Future sustainable mobility identifies hybrid electric vehicles as the core of the next urban transportation system [
1,
2,
3,
4]. To address the challenges of this transition [
5,
6,
7,
8] it is necessary to control one of the main problems of batteries which is thermal management. The management of the thermal behavior of the battery is crucial to the safety, efficiency, and life of the battery pack, in both hybrid and fully electric vehicles. The heating of the battery is a well-known aspect [
6,
9,
10] and is linked to its operation; the greater the load, the greater the heat generation [
11,
12]. Uneven temperature distribution and its local peaks are critical during charge and discharge cycles. Batteries are exposed to the risk of thermal runaway, a catastrophic event triggered by local heat concentrations, that has greatly raised public concerns [
13,
14]. Thermal management issues [
8] have been a limitation to the diffusion of lithium-ion batteries (LIB) in electric vehicles (EVs) and for this reason there is an urgent need to design safer batteries. Internal short circuit (ISC) is frequent among battery failures [
15]. ISC may trigger or accelerate thermal runaway (TR) by contributing to the temperature increment and the start of a series of exothermic side reactions. To manage the thermal status of the battery pack, a cooling system can be applied. Different configurations are widely tested according to the operating conditions, available volume, and battery pack performance. Chen et al. [
16] carried out a numerical investigation to compare different cooling methods for lithium battery cells, obtaining interesting insights to consider for the cooling system selection and design. For example, the system with fins was seen to add the most extra weight; whereas the air cooling consumed the most parasitic power, and the indirect liquid cooling system had the highest maximum temperature difference. Numerical investigations are very helpful for the analysis of dangerous and unstable processes such as thermal runaway. To improve the management of thermal behavior and to run numerical simulations, it is essential to identify the main thermal properties of the batteries, such as specific thermal capacity, thermal conductivity, and convective heat transfer coefficient. Zhang et al. presented a way to simultaneously estimate these parameters using the inverse heat transfer formulation [
17]. In place of directly calculating them, a first simulation was run with initial values, then they made a comparison between calculated and measured temperatures, and the following refinement of the values of the parameters was made until the optimized set of values was found. Sheng et al. [
18] developed a novel method to determine the thermal conductivity and the specific heat simultaneously based on the quasi-steady state heat transfer analysis. They found that the operating temperature has a more significant influence on the cell-specific heat than the thermal conductivity, while the effect of the state of charge has a minimal effect on these two parameters. The importance of a reliable determination of the thermal parameters is crucial, as highlighted by Dong et al. [
19] who evaluated the thermal model inaccuracy caused by physical properties uncertainty. While providing reliable values of battery temperature both internal and external, the convective heat transfer coefficient can be relevant for the prediction and prevention of over-temperature risk. To improve the rate of heat exchange from the battery to the cooling means, independently of the heat transfer method used, high thermal properties materials can be used to coat the battery. In this regard, some experimental applications can be found with the use of carbon- and graphene-based materials [
20,
21,
22,
23] but only as coatings for the anode and the cathode. Carbon can be used to improve the performance of the electrodes, as shown by Wee et al. [
20], who carried out a systematic study on the microstructural effect of carbon materials on the electrochemical or electrocatalytic performance of the electrodes. Moreover, Park et al. [
21] investigated the effect of carbon coatings on the thermal stability of natural graphite spheres used as anode materials in lithium-ion batteries by applying differential scanning calorimetry and X-ray diffraction. Among carbon-based materials, graphene is of particular interest because of its outstanding mechanical flexibility and ultralow weight, which can adapt to different applications that can grant structural support and thermal dissipation properties at the same time. Graphene has an impressive capability of electrolyte infiltration, which could accelerate the Li-ion’s diffusion [
22] providing a solution for key issues affecting the cathode of future lithium-sulfur batteries. Graphene-based hybrid electrodes have been exploited to increase electron transport, specific capacity, C-rate, and cyclability. Graphene can be used as a substrate for the growth of anode/cathode nanomaterials to achieve higher rate performance electrodes compared to non-conducting materials [
24]. There are several methods for the synthesis of graphene, from electrochemical exfoliation through rapid thermal annealing to biomass pyrolysis, each of them with its benefits and challenges [
25]. Moreover, the electrochemical properties of graphene and carbon nanotubes can be tailored by introducing heteroatoms into the carbon crystalline lattice granting to improve the kinetics of the heterogeneous electron transfer reaction [
26]. Besides these typical applications of coatings inside the battery linked to the electrochemical aspects of carbon and graphene, there are also some uses outside as matrix structures for heat dissipation with phase change materials (PCM). Researchers have developed a variety of methods to increase the thermal conductivity of PCM by adding high thermal conductivity materials [
27] (e.g., adding carbon fiber, metal foam, expanded graphite [
28], or adding fin structure). They show that increasing the thermal conductivity of PCM can effectively improve the heat dissipation performance of BTMS. While poor, literature can be found about covering the battery surface with high-conductivity coatings. Saw et al. [
29] proposed a feasibility study of boron nitride on Li-ion battery casing to enhance thermal management, also paying attention to the effect of the surface roughness and the coating thickness on the heat transfer. However, there is no evidence in the literature of the use of carbon fiber-reinforced polymer (CFRP) composites that are largely widespread thanks to their excellent mechanical properties as low density, high specific strength, and modulus [
30,
31,
32]. Hence, from the thermal point of view, there is room for a wide experimental investigation on carbon and graphene-based surface coatings for heat dissipation to preserve battery health and ensure a safe operation.
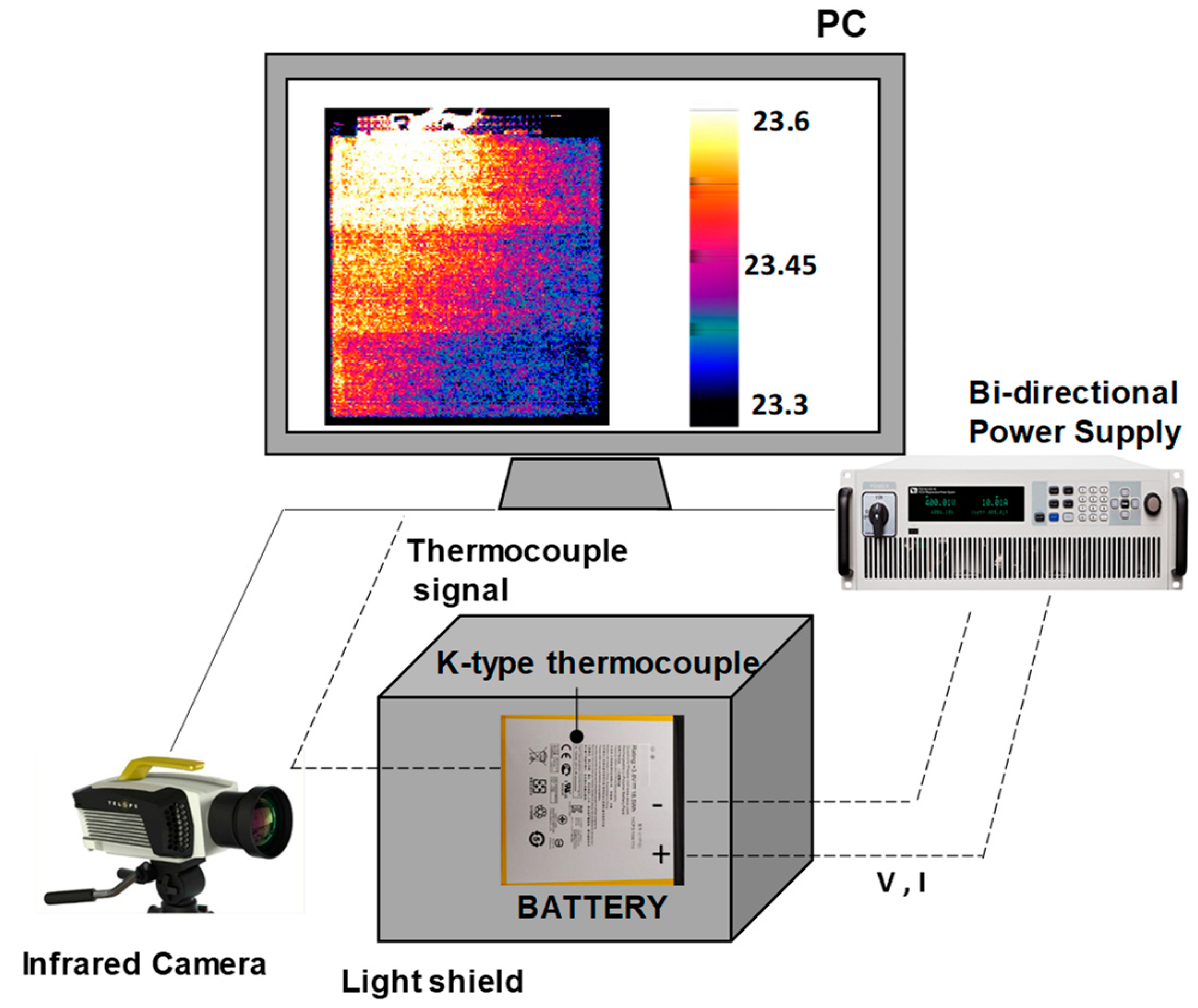
This work presents an experimental analysis of the thermal state of a flat-type battery coated with layers of carbon and graphene. The battery surface temperature is detected using infrared thermography during discharge processes at a constant current and a charging phase. Thermography has proven to be a reliable and practical tool for studying the thermal behavior of LIBs by performing non-invasive measurements of the surface temperature over time. This work presents and discusses the efficacy of two methods for the fast evaluation of the convective heat transfer coefficient h, and the specific heat capacity cp of the battery. The first is a 1d model based on the finite difference method, which can characterize the thermal status inside and on the surface of the battery. The second method uses Newton’s law of cooling for the direct calculation of the thermal parameters. The outputs of the two methods are compared using the normalized root mean squared error producing a positive agreement. This work provides numerical values of the main thermal parameters of a common Li-ion battery under different load conditions. By addressing the effect of the coating conductivity, it offers a comparative analysis that can be transferred to different types of cells and geometries for a preliminary analysis. Finally, also the proposed methodology is presented to be re-applied to different cases and battery configurations.