Influence of TiO2 and ZnO Nanoparticles on α-Synuclein and β-Amyloid Aggregation and Formation of Protein Fibrils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of TiO2 and ZnO Nanoparticles
2.2. Preparation of ZnO and TiO2 Nanoparticles
2.3. SERS Substrate Preparation
2.4. Sample Preparation for Raman and AFM Analysis
2.5. Samples Preparation for SERS Analysis
2.6. Sample Preparation for TERS Measurements
2.7. Acquisition of Spectra and Analysis
3. Results and Discussion
3.1. Analysis of the Structure of TiO2 and ZnO Nanoparticles
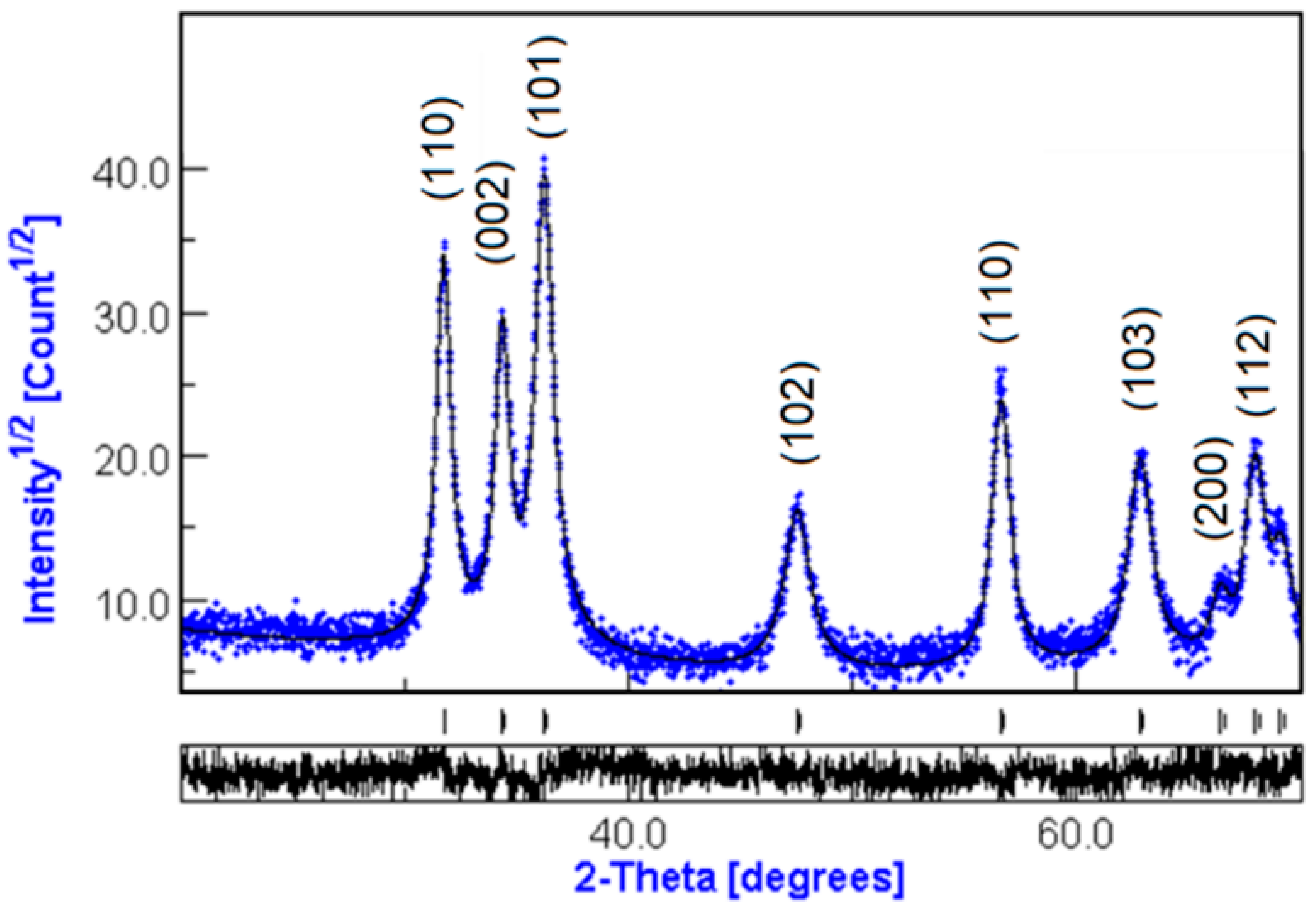
3.1.1. XRD Diffraction
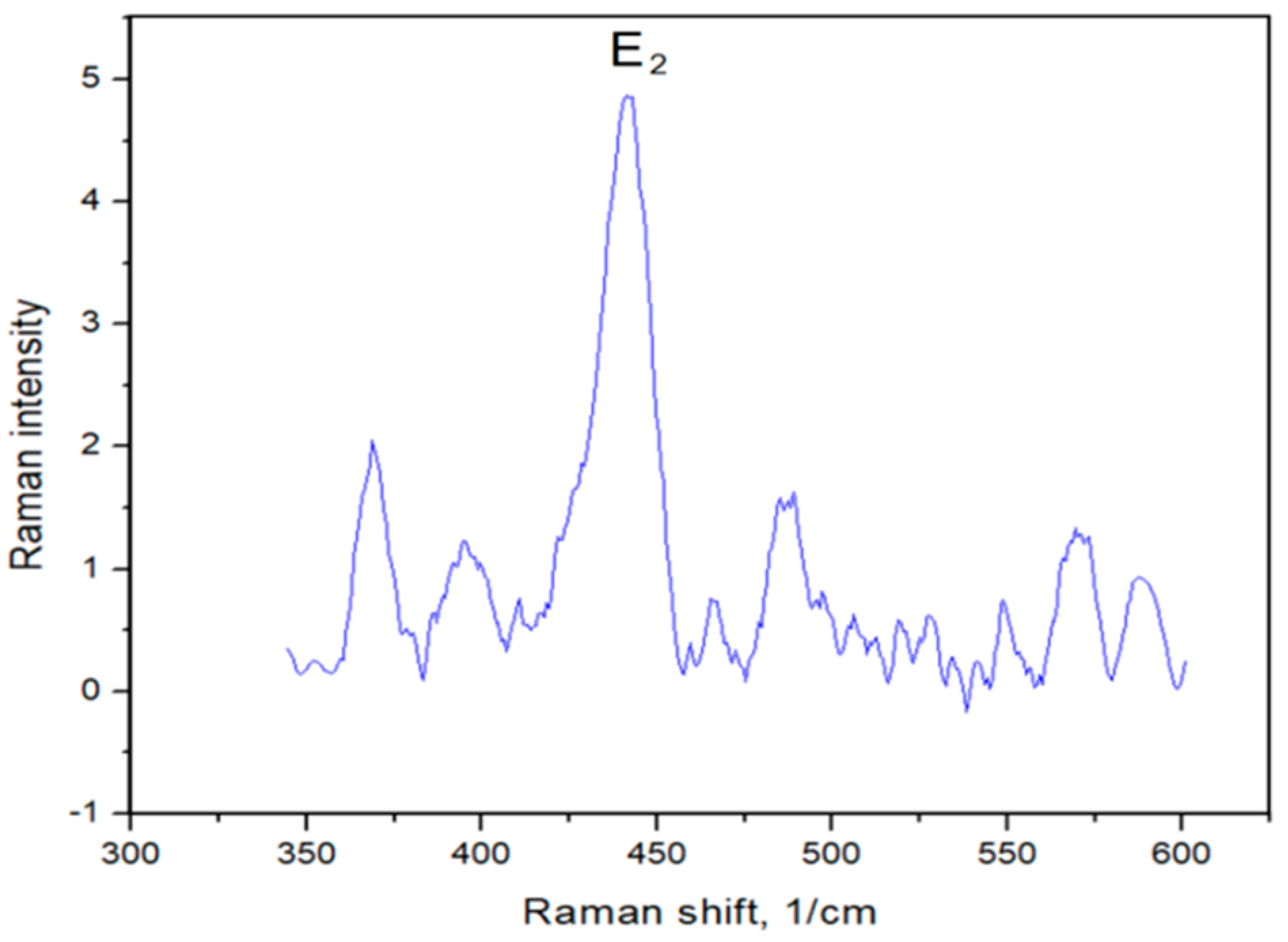
3.1.2. Raman Spectroscopy Analysis
3.1.3. AFM Measurements of ZnO Nanoparticle Sizes
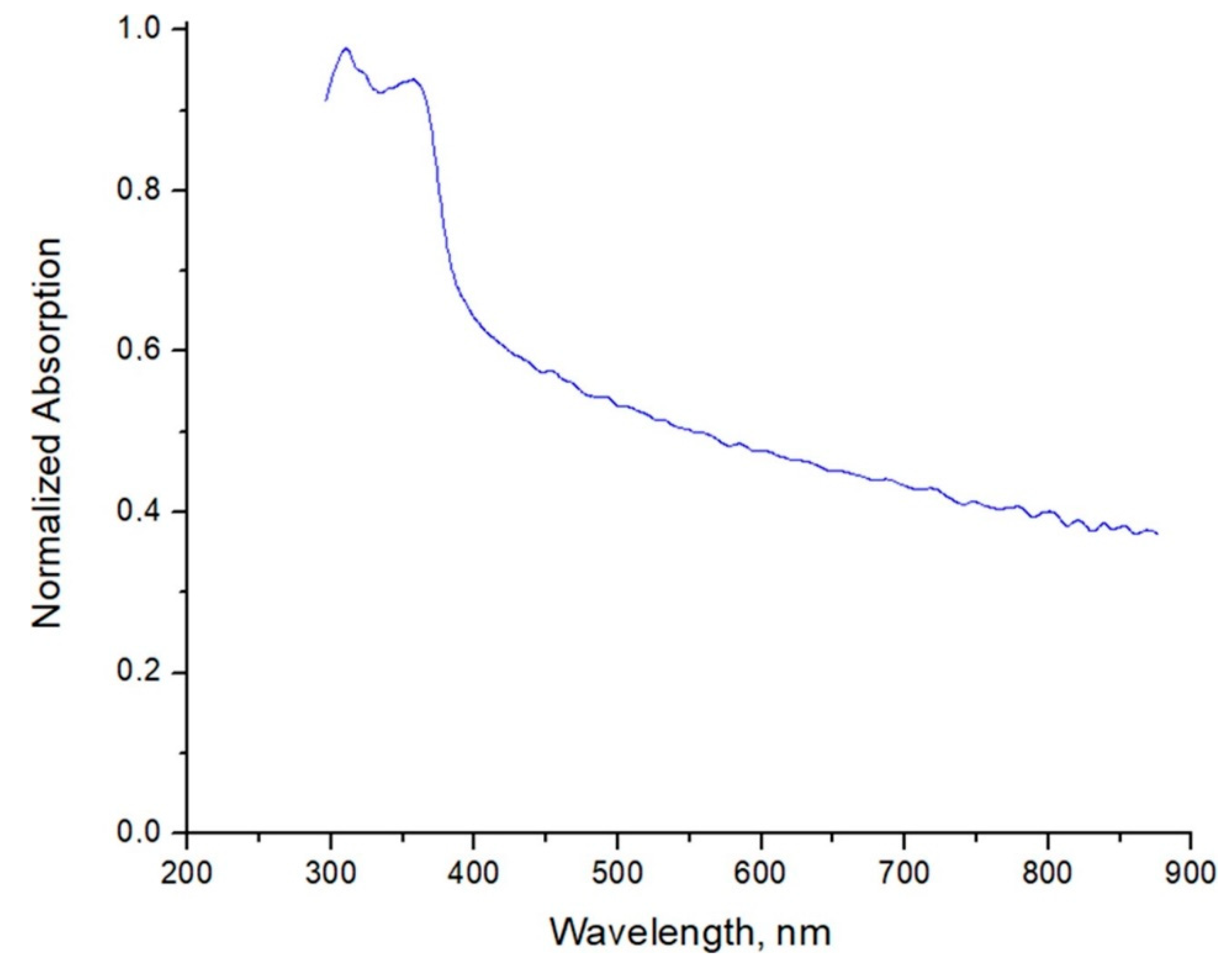
3.1.4. UV-Vis Analysis
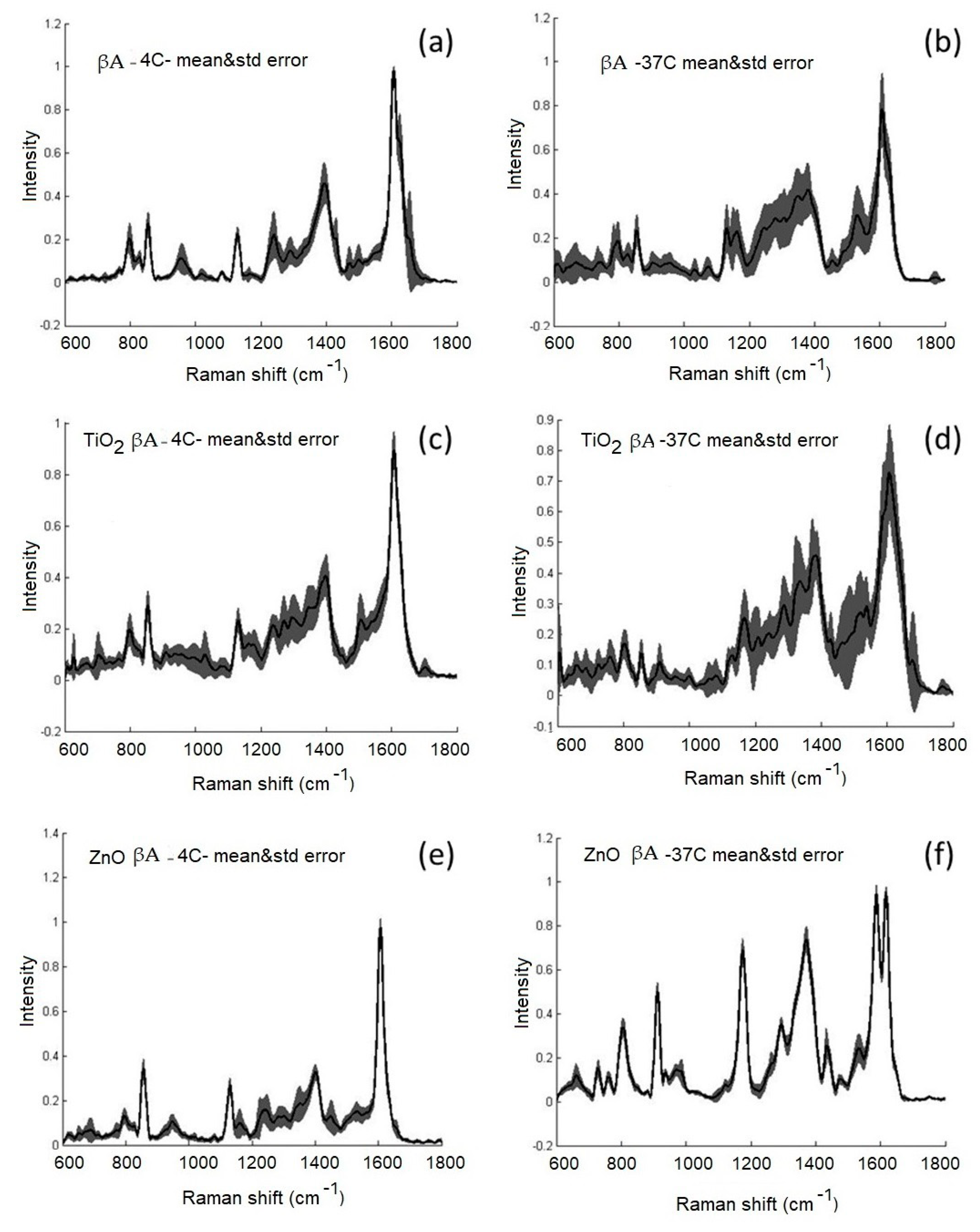
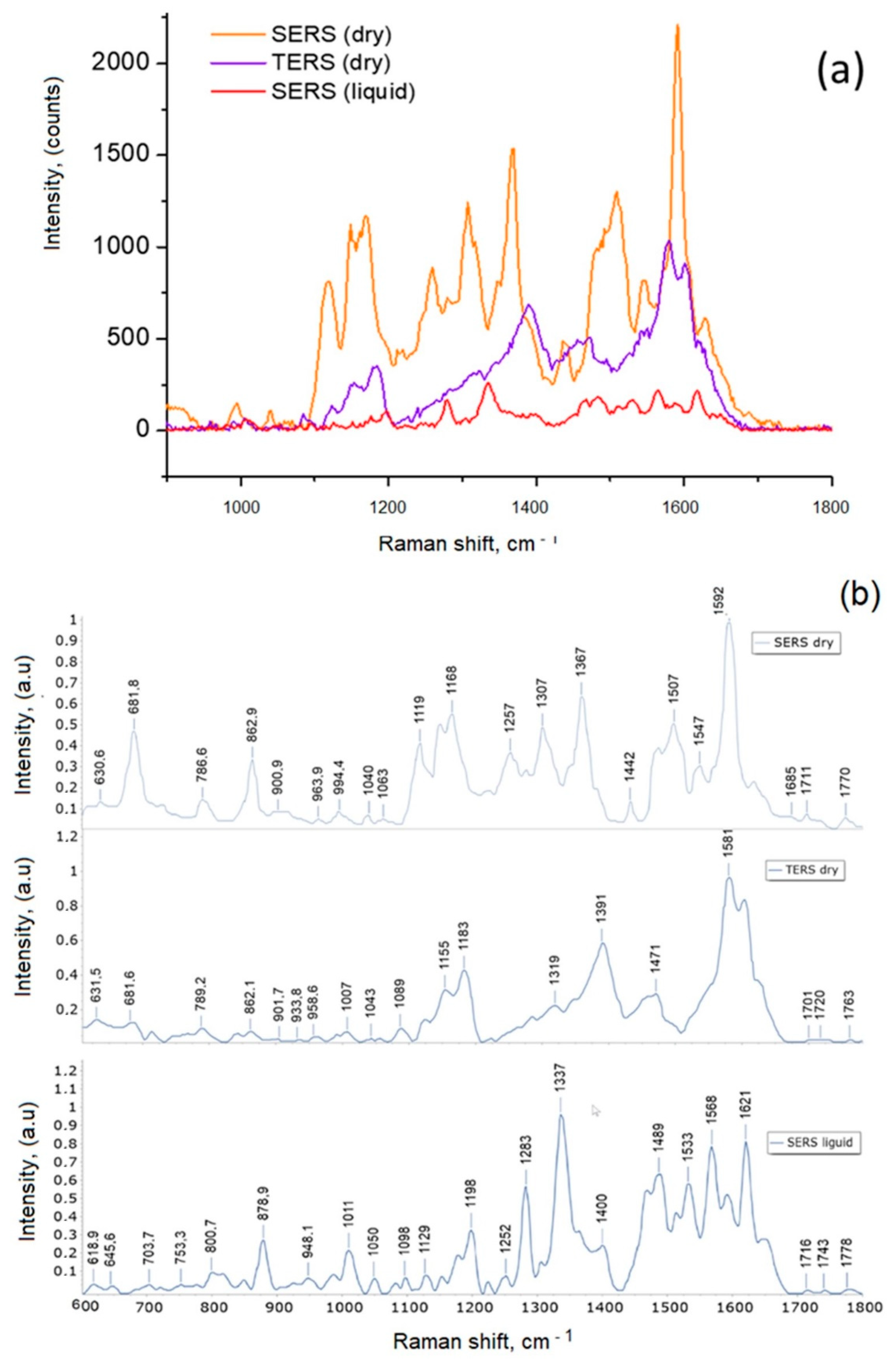
3.2. Investigation of the Effect of ZnO-NPs and TiO2-NPs on the Structure of βA 1-40 by SERS Spectroscopy at Temperatures of 4 °C and 37 °C
3.3. Investigation of the Effect of ZnO-NPs on the Structure of βA 1-40 by Raman and SERS Spectroscopy
3.4. Investigation of the Effect of TiO2-NPs on the Structure of α-Syn by Raman and SERS Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viter, R.; Savchuk, M.; Iatsunskyi, I.; Pietralik, Z.; Starodub, N.; Shyrka, N.; Ramanaviciene, A.; Ramanavicius, A. Analytical, thermodynamical and kinetic characteristics of photoluminescence immunosensor for the determination of Ochratoxin, A. Biosens. Bioelectron 2018, 99, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Turemis, M.; Rappi, D.; Giardi, M.T.; Basile, G.; Ramanaviciene, A.; Kapralovs, A.; Ramanavicius, A.; Viter, R. ZnO/polyaniline composite based photoluminescence sensor for the determination of acetic acid vapor. Talanta 2020, 211, 120658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorfman, A.; Kumar, N.; Hahm, J. Nanoscale ZnO-enhanced fluorescence detection of protein interactions. J. Adv. Mater. 2006, 18, 2685–2690. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, X.; Duan, J.; Wu, X.; Wu, J.; Zhang, P.; Liang, W.; Guo, J.; Cai, H.; Sun, P.; et al. Aptamer-based novel Ag-coated magnetic recognition and SERS nanotags with interior nanogap biosensor for ultrasensitive detection of protein biomarker. Sens. Actuators B Chem. 2021, 334, 129640. [Google Scholar] [CrossRef]
- Barucci, A.; D’Andrea, C.; Farnesi, E.; Banchelli, M.; Amicucci, C.; de Angelis, M.; Hwang, B.; Matteini, P. Label-free SERS detection of proteins based on machine learning classification of chemo-structural determinants. Analyst 2021, 2, 674–682. [Google Scholar] [CrossRef]
- Viter, R.; Savchuk, M.; Starodub, N.; Balevicius, Z.; Tumenas, S.; Ramanaviciene, A.; Jevdokimovs, D.; Earts, D.; Iatsuskyi, I.; Ramanavicius, A. Photoluminescence immunosensor based on bovine leukemia virus proteins immobilized on the ZnO nanorods. Sens. Actuators B Chem. 2019, 285, 601–606. [Google Scholar] [CrossRef] [Green Version]
- Fedorenko, V.; Damberga, D.; Grundsteins, K.; Ramanavicius, A.; Ramanavicius, S.; Coy, E.; Iatsunskyi, I.; Viter, R. Application of polydopamine functionalized Zinc oxide for glucose biosensor design. Polymers 2021, 13, 2918. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Rao, C.M.; Ahmad, M.F. Nanoparticle-protein interaction: The significance and the role of the protein corona. Cellular and Molecular Toxicology of Nanoparticles. Adv. Exp. Med. Biol. 2018, 1048, 175–198. [Google Scholar]
- D’Onofrio, M.; Munari, F.; Assfalg, M. Alpha-synuclein—nanoparticle interactions: Understanding, controlling and exploiting conformational plasticity. Molecules 2020, 25, 5625. [Google Scholar] [CrossRef]
- Mohammad-Beigi, H.; Zanganeh, M.; Scavenius, C.; Eskandari, H.; Farzadfard, A.; Shojaosadati, S.A.; Enghild, J.J.; Otzen, D.E.; Buell, A.K.; Sutherland, D.S. A Protein Corona Modulates Interactions of α-Synuclein with Nanoparticles and Alters the Rates of the Microscopic Steps of Amyloid Formation. ACS Nano 2022, 16, 1102–1118. [Google Scholar] [CrossRef]
- Re, F.; Airoldi, C.; Zona, C.; Masserini, M.; Ferla, B.L.; Quattrocchi, N.; Nicotra, F. Beta-amyloid aggregation inhibitors: Small molecules as candidate drugs for therapy of Alzheimer’s disease. Curr. Med. Chem. 2010, 17, 2990–3006. [Google Scholar] [CrossRef] [PubMed]
- Howlett, D.R.; Jennings, K.H.; Lee, D.C.; Clark, M.S.; Brown, F.; Wetzel, R.; Roberts, G.W. Aggregation state and neurotoxic properties of Alzheimer beta-amyloid peptide. Neurodegeneration 1995, 4, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Neuropathology, biochemistry, and biophysics of α-synuclein aggregation. J. Neurochem. 2007, 103, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Kågedal, K.; Halliday, G.M. Alpha-synuclein biology in Lewy body diseases. Alzheimer’s Res. Ther. 2014, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Roberts, H.L.; Schneider, B.L.; Brown, D.R. α-Synuclein increases β-amyloid secretion by promoting β-/γ-secretase processing of APP. PLoS ONE 2017, 12, e0171925. [Google Scholar] [CrossRef] [Green Version]
- Köppen, J.; Schulze, A.; Machner, L.; Wermann, M.; Eichentopt, R.; Guthardt, M.; Hähnel, A.; Klehm, J.; Kriegeskorte, M.C.; Hartlage-Rübsamen, M.; et al. Amyloid-beta peptides trigger aggregation of alfa-synuclein in vitro. Molecules 2020, 25, 580. [Google Scholar] [CrossRef] [Green Version]
- Bachhuber, T.; Katzmarski, N.; McCarter, J.F.; Loreth, D.; Tahirovic, S.; Kamp, F.; Abou-Ajram, C.; Nuscher, B.; Serrano-Pozo, A.; Müller, A.; et al. Inhibition of amyloid-β plaque formation by α-synuclein. Nat. Med. 2015, 21, 802–807. [Google Scholar] [CrossRef]
- Burdick, D.; Soreghan, B.; Kwon, M.; Kosmoski, J.; Knauer, M.; Henschen, A.; Glabe, C. Assembly and aggregation properties of synthetic Alzheimer’s A4/beta-amyloid peptide analogs. J. Biol. Chem. 1992, 267, 546–554. [Google Scholar] [CrossRef]
- Srinivasan, E.; Chandrasekhar, G.; Chandrasekar, P.; Anbarasu, K.; Vickram, A.S.; Karunakaran, R.; Srikumar, P.S. Alpha-synuclein aggregation in Parkinson’s disease. Front. Med. 2021, 8, 736978. [Google Scholar] [CrossRef]
- Gadhe, L.; Sakunthala, A.; Mukherjee, S.; Gahlot, N.; Bera, R.; Sawner, A.S.; Maji, S.K. Intermediates of α-synuclein aggregation: Implications in Parkinson’s disease pathogenesis. Biophys. Chem. 2021, 281, 106736. [Google Scholar] [CrossRef]
- Lynch, I.; Dawson, K.A. Protein–nanoparticle interactions. In Nano-Enabled Medical Applications, 1st ed.; Jenny Stanford Publishing: New York, NY, USA, 2020; pp. 231–250. [Google Scholar]
- Mushtaq, G.; Jalaluddin, K.A.; Joseph, E.; Mohammad, A.K. Nanoparticles, neurotoxicity and neurodegenerative diseases. Curr. Drug. Metab. 2015, 16, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Batiuskaite, D.; Bruzaite, I.; Snitka, V.; Ramanavicius, A. Assessment of TiO2 nanoparticle impact on the surface morphology of Chinese hamster ovary cells. Materials 2022, 15, 4570. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, L.; Xu, H.; Griskonis, E.; Batiuskaite, D.; Snitka, V. Comparison and evaluation of silver probe preparation techniques for Tip-Enhanced Raman spectroscopy. Plasmonics 2018, 13, 1907–1919. [Google Scholar] [CrossRef]
- Spectragryph-Optical Spectroscopy Software. Available online: https://www.effemm2.de/spectragryph/ (accessed on 28 May 2022).
- Reisner, L.A.; Cao, A.; Pandya, A.K. An integrated software system for processing, analyzing, and classifying Raman spectra. Chem. Intell. Lab. Syst. 2011, 105, 83–90. [Google Scholar] [CrossRef]
- Rajalakshmi, M.; Arora, A.K.; Bendre, B.S.; Mahamuni, S. Optical phonon confinement in zinc oxide nanoparticles. J. Appl. Phys. 2000, 87, 2445–2448. [Google Scholar] [CrossRef]
- Kripal, R.; Gupta, A.K.; Srivastava, R.K.; Mishra, S.K. Photoconductivity and photoluminescence of ZnO nanoparticles synthesized via co-precipitation method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 1605–1612. [Google Scholar] [CrossRef]
- Herbst, M.; Hofmann, E.; FÖrster, S. Nucleation and growth kinetics of ZnO nanoparticles studied by in situ microfluidic SAXS/WAXS/UV–Vis experiments. Langmuir 2019, 35, 11702–11709. [Google Scholar] [CrossRef]
- Sevgi, F.; Brauchle, E.M.; CarvajalBerrio, D.A.; Schenke-Layland, K.; Casadei, N.; Salker, M.S.; Ries, O.; Singh, Y. Imaging of α-Synuclein Aggregates in a Rat Model of Parkinson’s Disease Using Raman Microspectroscopy. Front. Cell Dev. Biol. 2021, 9, 1–17. [Google Scholar] [CrossRef]
- León-Bejarano, F.; Méndez, M.O.; Alba, A.; Rodríguez-Leyva, I.; González, F.J.; Rodríguez-Aranda, M.D.C.; Guevara, E.; Guirado-López, R.A.; Ramírez-Elías, M.G. Raman Spectroscopy Study of Skin Biopsies from Patients with Parkinson’s Disease: Trends in Alpha-Synuclein Aggregation from the Amide I Region. Appl. Spectros. 2022, 76, 1317–1328. [Google Scholar] [CrossRef]
- Lyu, J.; Long, X.; Xie, T.; Jiang, G.; Jiang, J.; Ye, L.; Li, Q. Copper oxide nanoparticles promote α-Synuclein oligomerization and underlying neurotoxicity as a model of Parkinson’s disease. J. Mol. Liq. 2021, 323, 115051. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Zhang, Y.; Bai, Y.; Cao, Y.; Yang, Y.; Zang, L.; Huang, M.; Sui, R. Nickel oxide nanoparticles increase the α-Synuclein amyloid formation and relevant overexpression of inflammatory mediators in microglia as a marker of Parkinson’s disease. Arab. J. Chem. 2021, 14, 103380. [Google Scholar] [CrossRef]
- Xie, H.; Wu, J. Silica nanoparticles induce alpha-synuclein induction and aggregation of PC12 cells. Chem. Biol. Interact. 2016, 258, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Pudlas, M.; Koch, S.; Bolwien, C.; Thude, S.; Jenne, N.; Hirth, T.; Walles, H.; Schenke-Layland, K. Raman spectroscopy: A noninvasive analysis tool for the discrimination of human skin cells. Tissue Eng. Part C Methods. 2011, 17, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Batiuskaite, D.; Grinceviciute, N.; Snitka, V. Impact of graphene oxide on the viability of Chinese hamster ovary and mouse hepatoma MH-22A cells. Toxicol. Vitr. 2015, 29, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Slekiene, N.; Snitka, V. Impact of graphene oxide functionalized with doxorubicin on the viability of mouse hepatoma MH-22A cells. Toxicol. Vitr. 2020, 65, 104821. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Raheb, J.; Akhavan, O.; Arjmand, S.; Mashinchian, O.; Rahman, M.; Mahmoudi, M. Personalized disease-specific protein corona influences the therapeutic impact of graphene oxide. Nanoscale 2015, 7, 8978–8994. [Google Scholar] [CrossRef]
- Mirsadeghi, S.; Dinarvand, R.; Ghahremani, M.H.; Hormozi-Nezhad, M.R.; Mahmoudi, Z.; Hajipour, M.J.; Mahmoudi, M. Protein corona composition of gold nanoparticles/nanorods affects amyloid beta fibrillation process. Nanoscale 2015, 7, 5004–5013. [Google Scholar] [CrossRef]
- Kurouski, D.; Van Duyne, R.P.; Lednev, I.K. Exploring the structure and formation mechanism of amyloid fibrils by Raman spectroscopy: A review. Analyst 2015, 140, 4967–4980. [Google Scholar] [CrossRef]
- Hernández, B.; Coïc, Y.M.; Pflüger, F.; Kruglik, S.G.; Ghomi, M. All characteristic Raman markers of tyrosine and tyrosinate originate from phenol ring fundamental vibrations. J. Raman Spectrosc 2016, 47, 210–220. [Google Scholar] [CrossRef]
- Eravuchira, P.J.; Banchelli, M.; D’Andrea, C.; De Angelis, M.; Matteini, P.; Gannot, I. Hollow core photonic crystal fiber-assisted Raman spectroscopy as a tool for the detection of Alzheimer’s disease biomarkers. J. Biomed. Opt. 2020, 25, 077001. [Google Scholar] [CrossRef]
- Skvortsov, A.; Babich, E.; Lipovskii, A.; Redkov, A.; Yang, G.; Zhurikhina, V. Raman Scattering Study of Amino Acids Adsorbed on a Silver Nanoisland Film. Sensors 2022, 22, 5455. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Chandra, K.; Dolui, S.; Maiti, N.C. Envisaging the Structural Elevation in the Early Event of Oligomerization of Disordered Amyloid β Peptide. ACS Omega 2017, 2, 4316–4327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, B.; Pflüger, F.; Nsangou, M.; Ghomi, M. Vibrational analysis of amino acids and short peptides in hydrated media. IV. amino acids with hydrophobic side chains: L-Alanine, L-Valine, and L-Isoleucine. J. Phys. Chem. B 2009, 113, 3169–3178. [Google Scholar] [CrossRef] [PubMed]
- Swati, J.; Sruti, C. (Eds.) Spectroscopy: Biosensing and Diagnostic Technique for Healthcare Applications; Bentham Science Publishers: Sharjah, United Arab Emirates, 2021; p. 106. ISBN 978-981-5039-12-2. [Google Scholar]
- Ridgley, D.M.; Claunch, E.C.; Barone, J.R. Characterization of large amyloid fibers and tapes with Fourier transform infrared (FT-IR) and Raman spectroscopy. Appl Spectrosc. 2013, 67, 1417–1426. [Google Scholar] [CrossRef]
- Shashilov, V.A.; Sikirzhytski, V.; Popova, L.A.; Lednev, I.K. Quantitative methods for structural characterization of proteins based on deep UV resonance Raman spectroscopy. Methods 2010, 52, 23–37. [Google Scholar] [CrossRef]
- Suga, K.; Lai, Y.C.; Faried, M.; Umakoshi, H. Direct observation of amyloid β behaviour at phospholipid membrane constructed on gold nanoparticles. Int. J Environ. Anal. Chem. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Purrello, R.; Jordan, T.; Spiro, T.G. UVRR spectroscopy of the peptide bond. 1. Amide S, a nonhelical structure marker, is a C. alpha. H bending mode. J. Am. Chem. Soc. 1991, 113, 6359–6368. [Google Scholar] [CrossRef]
- Takeuchi, H. Raman structural markers of tryptophan and histidine side chains in proteins. Biopolymers 2003, 72, 305–317. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 1187–1195. [Google Scholar] [CrossRef]
- Yu, X.; Hayden, E.Y.; Xia, M.; Liang, O.; Cheah, L.; Teplow, D.B.; Xie, Y.H. Surface-enhanced Raman spectroscopy distinguishes amyloid Β-protein isoforms and conformational states. Protein Sci. 2018, 27, 1427–1438. [Google Scholar] [CrossRef] [Green Version]
- Matanfack, A.G.; Pistiki, A.; Rösch, P.; Popp, J. Raman Stable Isotope Probing of Bacteria in Visible and Deep UV-Ranges. Life 2021, 11, 1003. [Google Scholar] [CrossRef] [PubMed]
- Krekora, M.; Nawrocka, A. Effect of a polyphenol molecular size on the gluten proteins–polyphenols interactions studied with FT-Raman spectroscopy. Food Biophys. 2022, 17, 535–544. [Google Scholar] [CrossRef]
- Imanbekova, M.; Suarasan, S.; Rojalin, T.; Mizenko, R.R.; Hilt, S.; Mathur, M.; Lepine, P.; Nicouleau, M.; Mohamed, N.; Durcan, T.M.; et al. Identification of amyloid beta in small extracellular vesicles via Raman spectroscopy. Nanoscale Adv. 2021, 3, 4119–4132. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.H.; Sun, X.; Yu, Y.P.; Hu, J.; Zhao, L.; Liu, Q.; Zhao, Y.; Li, Y.M. TiO2 nanoparticles promote β-amyloid fibrillation in vitro. Biochem. Biophys. Res. Commun. 2008, 373, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M. Potential role of nanoparticles in treating the accumulation of amyloid-beta peptide in Alzheimer’s patients. Polymers 2021, 13, 1051. [Google Scholar] [CrossRef]
- Dai, X.; Fu, W.; Chi, H.; Mesias, V.S.D.; Zhu, H.; Leung, C.W.; Liu, W.; Huang, J. Optical tweezers-controlled hotspot for sensitive and reproducible surface-enhanced Raman spectroscopy characterization of native protein structures. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Lee, D.; Choe, Y.J.; Lee, M.; Jeong, D.H.; Paik, S.R. Protein-based SERS technology monitoring the chemical reactivity on an α-Synuclein-mediated two-dimensional array of gold nanoparticles. Langmuir 2011, 27, 12782–12787. [Google Scholar] [CrossRef]
- Zukiene, R.; Snitka, V. Zinc oxide nanoparticle and bovine serum albumin interaction and nanoparticles influence on cytotoxicity in vitro. Colloids Surf. B Biointerfaces 2016, 135, 316–323. [Google Scholar] [CrossRef]
- Asthana, S.; Bhattacharyya, D.; Kumari, S.; Nayak, P.S.; Saleem, M.; Bhunia, A.; Jha, S. Interaction with zinc oxide nanoparticle kinetically traps α-synuclein fibrillation into off-pathway non-toxic intermediates. Int. J. Biol. Macromol. 2020, 150, 68–79. [Google Scholar] [CrossRef]


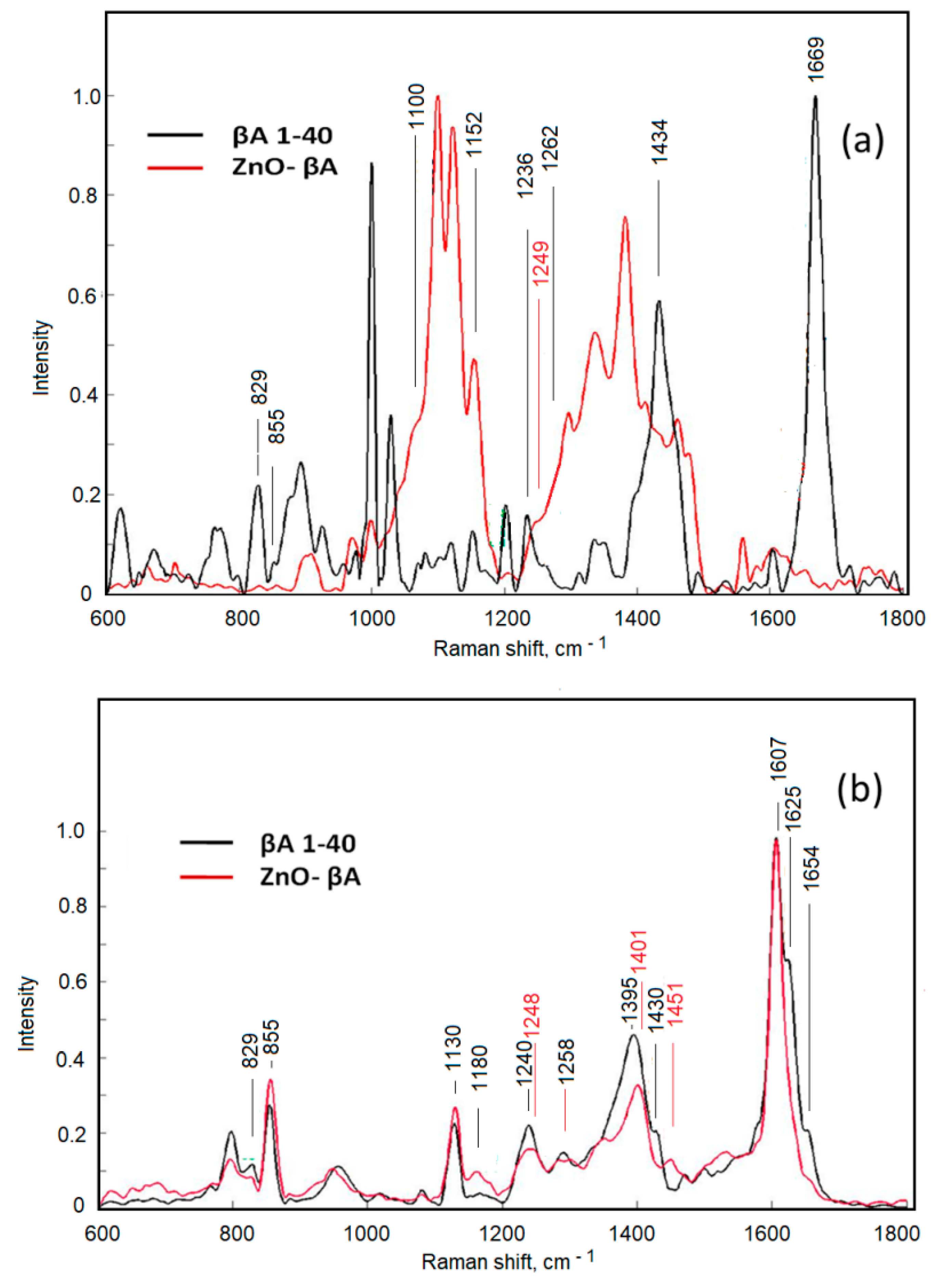
| Wave Number (cm−1) | Assignment | References |
|---|---|---|
| 829, 855 | Tyrosine (Tyr) | [41,42] |
| 1100 | Alanine (Ala), νCC, νCN, νCO | [43,44] |
| 1130 | δ(CCH) | [45] |
| 1152 | νCC, Isoleucine (Ile) | [44] |
| 1180 | Tyrosine (Tyr), phenylalanine (Phe) | [41,46] |
| 1236, 1240 | Amide III, β-sheet | [47,48] |
| 1248, 1249 | Amide III | [46] |
| 1258, 1262, 1654 | α-Helix band, amide III | [46,49] |
| 1395, 1401 | Amide S | [50] |
| 1430, 1434 | Tryptophan (Trp)doublet, CH2, CH3 deformation | [46,51] |
| 1451 | CH3 and CH2 symmetric stretching, phenylalanine (Phe) | [52,53] |
| 1607 | Tyrosine (Tyr), phenylalanine (Phe) | [54] |
| 1625 | Tyrosine (Tyr), tryptophan (Trp) | [55] |
| 1669 | Amide I, β-sheet | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slekiene, N.; Snitka, V.; Bruzaite, I.; Ramanavicius, A. Influence of TiO2 and ZnO Nanoparticles on α-Synuclein and β-Amyloid Aggregation and Formation of Protein Fibrils. Materials 2022, 15, 7664. https://doi.org/10.3390/ma15217664
Slekiene N, Snitka V, Bruzaite I, Ramanavicius A. Influence of TiO2 and ZnO Nanoparticles on α-Synuclein and β-Amyloid Aggregation and Formation of Protein Fibrils. Materials. 2022; 15(21):7664. https://doi.org/10.3390/ma15217664
Chicago/Turabian StyleSlekiene, Nora, Valentinas Snitka, Ingrida Bruzaite, and Arunas Ramanavicius. 2022. "Influence of TiO2 and ZnO Nanoparticles on α-Synuclein and β-Amyloid Aggregation and Formation of Protein Fibrils" Materials 15, no. 21: 7664. https://doi.org/10.3390/ma15217664








