A Comparative Density Functional Theory Study of Hydrogen Storage in Cellulose and Chitosan Functionalized by Transition Metals (Ti, Mg, and Nb)
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. Interaction of Hydrogen with Pristine Cellulose and Chitosan
3.2. Hydrogen Storage of Cellulose Coated with Magnesium, Niobium, and Titanium
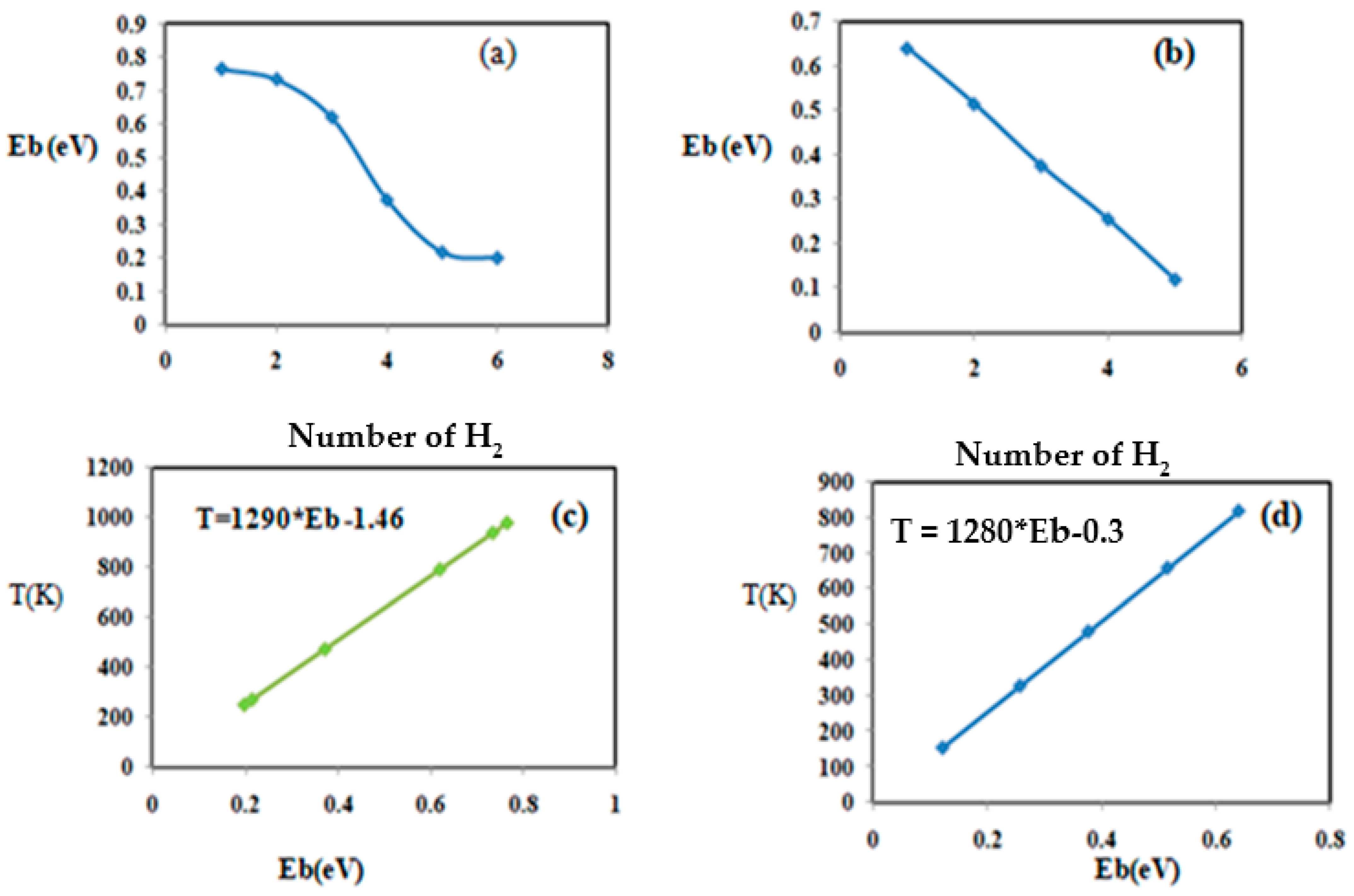
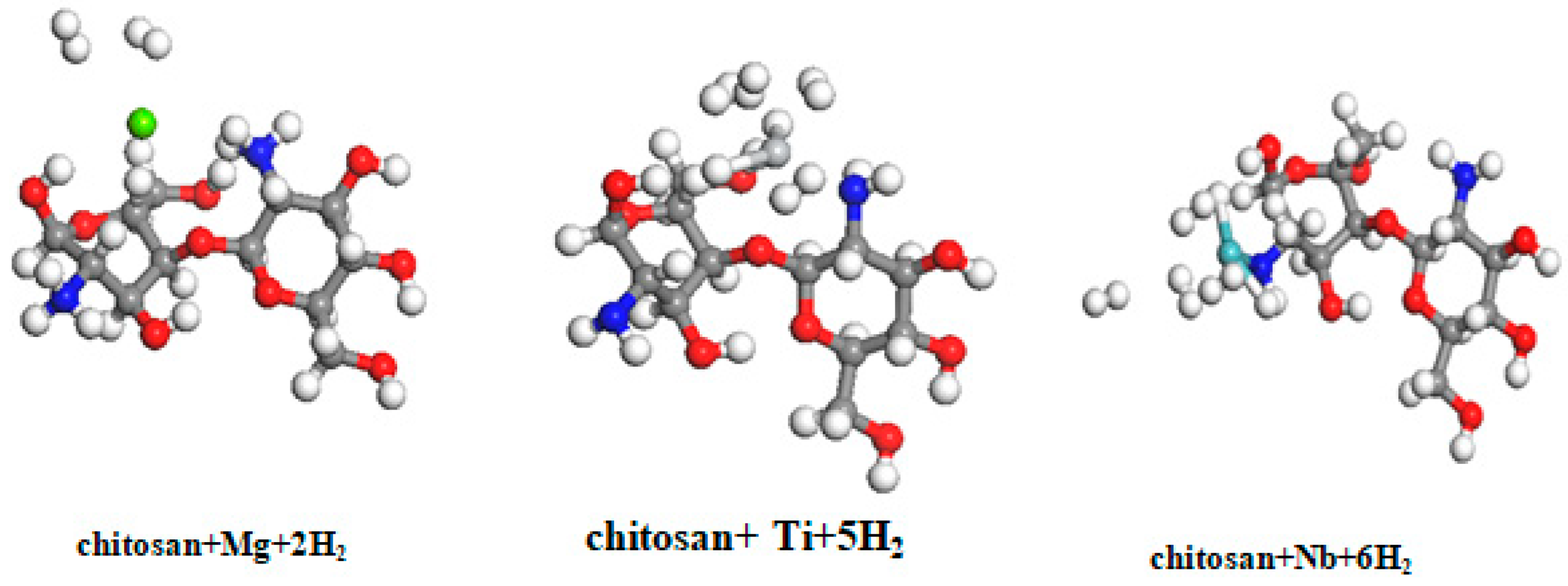
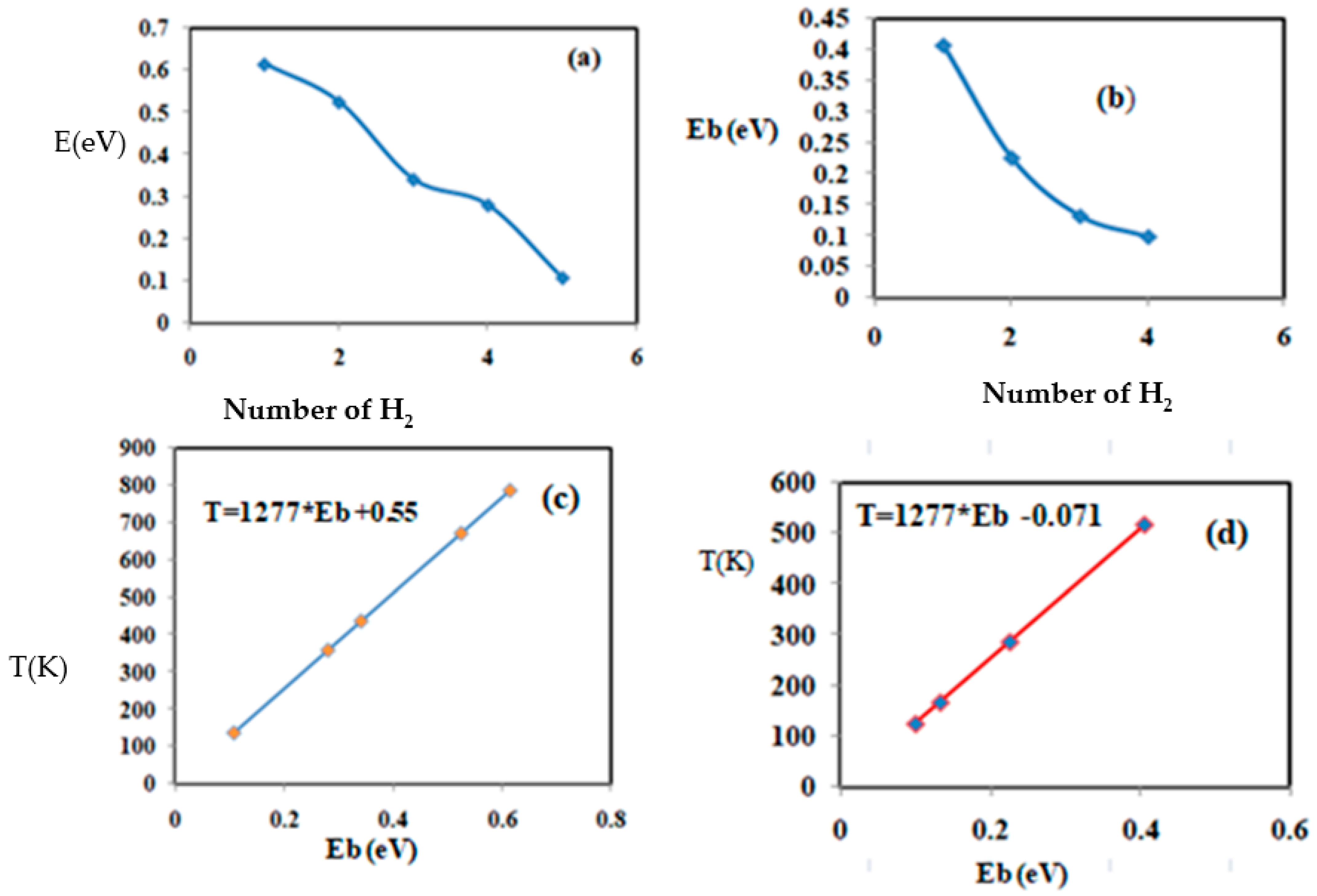
3.3. Hydrogen Storage on Chitosan Coated with Magnesium, Niobium, and Titanium
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faye, O.; Eduok, U.; Szpunar, J.A.; Beye, A.C. Two-dimensional carbon nitride (C3N) nanosheets as promising materials for H2S and NH3 elimination: A computational approach. Phys. E Low-Dimens. Syst. Nanostructures 2020, 117, 113794. [Google Scholar] [CrossRef]
- Faye, O.; Szpunar, J.A. An Efficient Way To Suppress the Competition between Adsorption of H2 and Desorption of nH2–Nb Complex from Graphene Sheet: A Promising Approach to H2 Storage. J. Phys. Chem. C 2018, 122, 28506–28517. [Google Scholar] [CrossRef]
- Cousins, K.; Zhang, R. Highly Porous Organic Polymers for Hydrogen Fuel Storage. Polymers 2019, 11, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jena, P. Materials for Hydrogen Storage: Past, Present, and Future. J. Phys. Chem. Lett. 2011, 2, 206–211. [Google Scholar] [CrossRef]
- Von Colbe, J.B.; Ares, J.-R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Satyapal, S.; Petrovic, J.; Read, C.; Thomas, G.; Ordaz, G. The U.S. Department of Energy’s National Hydrogen Storage Project: Progress towards meeting hydrogen-powered vehicle requirements. Catal. Today 2007, 120, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Wu, X.; Zeng, X.C. Designs of fullerene-based frameworks for hydrogen storage. J. Mater. Chem. A 2014, 2, 5910–5914. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; North, B.C.; Mathe, M.; Kang, X.; Liao, S. Hydrogen storage in Zr-fumarate MOF. Int. J. Hydrogen Energy 2015, 40, 10542–10546. [Google Scholar] [CrossRef]
- Mahmood, A.; Khan, S.U.-D.; Rana, U.A.; Tahir, M.H. Red shifting of absorption maxima of phenothiazine based dyes by incorporating electron-deficient thiadiazole derivatives as π-spacer. Arab. J. Chem. 2019, 12, 1447–1453. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, A.; HussainTahir, M.; Irfan, A.; Khalid, B.; Al-Sehemi, A.G. Computational Designing of Triphenylamine Dyes with Broad and Red-shifted Absorption Spectra for Dye-sensitized Solar Cells using Multi-Thiophene Rings in π-Spacer. Bull. Korean Chem. Soc. 2015, 36, 2615–2620. [Google Scholar] [CrossRef]
- Mahmood, A.; Irfan, A. Effect of fluorination on exciton binding energy and electronic coupling in small molecule acceptors for organic solar cells. Comput. Theor. Chem. 2020, 1179, 112797. [Google Scholar] [CrossRef]
- Mahmood, A.; Irfan, A.; Ahmad, F.; Janjua, M.R.S.A. Quantum chemical analysis and molecular dynamics simulations to study the impact of electron-deficient substituents on electronic behavior of small molecule acceptors. Comput. Theor. Chem. 2021, 1204, 113387. [Google Scholar] [CrossRef]
- Mahmood, A.; Khan, S.U.-D.; Rana, U.A.; Janjua, M.R.S.A.; Tahir, M.H.; Nazar, M.F.; Song, Y. Effect of thiophene rings on UV/visible spectra and non-linear optical (NLO) properties of triphenylamine based dyes: A quantum chemical perspective. J. Phys. Org. Chem. 2015, 28, 418–422. [Google Scholar] [CrossRef]
- Wei, T.; Lim, K.; Tseng, Y.; Chan, S. A review on the characterization of hydrogen in hydrogen storage materials. Renew. Sustain. Energy Rev. 2017, 79, 1122–1133. [Google Scholar] [CrossRef]
- Ozturk, Z.; Baykasoglu, C.; Celebi, A.T.; Kirca, M.; Mugan, A.; To, A.C. Hydrogen storage in heat welded random CNT network structures. Int. J. Hydrogen Energy 2015, 40, 403–411. [Google Scholar] [CrossRef]
- Manilov, A.; Skryshevsky, V. Hydrogen in porous silicon—A review. Mater. Sci. Eng. B 2013, 178, 942–955. [Google Scholar] [CrossRef]
- Faye, O.; Hussain, T.; Karton, A.; Szpunar, J.A. Tailoring the capability of carbon nitride (C3N) nanosheets toward hydrogen storage upon light transition metal decoration. Nanotechnology 2019, 30, 075404. [Google Scholar] [CrossRef]
- Kato, R.; Nishide, H. Polymers for carrying and storing hydrogen. Polym. J. 2018, 50, 77–82. [Google Scholar] [CrossRef]
- Xiong, R.; Sang, G.; Zhang, G.; Yan, X.; Li, P.; Yao, Y.; Luo, D.; Chen, C.; Tang, T. Evolution of the active species and catalytic mechanism of Ti doped NaAlH 4 for hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 6088–6095. [Google Scholar] [CrossRef]
- Aizat, M.A.; Aziz, F. Chitosan nanocomposite application in wastewater treatments. In Nanotechnology in Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–265. [Google Scholar] [CrossRef]
- Abou-Zeid, R.E.; Khiari, R.; El-Wakil, N.; Dufresne, A. Current State and New Trends in the Use of Cellulose Nanomaterials for Wastewater Treatment. Biomacromolecules 2019, 20, 573–597. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Self-Assembled and Cross-Linked Animal and Plant-Based Polysaccharides: Chitosan–Cellulose Composites and Their Anion Uptake Properties. ACS Appl. Mater. Interfaces 2016, 8, 33197–33209. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Kim, J.; Ide, Y.; Kim, J.H.; Hossain, M.S.A.; Bando, Y.; Yamauchi, Y.; Wu, K.C.-W. 3D network of cellulose-based energy storage devices and related emerging applications. Mater. Horiz. 2017, 4, 522–545. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Yu, H.; Lee, S.-Y.; Wei, T.; Li, J.; Fan, Z. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef]
- Nainggolan, I.; Shantini, D.; Nasution, T.I.; Derman, M.N. Role of Metals Content in Spinach in Enhancing the Conductivity and Optical Band Gap of Chitosan Films. Adv. Mater. Sci. Eng. 2015, 2015, 702815. [Google Scholar] [CrossRef] [Green Version]
- Trache, D.; Thakur, V.; Boukherroub, R. Cellulose Nanocrystals/Graphene Hybrids—A Promising New Class of Materials for Advanced Applications. Nanomaterials 2020, 10, 1523. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- Vidal, R.R.L.; Moraes, J.S. Removal of organic pollutants from wastewater using chitosan: A literature review. Int. J. Environ. Sci. Technol. 2019, 16, 1741–1754. [Google Scholar] [CrossRef]
- Peter, S.; Lyczko, N.; Gopakumar, D.; Maria, H.J.; Nzihou, A.; Thomas, S. Chitin and Chitosan Based Composites for Energy and Environmental Applications: A Review. Waste Biomass-Valorization 2020, 12, 4777–4804. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Erratum: Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 48, 4978. [Google Scholar] [CrossRef]
- Fritsch, D.; Koepernik, K.; Richter, M.; Eschrig, H. Transition Metal Dimers as Potential Molecular Magnets. J. Comput. Chem. 2008, 1145, 2210–2219. [Google Scholar] [CrossRef]
- Simão, C.D.; Reparaz, J.S.; Wagner, M.R.; Graczykowski, B.; Kreuzer, M.; Ruiz-Blanco, Y.B.; García, Y.; Malho, J.-M.; Goñi, A.R.; Ahopelto, J.; et al. Optical and mechanical properties of nanofibrillated cellulose: Toward a robust platform for next-generation green technologies. Carbohydr. Polym. 2015, 126, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elrahman, M.I.; Ahmed, M.O.; Abdel-Aleem, J.A. Optical properties of cellulose derivatives blend film carrying a chalcogenide material. Mater. Sci. Semicond. Process. 2013, 16, 1052–1056. [Google Scholar] [CrossRef]
- Hai, T.A.P.; Sugimoto, R. Fluorescence control of chitin and chitosan fabricated via surface functionalization using direct oxidative polymerization. RSC Adv. 2018, 8, 7005–7013. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, A. Photovoltaic and Charge Transport Behavior of Diketopyrrolopyrrole Based Compounds with A–D–A–D–A Skeleton. J. Clust. Sci. 2019, 30, 1123–1130. [Google Scholar] [CrossRef]
- Mahmood, A.; Abdullah, M.I.; Nazar, M.F. Quantum Chemical Designing of Novel Organic Non-Linear Optical Compounds. Bull. Korean Chem. Soc. 2014, 35, 1391–1396. [Google Scholar] [CrossRef] [Green Version]
- Khalid, M.; Khan, M.U.; Shafiq, I.; Hussain, R.; Ali, A.; Imran, M.; Braga, A.A.C.; Rehman, M.F.U.; Akram, M.S. Structural modulation of π-conjugated linkers in D–π–A dyes based on triphenylamine dicyanovinylene framework to explore the NLO properties. R. Soc. Open Sci. 2021, 8, 210570. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Khan, M.U.; Shafiq, I.; Hussain, R.; Mahmood, K.; Hussain, A.; Jawaria, R.; Hussain, A.; Imran, M.; Assiri, M.A.; et al. NLO potential exploration for D–π–A heterocyclic organic compounds by incorporation of various π-linkers and acceptor units. Arab. J. Chem. 2021, 14, 103295. [Google Scholar] [CrossRef]
- Khalid, M.; Lodhi, H.M.; Khan, M.U.; Imran, M. Structural parameter-modulated nonlinear optical amplitude of acceptor–π–D–π–donor-configured pyrene derivatives: A DFT approach. RSC Adv. 2021, 11, 14237–14250. [Google Scholar] [CrossRef]
- Singla, M.; Jaggi, N. Enhanced hydrogen sensing properties in copper decorated nitrogen doped defective graphene nanoribbons: DFT study. Phys. E Low-Dimens. Syst. Nanostructures 2021, 131, 114756. [Google Scholar] [CrossRef]
- Lousada, C.M. Wood cellulose as a hydrogen storage material. Int. J. Hydrogen Energy 2020, 45, 14907–14914. [Google Scholar] [CrossRef]
- Lee, H.; Choi, W.I.; Ihm, J. Combinatorial Search for Optimal Hydrogen-Storage Nanomaterials Based on Polymers. Phys. Rev. Lett. 2006, 97, 056104. [Google Scholar] [CrossRef] [Green Version]
- Durgun, E.; Ciraci, S.; Yildirim, T. Functionalization of carbon-based nanostructures with light transition-metal atoms for hydrogen storage. Phys. Rev. B 2008, 77, 085405. [Google Scholar] [CrossRef] [Green Version]
- Alhameedi, K.; Karton, A.; Jayatilaka, D.; Hussain, T. Metal functionalized inorganic nano-sheets as promising materials for clean energy storage. Appl. Surf. Sci. 2019, 471, 887–892. [Google Scholar] [CrossRef]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
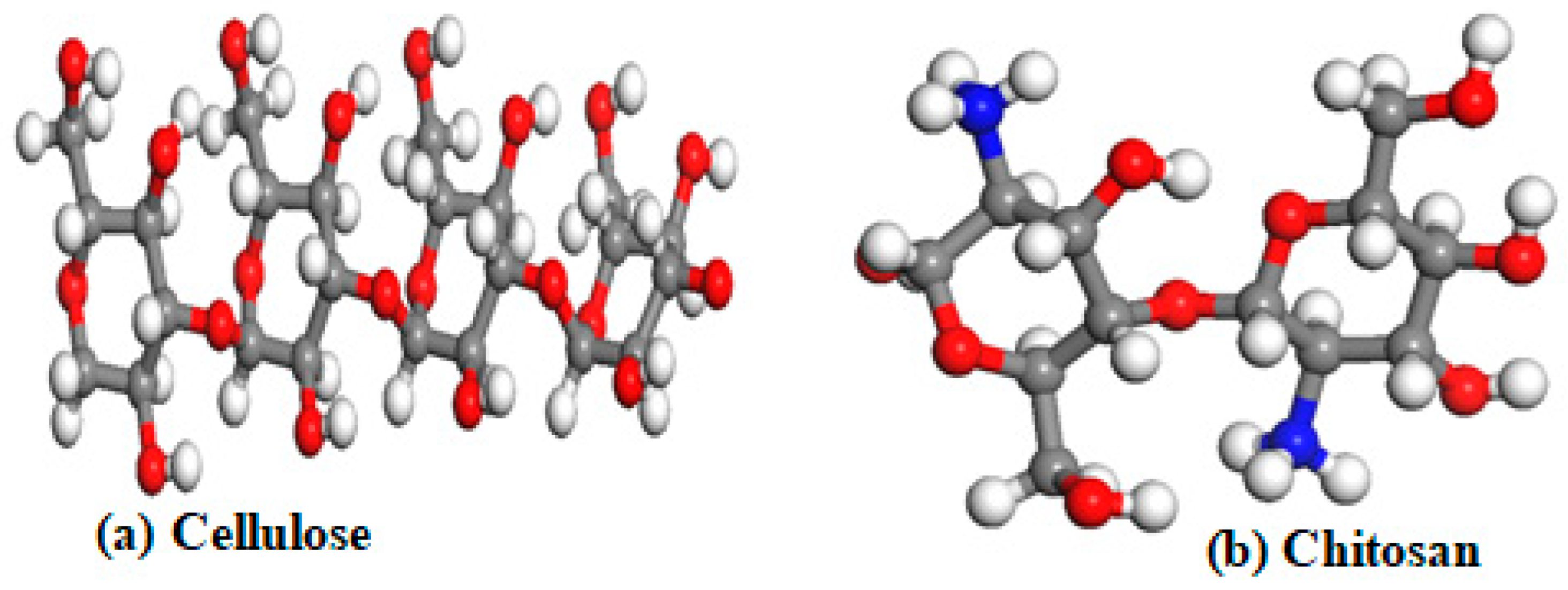

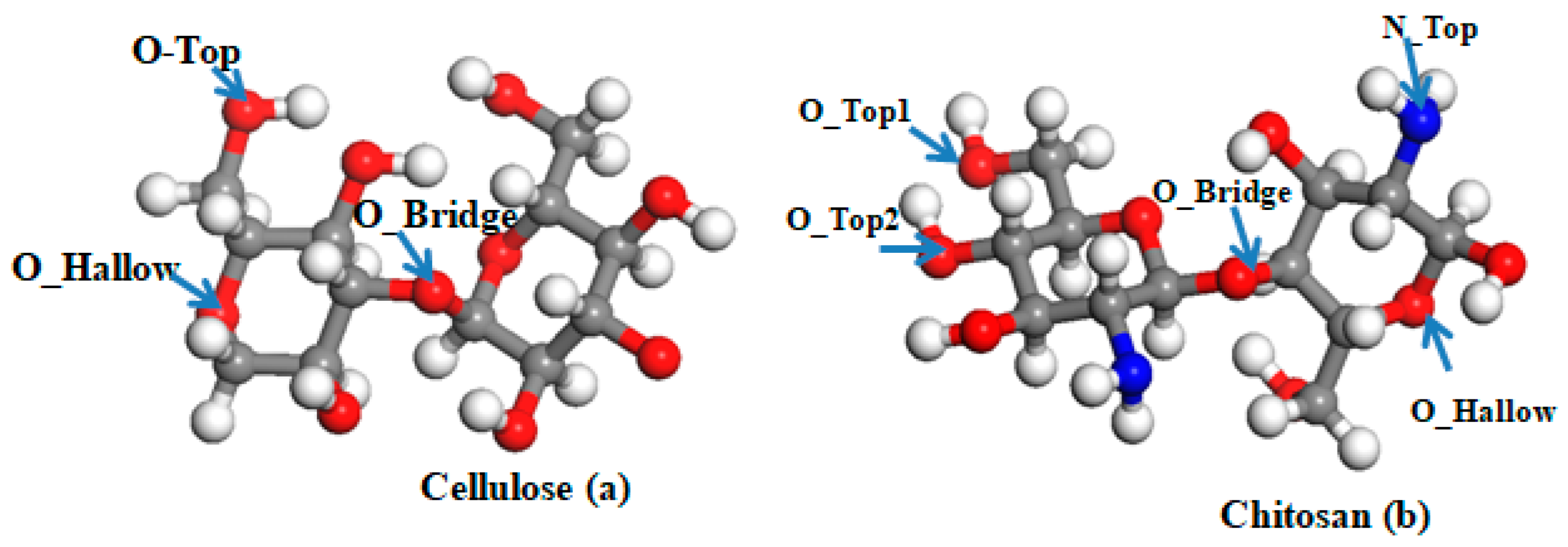
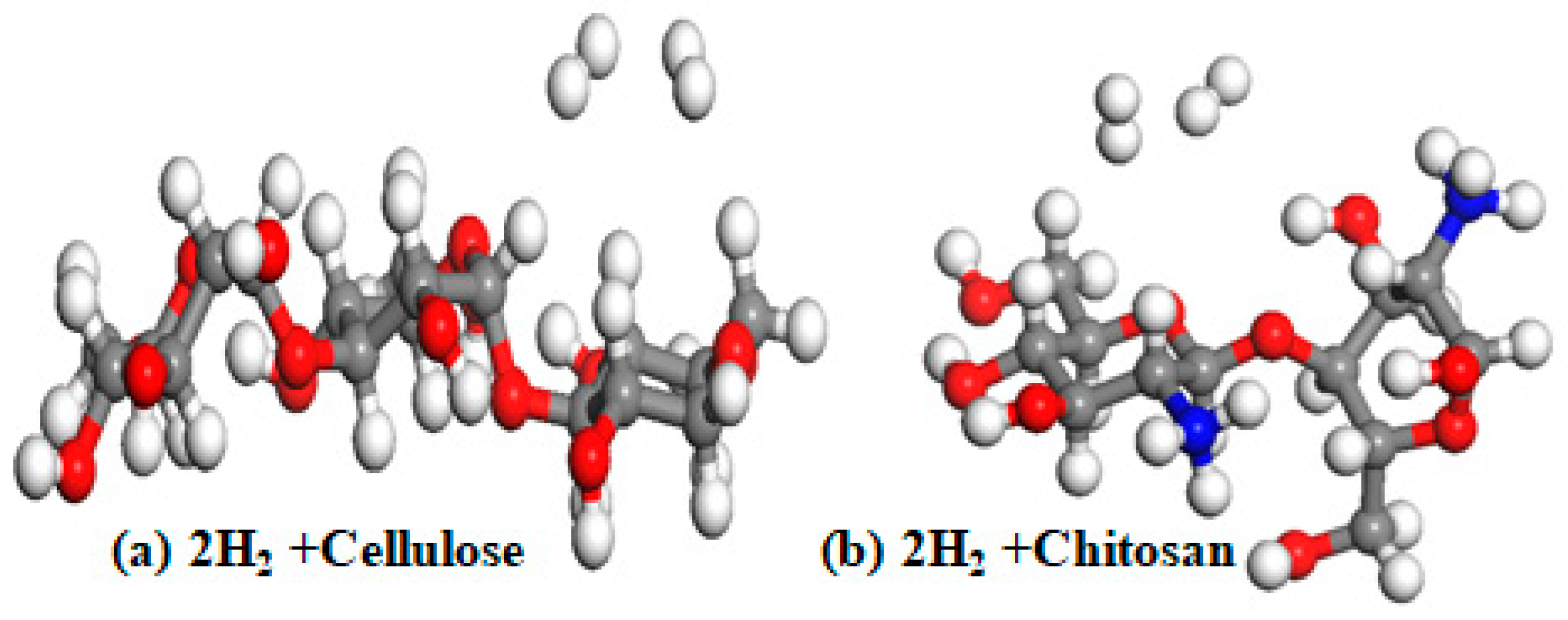
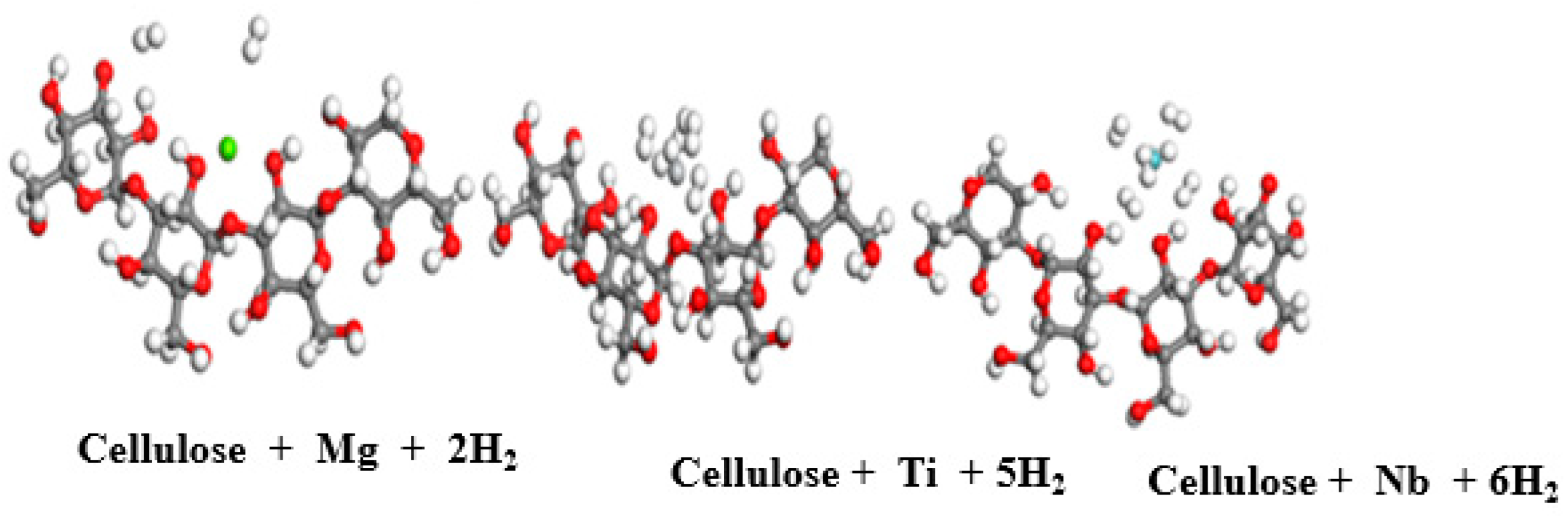
| Cellulose Parameters | |
|---|---|
| −16.899 | |
| −12.269 | |
| Ionization energy (I) | 16.899 |
| Electron affinity (A) | 12.269 |
| Energy gap (Eg) | 4.630 |
| Electronegativity (α) | 14.584 |
| Chemical potential (µ) | −14.584 |
| Chemical hardness (β) | 2.315 |
| Chemical softness (S) | 0.216 |
| Maximum charge transfer (Dn) | 3.149 |
| Chitosan parameters | |
| −5.918 | |
| −1.196 | |
| Ionization energy (I) | 5.918 |
| Electron affinity (A) | 1.196 |
| Energy gap (Eg) | 4.721 |
| Electronegativity (α) | 3.557 |
| Chemical potential (µ) | −3.557 |
| Chemical hardness (β) | 2.361 |
| Chemical softness (S) | 0.212 |
| Maximum charge transfer (Dn) | 1.507 |
| Substrate | Eb (eV) | d_F (Å) | Eg (eV) |
|---|---|---|---|
| cellulose + Mg | 1.890 | 2.544 | 2.46 |
| cellulose + Ti | 3.72 | 2.340 | 1.50 |
| cellulose + Nb | 3.726 | 2.3 | 0.25 |
| pristine cellulose | 4.630 | ||
| pristine chitosan | 4.720 | ||
| chitosan + Mg | 1.547 | 3.666 | 2.770 |
| chitosan +Ti | 5.450 | 3.255 | 1.570 |
| chitosan + Nb | 8.185 | 2.307 | 2.980 |
| Cellulose + H2 | 0.095 |
| Cellulose + 2H2 | 0.083 |
| Chitosan + H2 | 0.090 |
| Chitosan +2H2 | 0.050 |
| Number of H2 Molecules | (eV) | d_H-H (Å) | d_TM-H (Å) | TD (K) |
|---|---|---|---|---|
| Cell + H2 + Nb | 0.765 | 0.896 | 1.900 | 978 |
| 0.734 | 0.897 | 1.910 | 938 | |
| 0.620 | 0.870 | 1.996 | 792 | |
| 0.372 | 0.840 | 1.999 | 475 | |
| 0.215 | 0.831 | 2.020 | 274 | |
| 0.198 | 0.789 | 2.250 | 253 | |
| Cell + Ti + H2 | 0.640 | 0.850 | 1.920 | 818 |
| 0.515 | 0.858 | 1.895 | 658 | |
| 0.376 | 0.831 | 1.997 | 480 | |
| 0.256 | 0.807 | 2.010 | 327 | |
| 0.120 | 0.792 | 2.231 | 153 | |
| Cell + Mg + H2 | 0.112 | 0.758 | 3.050 | 143 |
| 0.086 | 0.757 | 3.125 | 109 |
| Number of H2 Molecules | Binding Energy | d_H-H (Å) | d_H-M (Å) | TD (K) |
|---|---|---|---|---|
| Ch + Mg + H2 | 0.142 | 0.752 | 3.141 | 99 |
| 0.078 | 0.761 | 3.304 | 181 | |
| 0.101 | 0.756 | 3.280 | 129 | |
| Ch + Ti +H2 | 0.405 | 0.794 | 2.025 | 517 |
| 2H2 | 0.225 | 0.790 | 2.068 | 287 |
| 0.132 | 0.799 | 2.011 | 168 | |
| 0.099 | 0.787 | 2.076 | 126 | |
| Ch + Nb + H2 | ||||
| 0.615 | 0.816 | 2.012 | 786 | |
| 0.525 | 0.825 | 1.995 | 671 | |
| 0.341 | 0.823 | 2.020 | 436 | |
| 0.280 | 0.835 | 2.045 | 358 | |
| 0.107 | 0.800 | 2.890 | 136 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faye, O.; Szpunar, J.A.; Eduok, U. A Comparative Density Functional Theory Study of Hydrogen Storage in Cellulose and Chitosan Functionalized by Transition Metals (Ti, Mg, and Nb). Materials 2022, 15, 7573. https://doi.org/10.3390/ma15217573
Faye O, Szpunar JA, Eduok U. A Comparative Density Functional Theory Study of Hydrogen Storage in Cellulose and Chitosan Functionalized by Transition Metals (Ti, Mg, and Nb). Materials. 2022; 15(21):7573. https://doi.org/10.3390/ma15217573
Chicago/Turabian StyleFaye, Omar, Jerzy A. Szpunar, and Ubong Eduok. 2022. "A Comparative Density Functional Theory Study of Hydrogen Storage in Cellulose and Chitosan Functionalized by Transition Metals (Ti, Mg, and Nb)" Materials 15, no. 21: 7573. https://doi.org/10.3390/ma15217573
APA StyleFaye, O., Szpunar, J. A., & Eduok, U. (2022). A Comparative Density Functional Theory Study of Hydrogen Storage in Cellulose and Chitosan Functionalized by Transition Metals (Ti, Mg, and Nb). Materials, 15(21), 7573. https://doi.org/10.3390/ma15217573





