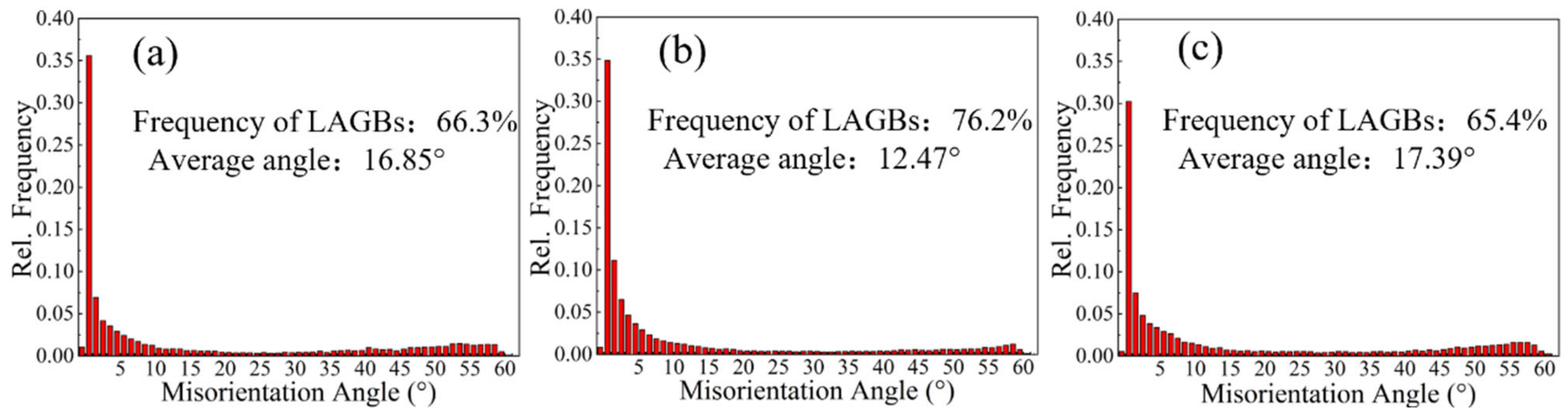
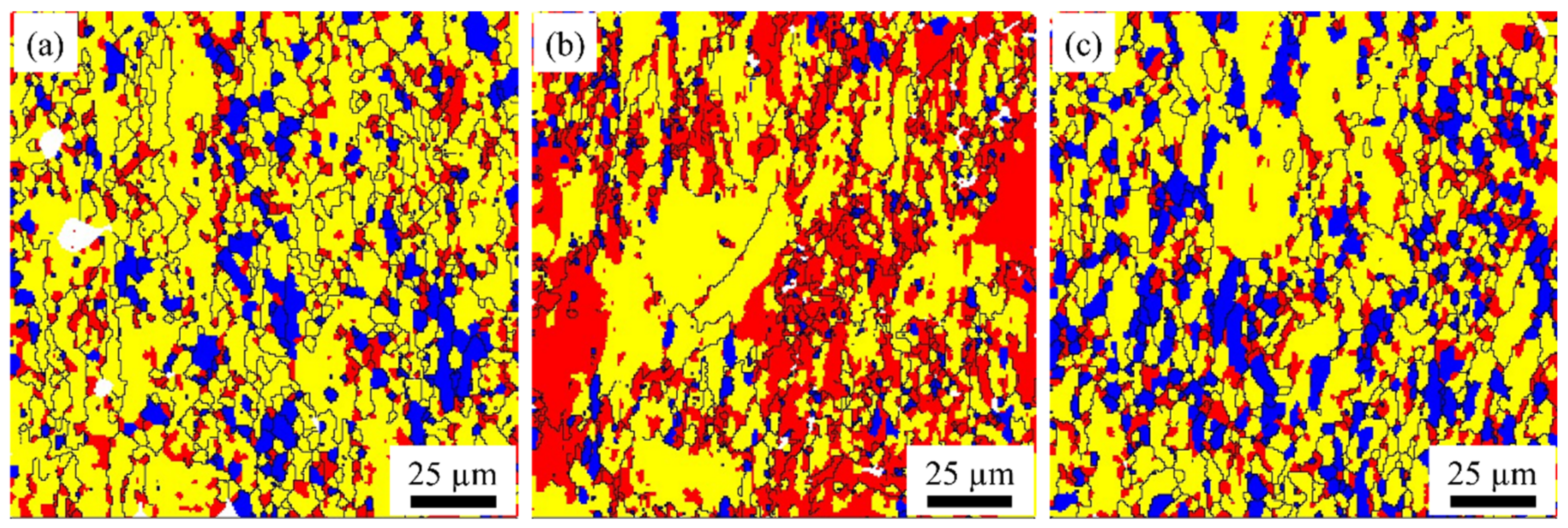
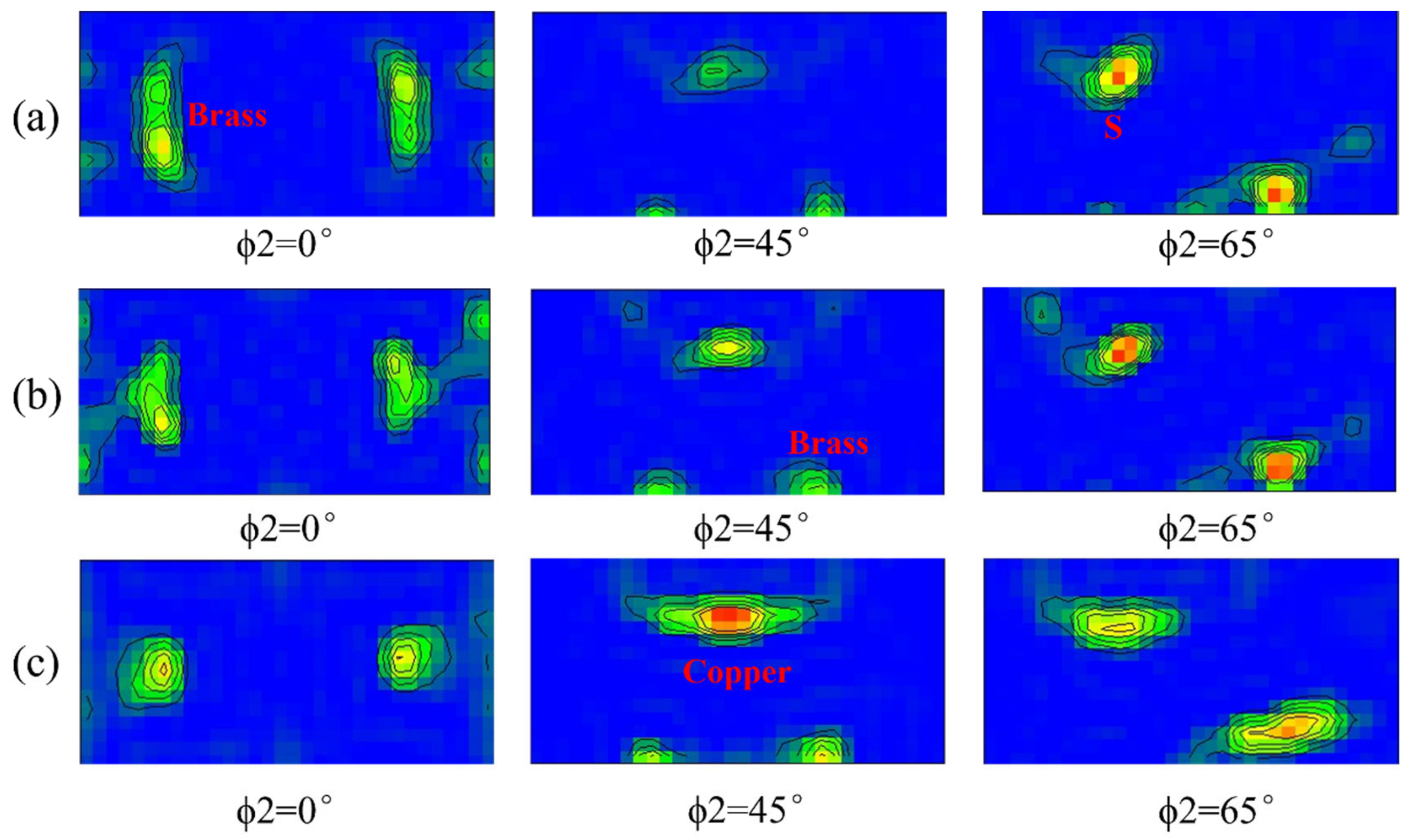
3.1. Grain Structure and Texture Characterization
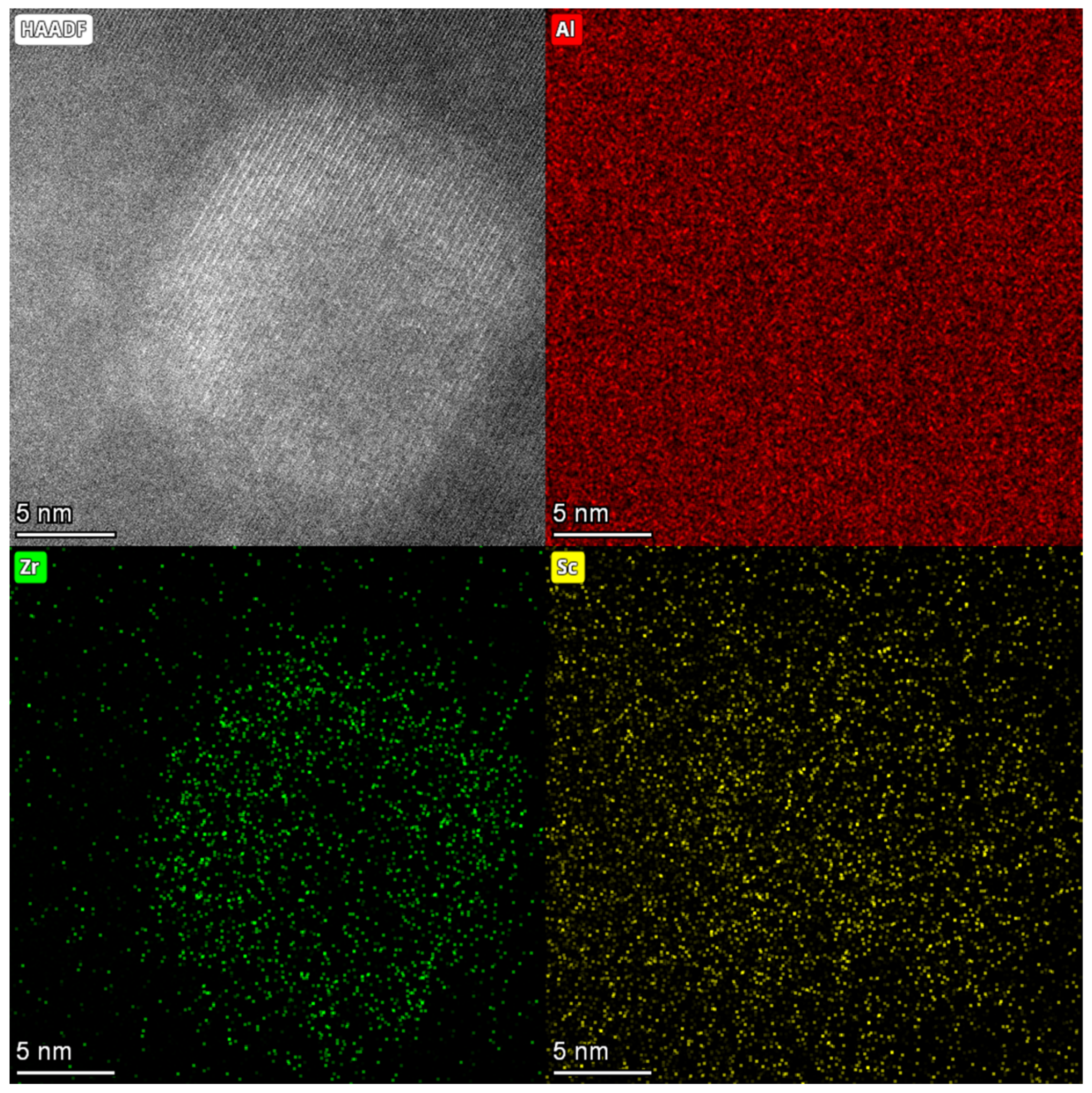
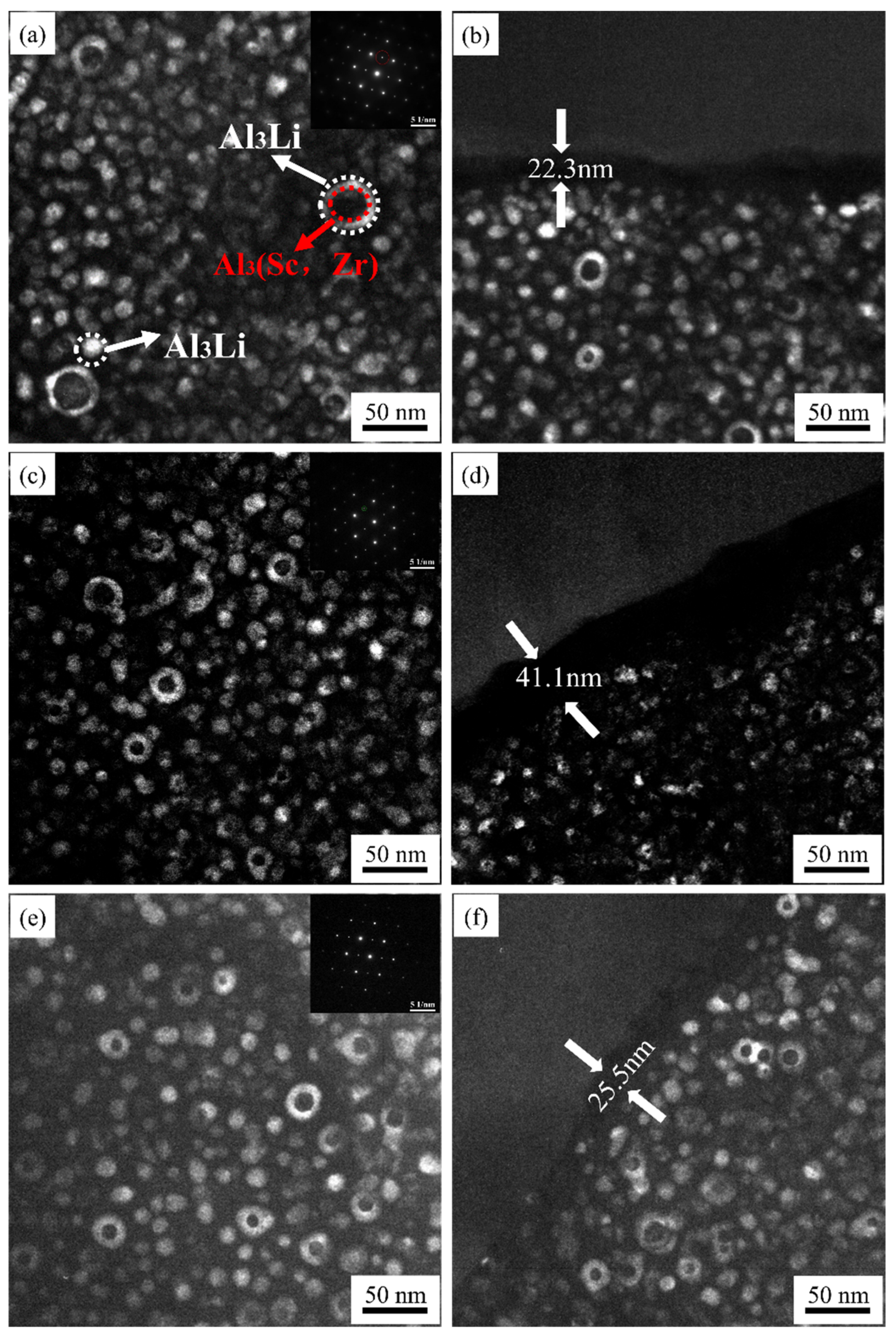
3.2. Precipitation Behavior
3.3. Tensile Properties
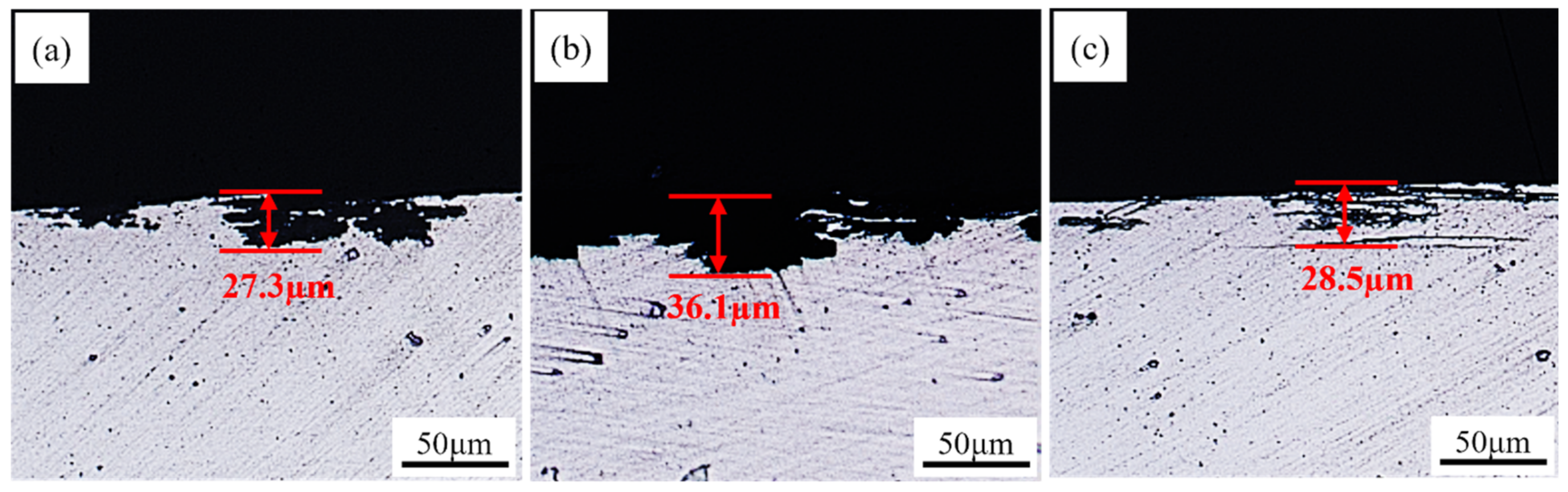
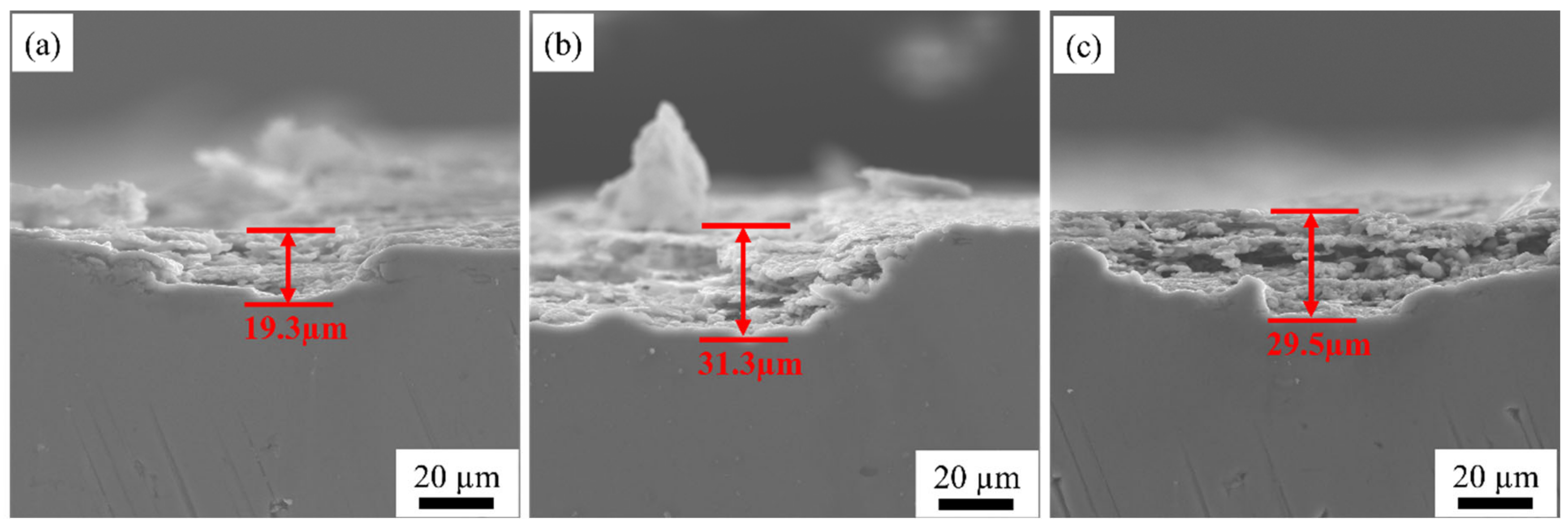
3.4. Intergranular Corrosion Properties
4. Discussion
4.1. The Effect of Rolling Temperature on the Mechanical Properties of the Alloy
4.2. The Effect of Rolling Temperature on the Corrosion Performance of the Alloy
5. Conclusions
- (1)
- With the increase in rolling temperature, the ultimate tensile strengths of the S420, S450, and S480 samples are 477 MPa, 486 MPa, and 496 MPa, respectively. The yield strengths are 377 MPa, 375 MPa, and 412 MPa, respectively, and the elongations are 5%, 10%, and 6%. Thus, the strength of the alloy gradually increases with the increase in the hot rolling temperature, while there is a partial loss of elongation.
- (2)
- With the increase in rolling temperature, the maximum grain boundary corrosion depths of the S420, S450, and S480 samples are 27.3 μm, 36.1 μm, and 28.5 μm. The corrosion resistance of the samples is in the order of S420 > S480 > S450. With the increase in rolling temperature, the degree of recrystallization and the recrystallized grain size of the sample become higher. More large-angle grain boundaries are formed between recrystallized grains. The large-angle grain boundary is the corrosion-prone part. It causes the PFZ width of the alloys to increase. Meanwhile, the precipitated phase affects the dislocation density. The dislocation density of the sample increases with the increasing rolling temperature, which worsens the corrosion resistance of alloys. As the rolling temperature rises, the intergranular corrosion resistance gradually becomes worse.
- (3)
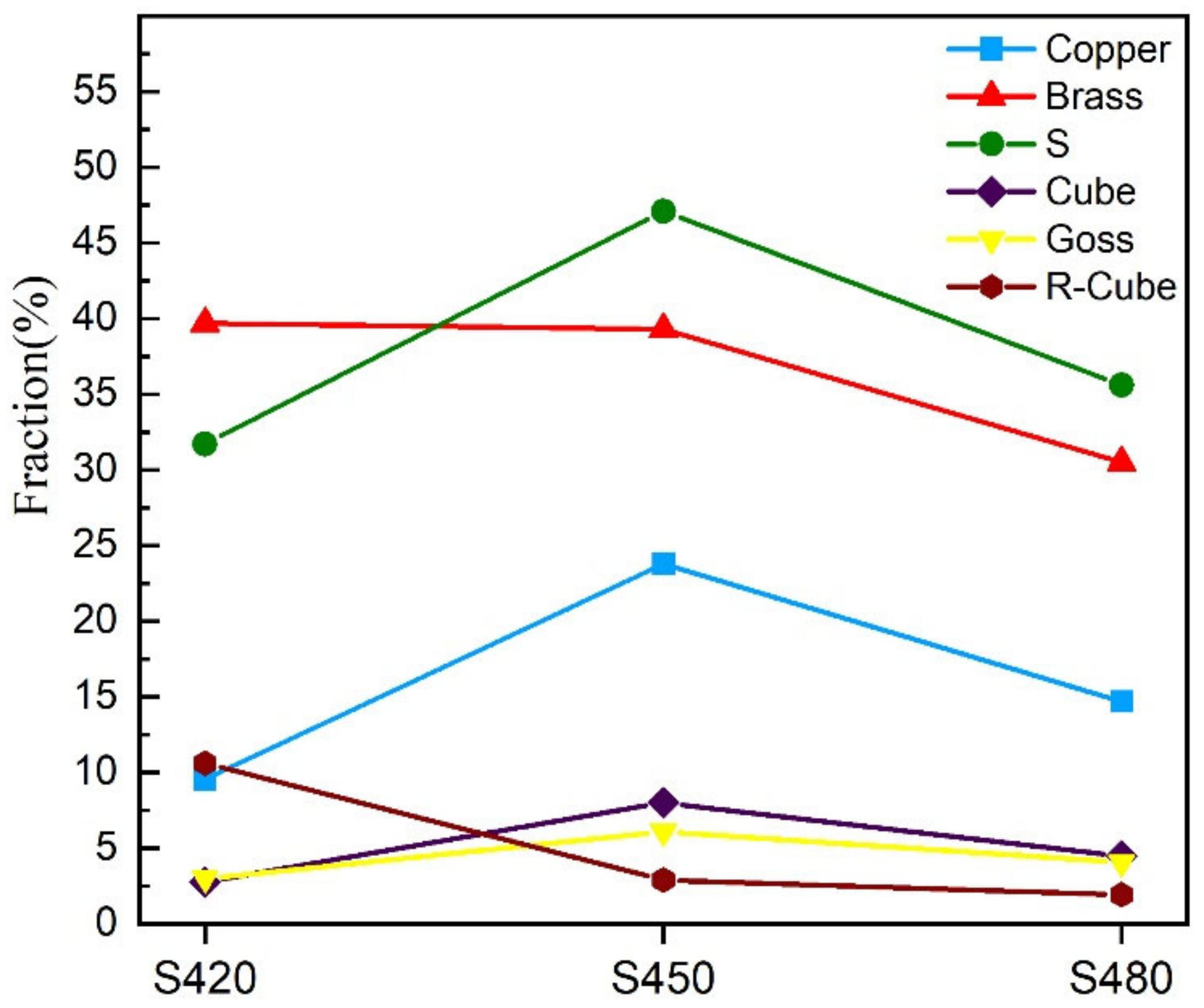
- As the rolling temperature increases, the strength of the Brass texture in the alloy decreases, while the strength of S and Copper textures increases. The evolution of the texture increases the value of Taylor factor M; thus, the strength of the alloy increases. The increasing rolling temperature also raises the number density of the sample’s Al3(Sc, Zr)/Al3Li core–shell structure composite phase. The presence of this composite particle not only inhibits grain growth but also changes the dislocation cutting mechanism to the Orowan bypassing mechanism, and the combined effect of the two increases the strength of the material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steuwer, A.; Dumont, M.; Altenkirch, J.; Birosca, S.; Deschamps, A.; Prangnell, P.B.; Withers, P.J. A combined approach to microstructure mapping of an Al-Li AA2199 friction stir weld. Acta Mater. 2011, 59, 3002–3011. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminum alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Gupta, R.K.; Nayan, N.; Nagasireesha, G.; Sharma, S.C. Development and characterization of Al-Li alloys. Mater. Sci. Eng. 2006, 420, 228–234. [Google Scholar] [CrossRef]
- Rioja, R.J.; Liu, J. The evolution of Al-Li base products for aerospace and space applications. Metall. Mater. Trans. 2012, 43, 3325–3337. [Google Scholar] [CrossRef]
- Khokhlatova, L.B.; Lukin, V.I.; Kolobnev, N.I.; Ioda, E.N.; Mezentseva, E.A. Advanced aluminum-lithium 1420 alloy for welded structures for aerospace technology. Weld. Int. 2010, 24, 783–786. [Google Scholar] [CrossRef]
- Fridlyander, I.N.; Khokhlatova, L.B.; Kolobnev, N.I.; Rendiks, K.; Tempus, G. Thermally stable aluminum-lithium alloy 1424 for application in welded fuselage. Met. Sci. Heat. Treat. 2002, 44, 3–8. [Google Scholar] [CrossRef]
- Annin, B.D.; Fomin, V.M.; Antipov, V.V.; Ioda, E.N.; Karpov, E.V.; Malikov, A.G.; Orishich, A.M.; Cherepanov, A.N. Investigation of the technology of laser welding of aluminum alloy 1424. Dokl. Phys. 2015, 60, 533–538. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Jayaganthan, R. Effect of rolling temperature on microstructure and mechanical properties of 6063 Al alloy. Mater. Sci. Eng. 2008, 492, 300–305. [Google Scholar] [CrossRef]
- Zuiko, I.S.; Mironov, S.; Kaibyshev, R. Microstructural evolution and strengthening mechanisms operating during cryogenic rolling of solutionized Al-Cu-Mg alloy. Mater. Sci. Eng. 2019, 745, 82–89. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Liu, T.; Ma, Y.; Iwaoka, H.; Hirosawa, S.; Zhang, K.; Zhang, R. Microstructure evolution and mechanical properties of Al-Cu-Li alloys with different rolling schedules and subsequent artificial ageing heat treatment. Mater. Charact. 2020, 170, 110676. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Z.; Jia, P.; Bai, S.; Yan, P.; Hu, Y. Dynamic dissolution and texture evolution of an Al-Cu-Mg-Ag alloy during hot rolling. J. Alloy Compd. 2020, 827, 154254. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Z.; Hu, Y.; Li, S.; Bai, S. Evolution of Goss texture in an Al-Cu-Mg alloy during cold rolling. Arch. Civ. Mech. Eng. 2020, 20, 24. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, J. Textures in industrial processes and products. Mater. Sci. Forum. 2011, 702, 18–25. [Google Scholar] [CrossRef]
- Hirsch, J. Texture and anisotropy in industrial applications of aluminum alloys. Arch. Metall. Mater. 2005, 50, 21–34. [Google Scholar]
- Betsofen, S.Y.; Antipov, V.V.; Knyazev, M.I. Al-Cu-Li and Al-Mg-Li alloys: Phase composition, texture, and anisotropy of mechanical properties (review). Russ. Metall. 2016, 4, 326–341. [Google Scholar] [CrossRef]
- Deschamps, A.; Sigli, C.; Mourey, T.; de Geuser, F.; Lefebvre, W.; Davo, B. Experimental and modelling assessment of precipitation kinetics in an Al-Li-Mg alloy. Acta Mater. 2012, 60, 1917–1928. [Google Scholar] [CrossRef]
- Nembach, E. Order strengthening: Recent developments, with special reference to aluminum-lithium-alloys. Prog. Mater. Sci. 2000, 45, 275–338. [Google Scholar] [CrossRef]
- Randle, V.; Engler, O. Gordon and Breach. In Introduction to Texture Analysis: Macrotexture, Microtexture and Orientation Mapping; CRC: Boca Raton, FL, USA, 2000. [Google Scholar]
- Zhang, X.L.; Zhang, L.; Wu, G.H.; Sun, J.W.; Rong, M.; Hsieh, C.C.; Yu, Y.X. Influence of Sc content on the microstructure and mechanical properties of cast Al-2Li-2Cu-0. 5Mg-0.2Zr alloy. Mater. Charact. 2018, 142, 223–236. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Wu, R.Z.; Sun, J.F.; Jiao, Y.L.; Hou, L.G.; Li, X.L.; Zhang, J.H.; Zhang, M.L. Ambient-temperature mechanical properties of isochronally aged 1420-Sc-Zr aluminum. Mater. Sci. Eng. 2019, 745, 411–419. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Precipitation evolution in Al-Zr and Al-Zr-Ti alloys during isothermal aging at 375–425 °C. Acta Mater. 2008, 56, 114–127. [Google Scholar] [CrossRef]
- Medjahed, A.; Moula, H.; Zegaoui, A.; Derradji, M.; Henniche, A.; Wu, R.Z.; Hou, L.G.; Zhang, J.H.; Zhang, M.L. Influence of the rolling direction on the microstructure, mechanical, anisotropy and gamma rays shielding properties of an Al-Cu-Li-Mg-X alloy. Mater. Sci. Eng. 2018, 732, 129–137. [Google Scholar] [CrossRef]
- Kuang, Q.; Wang, R.; Peng, C.; Cai, Z. Comparison of microstructure and mechanical properties of Al-Mg-Li-Sc-Zr alloys processed by ingot metallurgy and rapid solidification. J. Alloy Compd. 2021, 883, 160937. [Google Scholar] [CrossRef]
- Tzeng, Y.C.; Chung, C.Y.; Chien, H.C. Effects of trace amounts of Zr and Sc on the recrystallization behavior and mechanical properties of Al-4.5Zn-1.6Mg alloys. Mater. Lett. 2018, 228, 270–272. [Google Scholar] [CrossRef]
- Chen, A.T.; Yu, P.; Zhang, L.; Wu, G.H.; Li, Y.L. Microstructural evolution and mechanical properties of cast Al-3Li-1.5Cu-0.2Zr alloy during heat treatment. Mater. Charact. 2016, 114, 234–242. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, L.; Wu, G.H.; Liu, W.C.; Shi, C.C.; Tao, J.S.; Sun, J.W. Microstructural evolution and mechanical properties of cast Al-2Li-2Cu0.5Mg-0.2Zr alloy during heat treatment. Mater. Charact. 2017, 152, 312–317. [Google Scholar] [CrossRef]
- Furukawa, M.; Horita, Z.; Nemoto, M.; Valiev, R.Z.; Langdon, T.G. Microhardness measurements and the Hall-Petch relationship in an Al-Mg alloy with sub-micrometer grain size. Acta Mater. 1996, 44, 4619–4629. [Google Scholar] [CrossRef]
- Furukawa, M.; Iwahashi, Y.; Horita, Z.; Nemoto, M.; Tsenev, N.K.; Valiev, R.Z.; Langdon, T.G. Structural evolution and the Hall-Petch relationship in an Al-Mg-Li-Zr alloy with ultra-fine grain size. Acta. Mater. 1997, 45, 4751–4757. [Google Scholar] [CrossRef]
- Shanmugasundaram, T.; Heilmaier, M.; Murty, B.S.; Sarma, V.S. On the Hall-Petch relationship in a nanostructured Al-Cu alloy. Mater. Sci. Eng. 2010, 527, 7821–7825. [Google Scholar] [CrossRef]
- Starink, M.J.; Wang, S.C. A model for the yield strength of overaged Al-Zn-Mg-Cu alloys. Acta Mater. 2003, 51, 5131–5150. [Google Scholar] [CrossRef] [Green Version]
- Starink, M.J.; Deschamps, A.; Wang, S.C. The strength of friction stir welded and friction stir processed aluminum alloys. Scripta Mater. 2008, 58, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Qiu, Y.; Qi, Y.; Birbilis, N. A closer inspection of a grain boundary immune to intergranular corrosion in a sensitised Al-Mg alloy. Corros. Sci. 2018, 133, 1–5. [Google Scholar] [CrossRef]
- Lervik, A.; Wenner, S.; Lunder, O.; Marioara, C.D.; Holmestad, R. Grain boundary structures and their correlation with intergranular corrosion in an extruded Al-Mg-Si-Cu alloy. Mater. Charact. 2020, 170, 110695. [Google Scholar] [CrossRef]
- Xu, W.; Xin, Y.C.; Zhang, B.; Li, X.Y. Stress corrosion cracking resistant nanostructured Al-Mg alloy with low angle grain boundaries. Acta Mater. 2022, 225, 117607. [Google Scholar] [CrossRef]




| Mg | Li | Zn | Sc | Zr | Al |
|---|---|---|---|---|---|
| 4.75 | 1.77 | 0.53 | 0.06 | 0.11 | Bal |
| 5 Systems (Taylor Model) | 1 System (Sachs Model) | 3.5 Systems (According to Hutchinson’s Model) | |
|---|---|---|---|
| Typical rolling textures | |||
| Copper | 3.70 | 3.04 | 3.44 |
| S | 3.33 | 2.50 | 2.97 |
| Brass | 3.17 | 2.44 | 2.94 |
| Typical recrystallization textures | |||
| Goss | 2.45 | 2.45 | 2.45 |
| Cube | 2.45 | 2.45 | 2.45 |
| Texture-free FCC classical models | 3.07 | 2.24 | 2.60 |
| Different Samples | 5 Systems (Taylor Model) | 1 System (Sachs Model) | 3.5 Systems (According to Hutchinson’s Model) |
|---|---|---|---|
| S420 | 3.23 | 2.51 | 2.96 |
| S450 | 3.25 | 2.57 | 2.99 |
| S480 | 3.26 | 2.57 | 2.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wu, M.; Xiao, D.; Huang, L.; Liu, W.; Tang, S. Effect of Rolling Temperature on Microstructure and Properties of Al-Mg-Li Alloy. Materials 2022, 15, 7517. https://doi.org/10.3390/ma15217517
Li W, Wu M, Xiao D, Huang L, Liu W, Tang S. Effect of Rolling Temperature on Microstructure and Properties of Al-Mg-Li Alloy. Materials. 2022; 15(21):7517. https://doi.org/10.3390/ma15217517
Chicago/Turabian StyleLi, Weiwei, Mingdong Wu, Daihong Xiao, Lanping Huang, Wensheng Liu, and Sai Tang. 2022. "Effect of Rolling Temperature on Microstructure and Properties of Al-Mg-Li Alloy" Materials 15, no. 21: 7517. https://doi.org/10.3390/ma15217517





