Quasi-Solid-State Polymer Electrolyte Based on Electrospun Polyacrylonitrile/Polysilsesquioxane Composite Nanofiber Membrane for High-Performance Lithium Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of PAN@PSiO Composite Nanofibers Membrane
2.3. Methods and Characterizations
2.4. Battery Assembly and Electrochemical Tests
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Lee, K.; Kim, S.; Park, J.; Park, S.H.; Coskun, A.; Jung, D.S.; Cho, W.; Choi, J.W. Selection of Binder and Solvent for Solution-Processed All-Solid-State Battery. J. Electrochem. Soc. 2017, 164, A2075–A2081. [Google Scholar] [CrossRef]
- Li, Y.; Xu, B.; Xu, H.; Duan, H.; Lu, X.; Xin, S.; Zhou, W.; Xue, L.; Fu, G.; Manthiram, A.; et al. Hybrid Polymer/Garnet Electrolyte with a Small Interfacial Resistance for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 56, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ding, Y.; Li, Y.; Peng, L.; Byon, H.R.; Goodenough, J.B.; Yu, G. A chemistry and material perspective on lithium redox flow batteries towards high-density electrical energy storage. Chem. Soc. Rev. 2015, 44, 7968–7996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Bai, Y.; Sun, X.-G.; Li, Y.; Guo, B.; Chen, J.; Veith, G.M.; Hensley, D.K.; Paranthaman, M.P.; Goodenough, J.B.; et al. Superior Conductive Solid-like Electrolytes: Nanoconfining Liquids within the Hollow Structures. Nano Lett. 2015, 15, 3398–3402. [Google Scholar] [CrossRef]
- Li, M.; Zhu, W.; Zhang, P.; Chao, Y.; He, Q.; Yang, B.; Li, H.; Borisevich, A.; Dai, S. Graphene-Analogues Boron Nitride Nanosheets Confining Ionic Liquids: A High-Performance Quasi-Liquid Solid Electrolyte. Small 2016, 12, 3535–3542. [Google Scholar] [CrossRef]
- Yang, C.; Liu, B.; Jiang, F.; Zhang, Y.; Xie, H.; Hitz, E.; Hu, L. Garnet/polymer hybrid ion-conducting protective layer for stable lithium metal anode. Nano Res. 2017, 10, 4256–4265. [Google Scholar] [CrossRef]
- Liu, S.; Shan, H.; Xia, S.; Yan, J.; Yu, J.; Ding, B. Polymer Template Synthesis of Flexible SiO2 Nanofibers to Upgrade Composite Electrolytes. ACS Appl. Mater. Interfaces 2020, 12, 31439–31447. [Google Scholar] [CrossRef]
- Fergus, J.W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569. [Google Scholar] [CrossRef]
- MacGlashan, G.S.; Andreev, Y.G.; Bruce, P.G. Structure of the polymer electrolyte poly(ethylene oxide)6: LiAsF6. Nature 1999, 398, 792–794. [Google Scholar] [CrossRef]
- Chen, R.; Qu, W.; Guo, X.; Li, L.; Wu, F. The pursuit of solid-state electrolytes for lithium batteries: From comprehensive insight to emerging horizons. Mater. Horiz. 2016, 3, 487–516. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Lin, D.; Liu, W.; Liu, Y.; Lee, H.R.; Hsu, P.-C.; Liu, K.; Cui, Y. High Ionic Conductivity of Composite Solid Polymer Electrolyte via In Situ Synthesis of Monodispersed SiO2 Nanospheres in Poly(ethylene oxide). Nano Lett. 2015, 16, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, H.; Cui, Z.; Zhou, Q.; Shangguan, X.; Tian, S.; Zhou, X.; Cui, G. Siloxane-based polymer electrolytes for solid-state lithium batteries. Energy Storage Mater. 2019, 23, 466–490. [Google Scholar] [CrossRef]
- Jeong, K.; Park, S.; Lee, S.-Y. Revisiting polymeric single lithium-ion conductors as an organic route for all-solid-state lithium ion and metal batteries. J. Mater. Chem. A 2018, 7, 1917–1935. [Google Scholar] [CrossRef]
- Yang, G.; Chanthad, C.; Oh, H.; Ayhan, I.A.; Wang, Q. Organic–inorganic hybrid electrolytes from ionic liquid-functionalized octasilsesquioxane for lithium metal batteries. J. Mater. Chem. A 2017, 5, 18012–18019. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Y.; Dong, L.; Chen, L.; Lu, Q.; Li, M.; Zeng, X.; Dai, S.; Chen, G.; Shi, L. Multiclaw-shaped octasilsesquioxanes functionalized ionic liquids toward organic-inorganic composite electrolytes for lithium-ion batteries. Chem. Eng. J. 2020, 405, 126942. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.; Liu, C.; Yang, Y.; Li, Y. Quasi-Solid-State Electrolyte Membranes Based on Helical Mesoporous Polysilsesquioxane Nanofibers for High-Performance Lithium Batteries. J. Taiwan Inst. Chem. Eng. 2022, 135, 104399. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Deng, Y.; Pan, Y.; Zhang, Z.; Fu, Y.; Gong, L.; Liu, C.; Yang, J.; Zhang, H.; Cheng, X. Novel thermotolerant and flexible polyimide aerogel separator achieving advanced lithium-ion batteries. Adv. Funct. Mater. 2022, 32, 2106176. [Google Scholar] [CrossRef]
- Zhai, Y.; Xiao, K.; Yu, J.; Yang, J.; Ding, B. Thermostable and nonflammable silica–polyetherimide–polyurethane nanofibrous separators for high power lithium ion batteries. J. Mater. Chem. A 2015, 3, 10551–10558. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Jian, J.; Lu, Y.; Zhang, B.; Liu, H.; Li, L.; Wang, X.; Kuang, C.; Zhai, Y. A dual-layer micro/nanostructured fibrous membrane with enhanced ionic conductivity for lithium-ion battery. Electrochim. Acta 2018, 292, 357–363. [Google Scholar] [CrossRef]
- Rao, E.; McVerry, B.; Borenstein, A.; Anderson, M.; Jordan, R.S.; Kaner, R.B. Roll-to-Roll Functionalization of Polyolefin Separators for High-Performance Lithium-Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 3292–3300. [Google Scholar] [CrossRef]
- Sheng, J.; Chen, T.; Wang, R.; Zhang, Z.; Hua, F.; Yang, R. Ultra-light cellulose nanofibril membrane for lithium-ion batteries. J. Membr. Sci. 2019, 595, 117550. [Google Scholar] [CrossRef]
- Shi, J.; Xia, Y.; Han, S.; Fang, L.; Pan, M.; Xu, X.; Liu, Z. Lithium ion conductive Li1.5Al0.5Ge1.5(PO4)3 based inorganic-organic composite separator with enhanced thermal stability and excellent electrochemical performances in 5 V lithium ion batteries. J. Power Sources 2015, 273, 389. [Google Scholar] [CrossRef]
- Yu, L.; Miao, J.; Lin, J.Y.S.; Jin, Y. A comparative study on polypropylene separators coated with different inorganic materials for lithium-ion batteries. Front. Chem. Sci. Eng. 2017, 11, 346–352. [Google Scholar] [CrossRef]
- Sheng, S.Z. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351. [Google Scholar]
- Jeong, H.-S.; Lee, S.-Y. Closely packed SiO2 nanoparticles/poly(vinylidene fluoride-hexafluoropropylene) layers-coated polyethylene separators for lithium-ion batteries. J. Power Sources 2011, 196, 6716–6722. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Lu, Y.; Zhu, J.; Zhang, X. Silica/polyacrylonitrile hybrid nanofiber membrane separators via sol-gel and electrospinning techniques for lithium-ion batteries. J. Power Sources 2016, 313, 205–212. [Google Scholar] [CrossRef]
- Kim, P.S.; Le Mong, A.; Kim, D. Thermal, mechanical, and electrochemical stability enhancement of Al2O3 coated polypropylene/polyethylene/polypropylene separator via poly(vinylidene fluoride)-poly(ethoxylated pentaerythritol tetraacrylate) semi-interpenetrating network binder. J. Membr. Sci. 2020, 612, 118481. [Google Scholar] [CrossRef]
- Xiao, K.; Zhai, Y.; Yu, J.; Ding, B. Nanonet-structured poly(m-phenylene isophthalamide)–polyurethane membranes with enhanced thermostability and wettability for high power lithium ion batteries. RSC Adv. 2015, 5, 55478–55485. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, L.; Toprakçi, O.; Liang, Y.; Alcoutlabi, M. Electrospun Nanofiber-Based Anodes, Cathodes, and Separators for Advanced Lithium-Ion Batteries. Polym. Rev. 2011, 51, 239–264. [Google Scholar] [CrossRef]
- Cho, T.-H.; Tanaka, M.; Onishi, H.; Kondo, Y.; Nakamura, T.; Yamazaki, H.; Tanase, S.; Sakai, T. Battery performances and thermal stability of polyacrylonitrile nano-fiber-based nonwoven separators for Li-ion battery. J. Power Sources 2008, 181, 155–160. [Google Scholar] [CrossRef]
- Nien, Y.-H.; Chang, C.-N.; Chuang, P.-L.; Hsu, C.-H.; Liao, J.-L.; Lee, C.-K. Fabrication and characterization of nylon 66/PAN nanofibrous film used as separator of lithium-ion battery. Polymers 2021, 13, 1984. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhang, W.; Muhammada, I.P.; Chen, X.; Zeng, Y.; Wang, B.; Zhang, S. Coaxially electrospun PAN/HCNFs@PVDF/UiO-66 composite separator with high strength and thermal stability for lithium-ion battery. Microporous Mesoporous Mater. 2021, 311, 110724. [Google Scholar] [CrossRef]
- Dong, G.X.; Li, H.J.; Wang, Y.; Jiang, W.J.; Ma, Z.S. Electrospun PAN/cellulose composite separator for high performance lithium-ion battery. Ionics 2021, 27, 2955–2965. [Google Scholar] [CrossRef]
- He, C.; Liu, J.; Li, J.; Zhu, F.; Zhao, H. Blending based polyacrylonitrile/poly(vinyl alcohol) membrane for rechargeable lithium ion batteries. J. Membr. Sci. 2018, 560, 30–37. [Google Scholar] [CrossRef]
- Carol, P.; Ramakrishnan, P.; John, B.; Cheruvally, G. Preparation and characterization of electrospun poly(acrylonitrile) fibrous membrane based gel polymer electrolytes for lithium-ion batteries. J. Power Sources 2011, 196, 10156–10162. [Google Scholar] [CrossRef]
- Yuan, X.; Razzaq, A.A.; Chen, Y.; Lian, Y.; Zhao, X.; Peng, Y.; Deng, Z. Polyacrylonitrile-based gel polymer electrolyte filled with Prussian blue for high-performance lithium polymer batteries. Chin. Chem. Lett. 2021, 32, 890. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, Z.; Qiu, Y.; Zhang, X. Fabrication and characterization of LATP/PAN composite fiber-based lithium-ion battery separators. Electrochim. Acta 2011, 56, 6474–6480. [Google Scholar] [CrossRef]
- Dong, T.; Arifeen, W.U.; Choi, J.; Yoo, K.; Ko, T. Surface-modified electrospun polyacrylonitrile nano-membrane for a lithium-ion battery separator based on phase separation mechanism. Chem. Eng. J. 2020, 398, 125646. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, Y.; Li, Y. Electrospun Core-Shell Nanofiber as Separator for Lithium-Ion Batteries with High Performance and Improved Safety. Energies 2019, 12, 3391. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Xiong, L.; Chen, X.; Guo, H.; Zhang, H.; Chen, X. Enhanced Electrochemical Properties of Gel Polymer Electrolyte with Hybrid Copolymer of Organic Palygorskite and Methyl Methacrylate. Materials 2018, 11, 1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shomura, R.; Tamate, R.; Matsuda, S. Lithium-Ion-Conducting Ceramics-Coated Separator for StableOperation of Lithium Metal-Based Rechargeable Batteries. Materials 2022, 15, 322. [Google Scholar] [CrossRef]
- Hu, J.; Wang, H.; Yang, Y.; Li, Y.; Wu, Q.-H. A highly conductive quasi-solid-state electrolyte based on helical silica nanofibers for lithium batteries. RSC Adv. 2021, 11, 33858–33866. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Cao, H.; Zhao, L.; Huang, Y.; Song, A.; Lin, Y.; Wang, M.; Li, X. A novel polyacrylonitrile-based porous structure gel polymer electrolyte composited by incorporating polyhedral oligomeric silsesquioxane by phase inversion method. J. Solid State Electrochem. 2018, 22, 1771–1783. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Sun, K.; Yang, K.; Chen, F. Preparation and characterization of gel polymer electrolyte based on electrospun polyhedral oligomeric silsesquioxane-poly(methylmethacrylate)/polyvinylidene fluoride hybrid nanofiber membranes for lithium-ion batteries. J. Solid State Electrochem. 2018, 22, 581. [Google Scholar] [CrossRef]



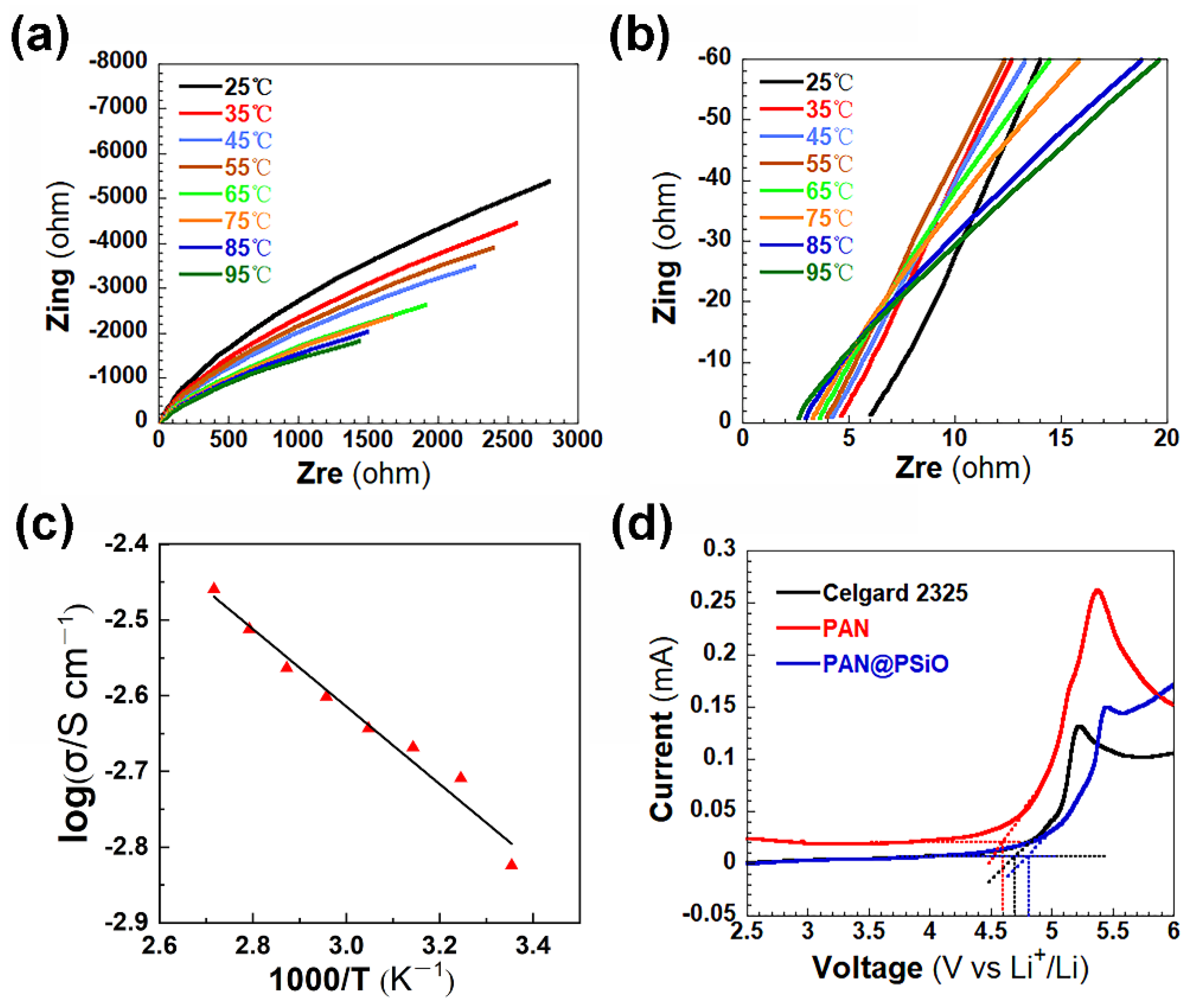
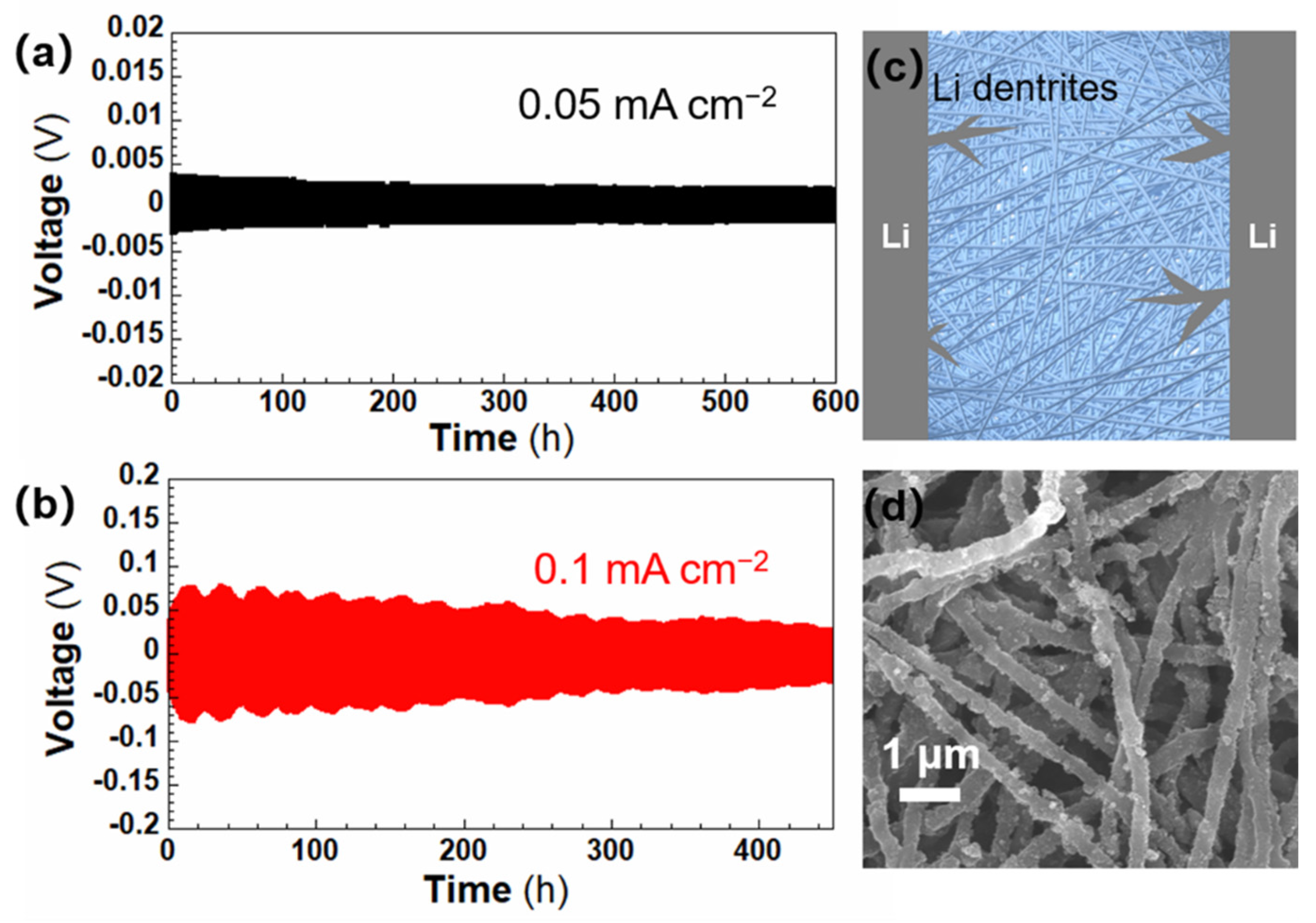
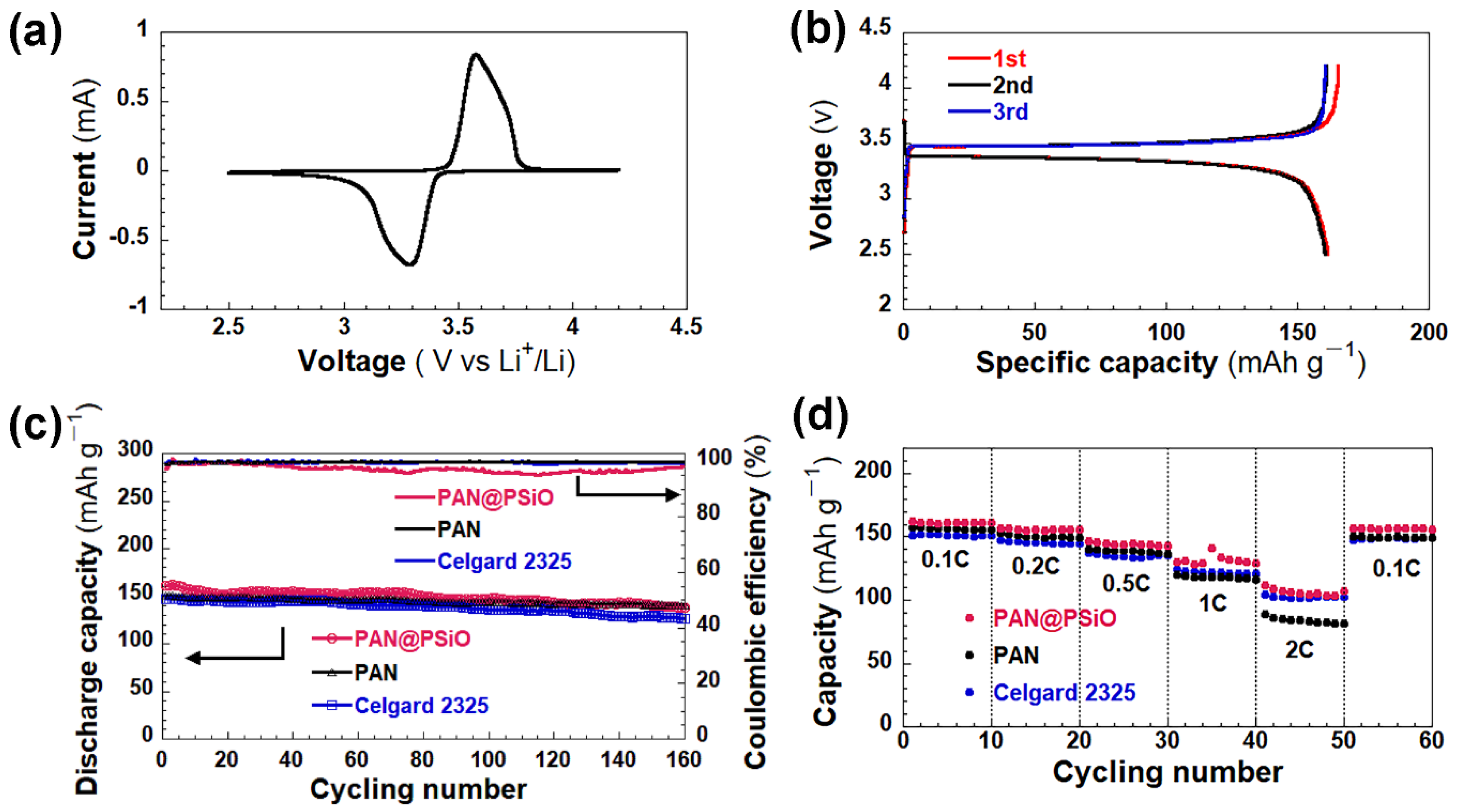
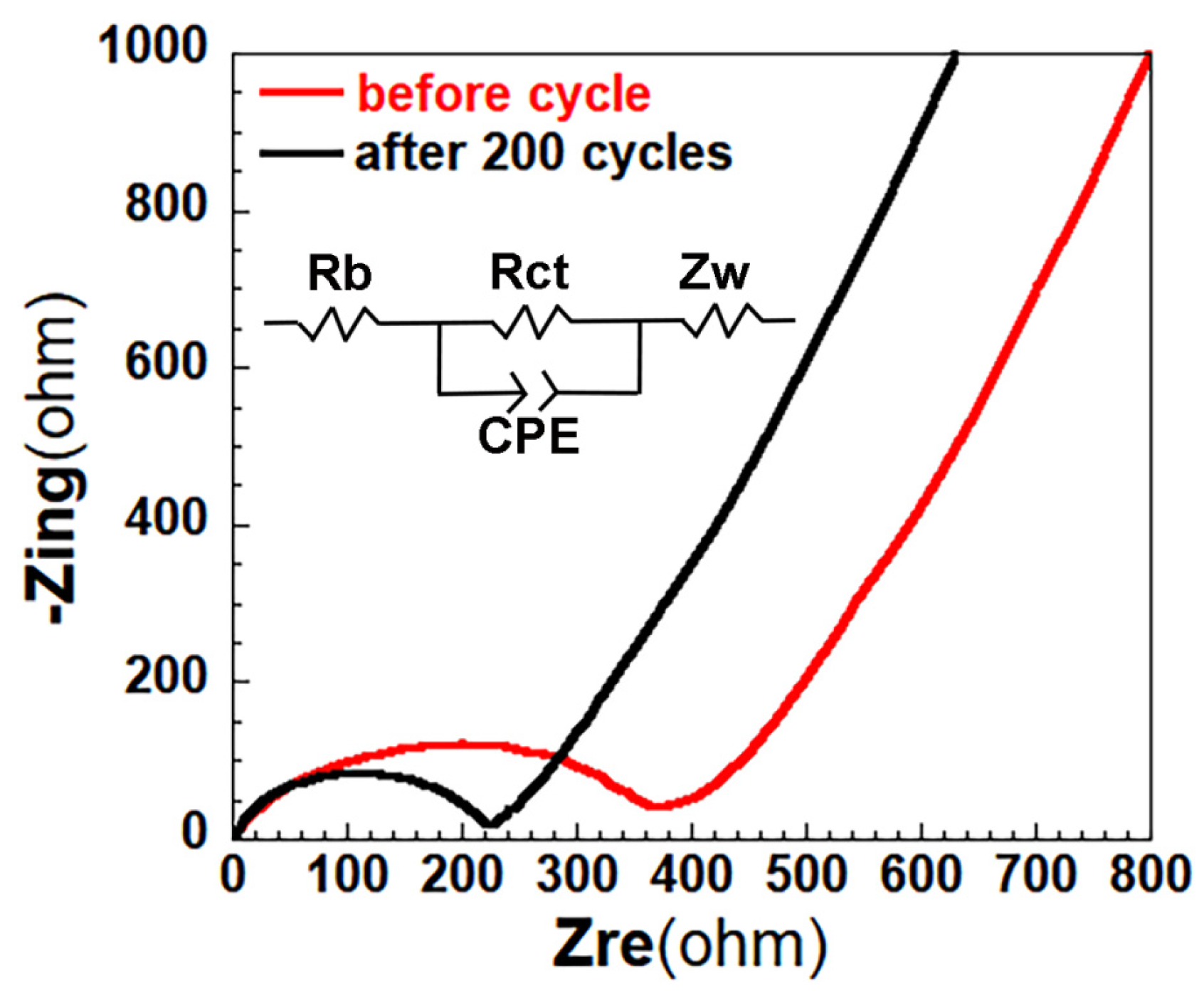
| Separator | Porosity (%) | Electrolyte * Intake (%) | Thickness (μm) | Ontology Impedance (ω) | Ionic Conductivity (mS cm−1) |
|---|---|---|---|---|---|
| Celgard 2325 | 46.9 | 90.5 | 25 | 3.57 | 0.35 |
| PAN | 70.0 | 261 | 170 | 7.49 | 1.13 |
| PAN@PSiO | 68.3 | 297 | 180 | 5.71 | 1.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Hu, J.; Zhu, Y.; Yang, Y.; Li, Y.; Wu, Q.-H. Quasi-Solid-State Polymer Electrolyte Based on Electrospun Polyacrylonitrile/Polysilsesquioxane Composite Nanofiber Membrane for High-Performance Lithium Batteries. Materials 2022, 15, 7527. https://doi.org/10.3390/ma15217527
Liu C, Hu J, Zhu Y, Yang Y, Li Y, Wu Q-H. Quasi-Solid-State Polymer Electrolyte Based on Electrospun Polyacrylonitrile/Polysilsesquioxane Composite Nanofiber Membrane for High-Performance Lithium Batteries. Materials. 2022; 15(21):7527. https://doi.org/10.3390/ma15217527
Chicago/Turabian StyleLiu, Caiyuan, Jiemei Hu, Yanan Zhu, Yonggang Yang, Yi Li, and Qi-Hui Wu. 2022. "Quasi-Solid-State Polymer Electrolyte Based on Electrospun Polyacrylonitrile/Polysilsesquioxane Composite Nanofiber Membrane for High-Performance Lithium Batteries" Materials 15, no. 21: 7527. https://doi.org/10.3390/ma15217527
APA StyleLiu, C., Hu, J., Zhu, Y., Yang, Y., Li, Y., & Wu, Q.-H. (2022). Quasi-Solid-State Polymer Electrolyte Based on Electrospun Polyacrylonitrile/Polysilsesquioxane Composite Nanofiber Membrane for High-Performance Lithium Batteries. Materials, 15(21), 7527. https://doi.org/10.3390/ma15217527








