Investigation of Low-Pressure Sn-Passivated Cu-to-Cu Direct Bonding in 3D-Integration
Abstract
:1. Introduction
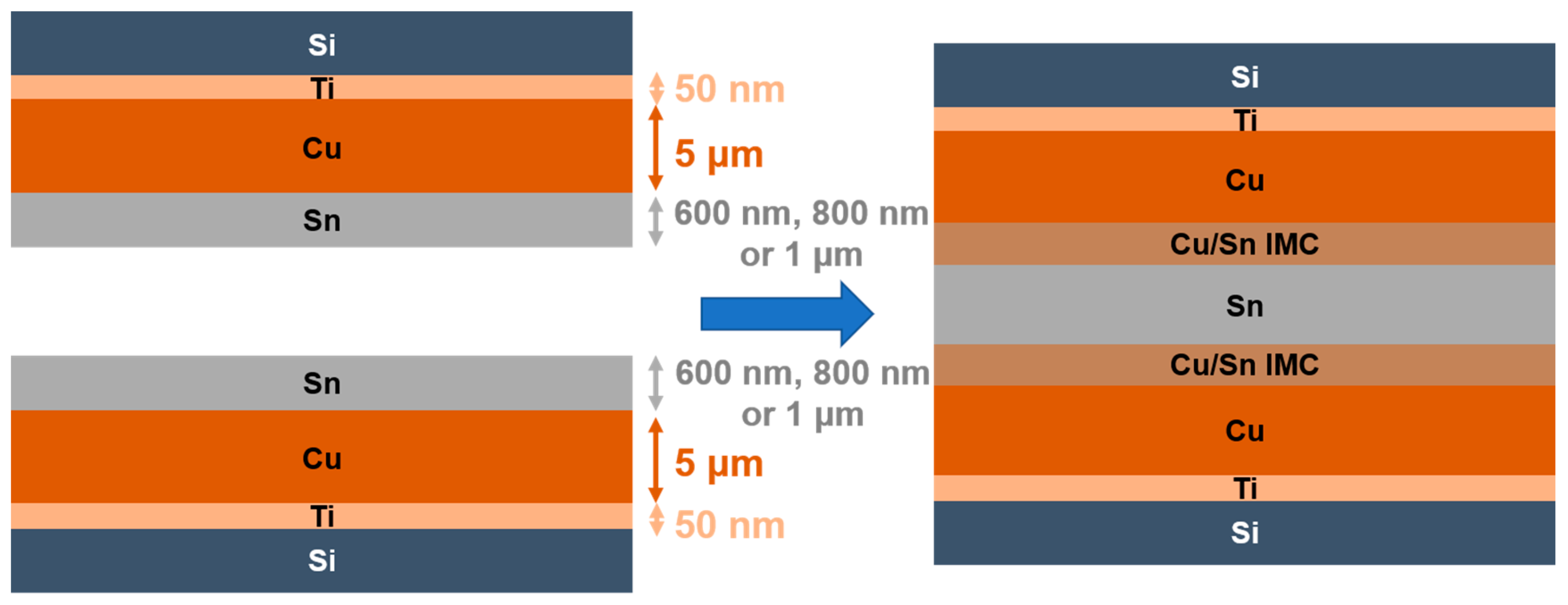
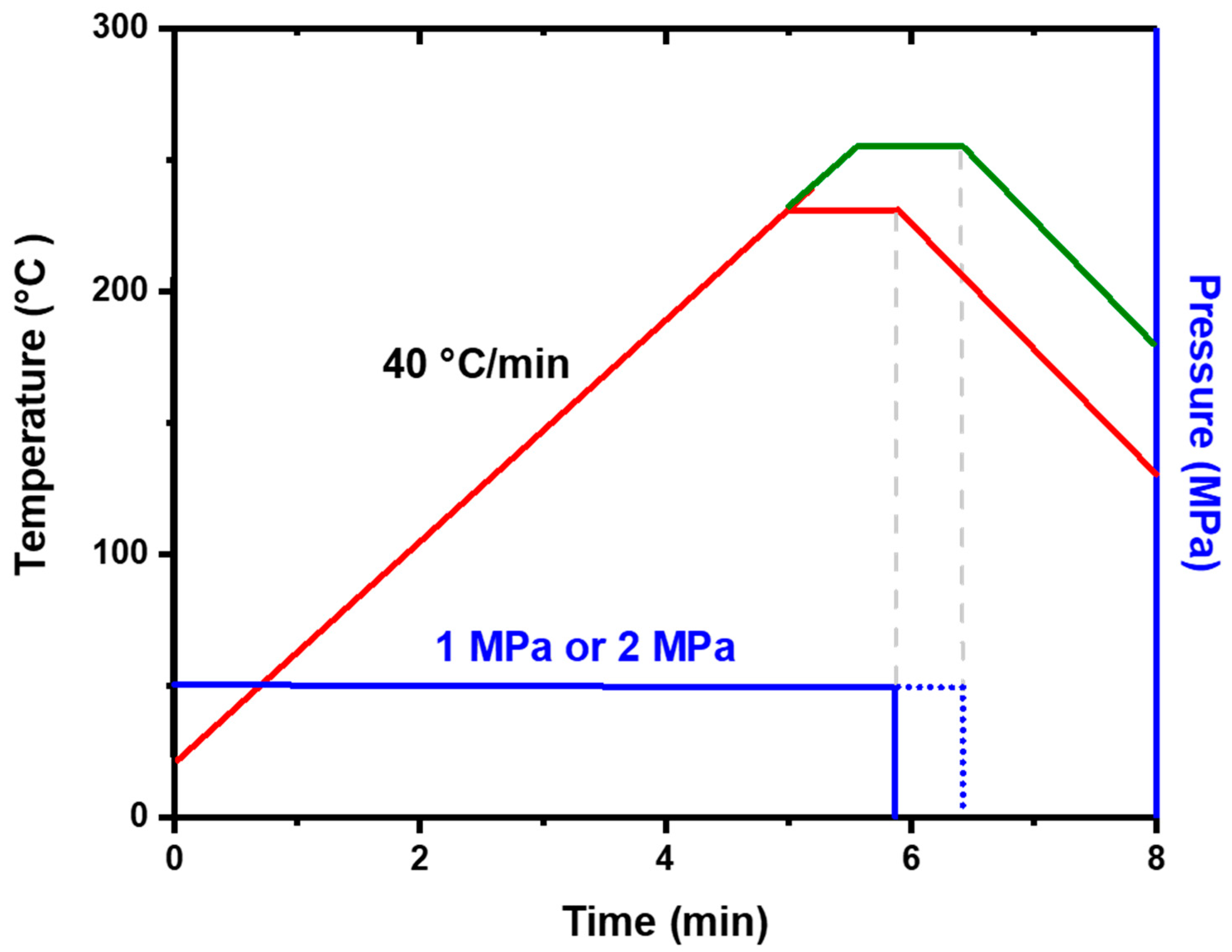
2. Experimental Methods
3. Results and Discussion
3.1. Bonding Parameter Optimization
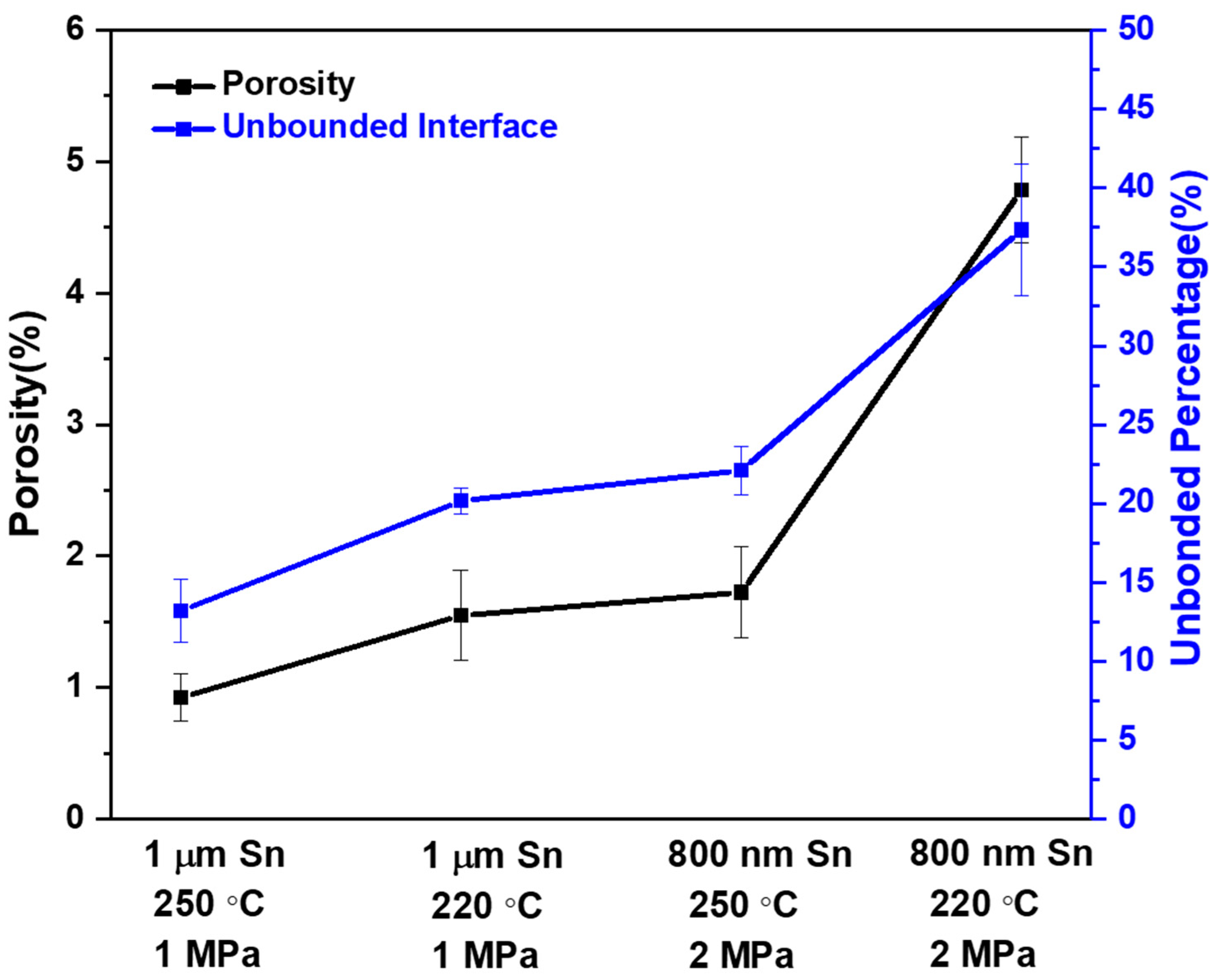
3.2. Porosity and Unbonded Interface Percentage
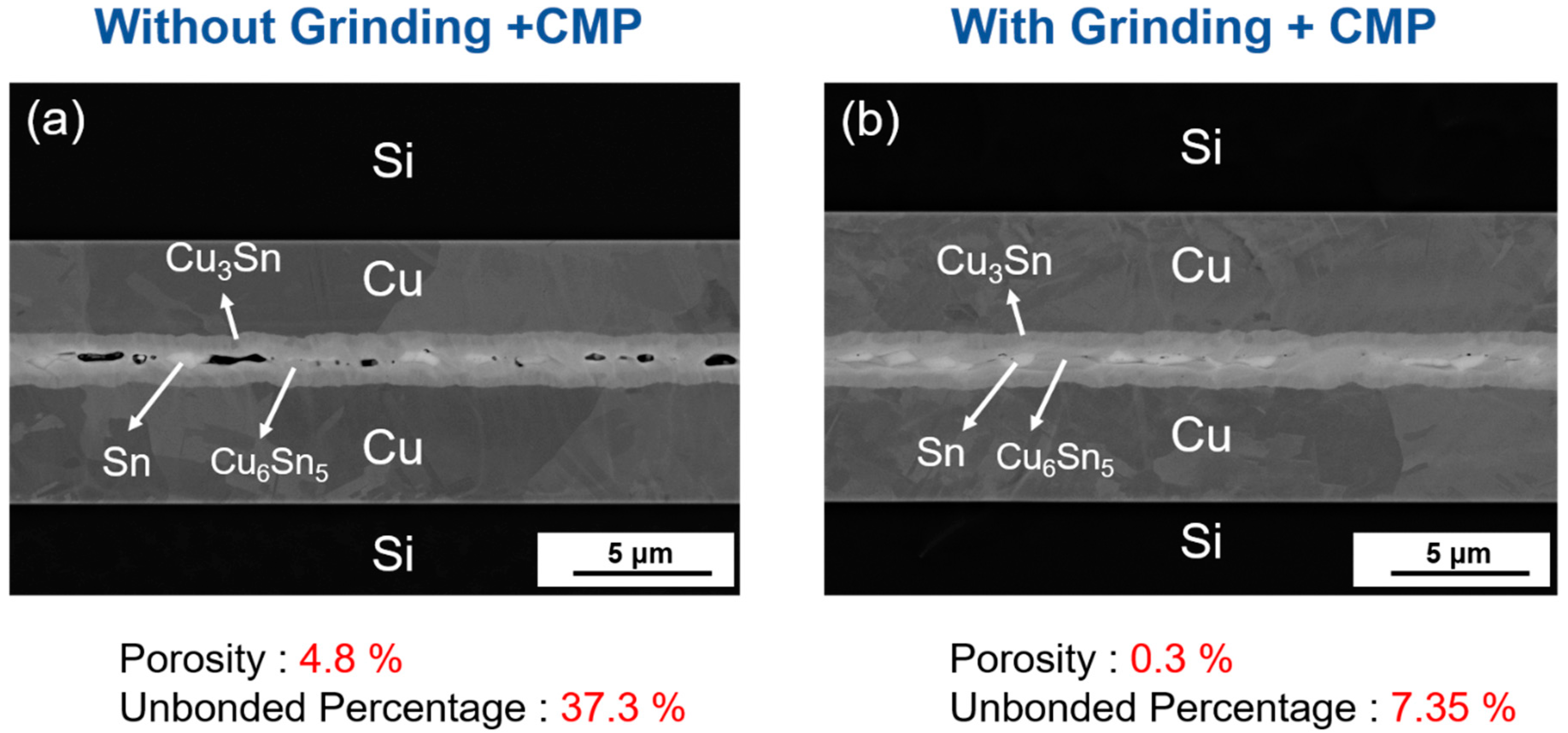
3.3. Bonding after Grinding and CMP
3.4. Summary of Bonding Parameters and Results
3.5. TEM Analysis
4. Conclusions
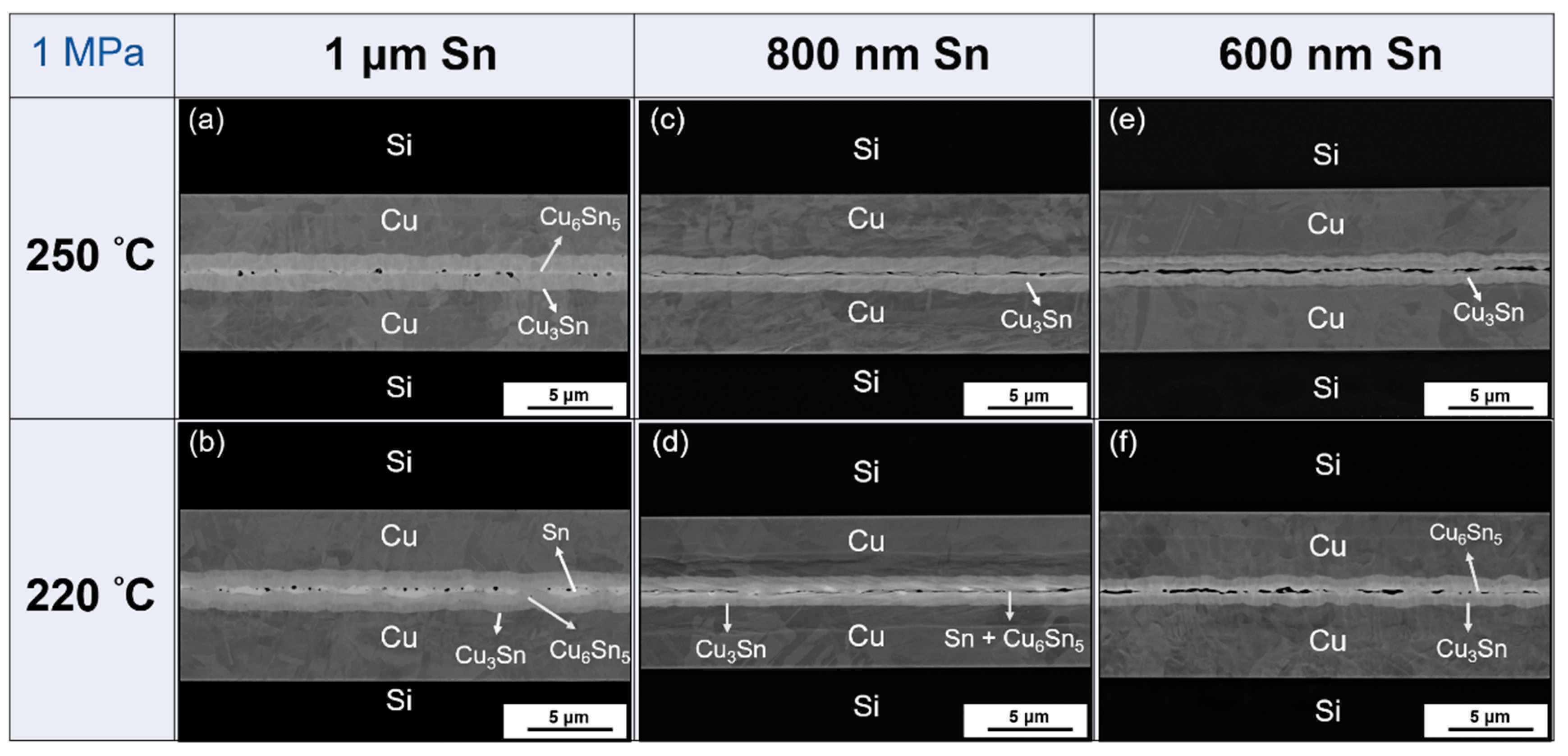
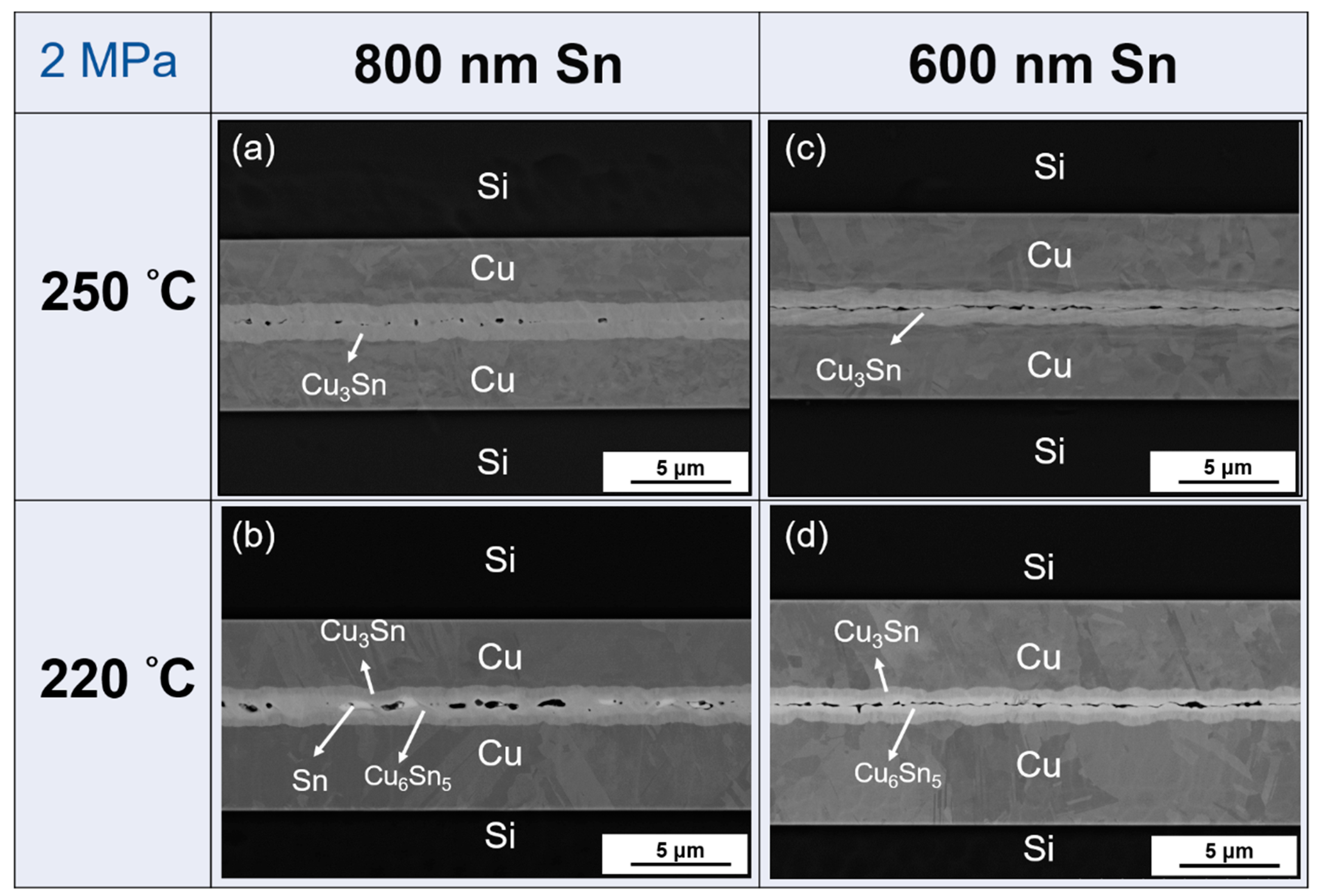
- As shown in Table 3, there are three parameter changes in this study: Sn thickness, bonding temperature, and bonding pressure. From the results, higher Sn thickness (1 μm) and higher temperature (250 °C) are recommended to achieve better bonding. Moreover, if a lower Sn thickness (800 nm) is used, 2 MPa pressure can still achieve a successful bond. However, the bonding quality is not as good as the parameters of 1 μm Sn under 1 MPa pressure at both 250 °C and 220 °C. Besides, 600 nm Sn is not thick enough to bond well without the planarization process in spite of the 250 °C temperature and 2 MPa pressure.
- Though the high surface flatness is not needed in the bonding after passivating soft Sn over Cu, the surface roughness of Cu will still affect the bonding quality. The comparison between planarization and no planarization of required bonding parameters and the bonding results are shown in Table 4.
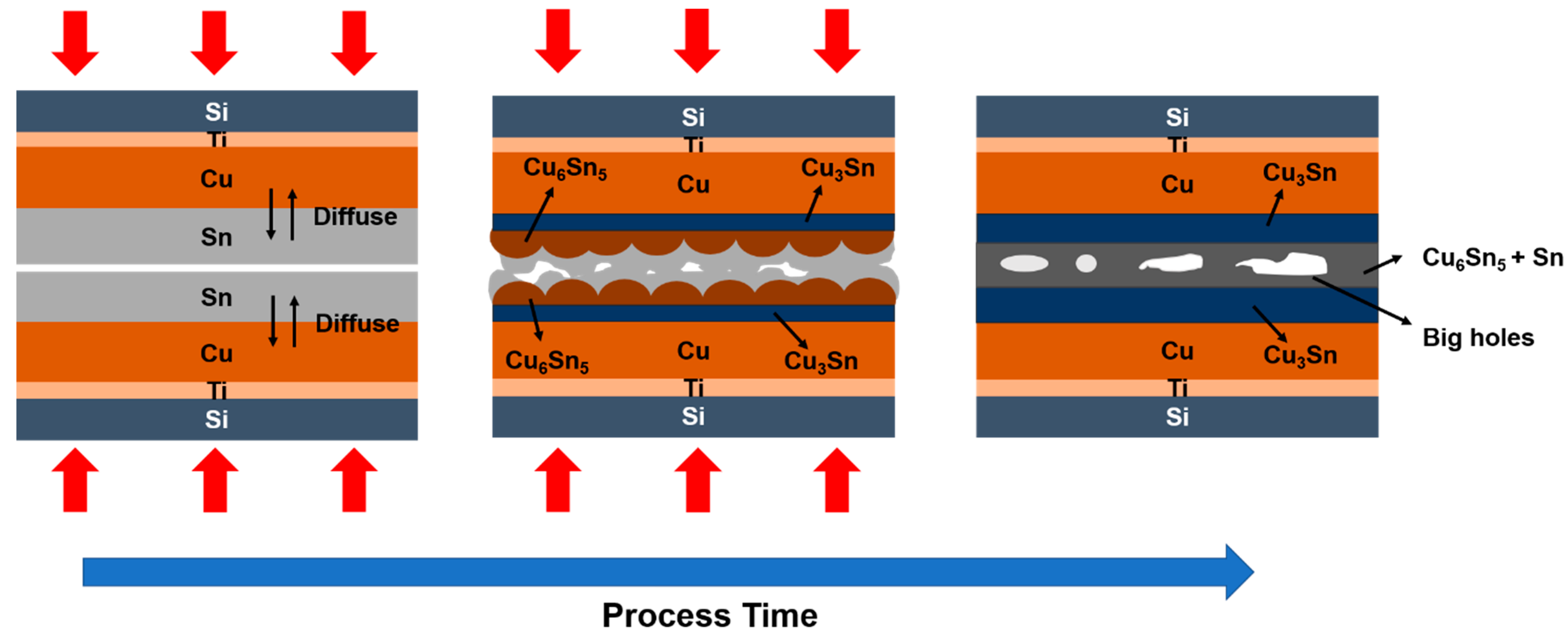
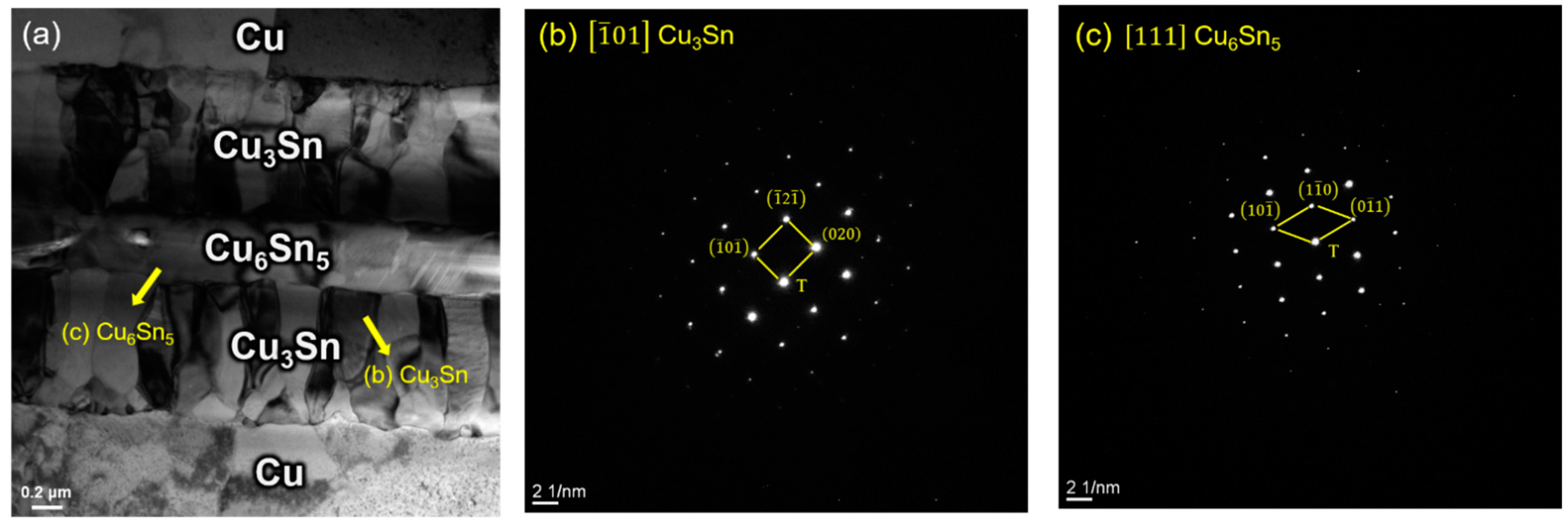
- As per the TEM analysis, there are no cracks or voids which occur at the Cu–Cu3Sn, Cu3Sn–Cu6Sn5, or Cu3Sn–Cu3Sn interfaces. This implies that there is good adhesion between different layers regardless of whether Cu6Sn5 is present at the interface. Therefore, the strength of this Cu–Sn joint would be sufficiently strong, because no defects occur between different layers. Thus, the defects at the bonding interface might be the dominant factor which affects the joint strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, S.; Fan, A.; Chen, K.-N.; Tan, C.S.; Checka, N.; Reif, R. Technology, performance, and computer-aided design of three-dimensional integrated circuits. In Proceedings of the 2004 International Symposium on Physical Design, Phoenix, AZ, USA, 18–21 April 2004. [Google Scholar]
- Liang, H.-W.; Chen, H.-C.; Lin, C.-H.; Lee, C.-L.; Yang, S.-C.; Chen, K.-N. The influence of device morphology on wafer-level bonding with polymer-coated layer. In Proceedings of the IEEE International 3D Systems Integration Conference (3DIC), San Francisco, CA, USA, 8–11 November 2016. [Google Scholar]
- Chen, K.; Tan, C. Integration schemes and enabling technologies for three-dimensional integrated circuits. IET Comput. Digit. Tech. 2011, 5, 160–168. [Google Scholar] [CrossRef]
- Swinnen, B.; Ruythooren, W.; de Moor, P.; Bogaerts, L.; Carbonell, L.; de Munck, K.; Eyckens, B.; Stoukatch, S.; Vaes, J. 3D integration by Cu-Cu thermo-compression bonding of extremely thinned bulk-Si die containing 10 μm pitch through-Si vias. In Proceedings of the 2006 International Electron Devices Meeting, San Francisco, CA, USA, 11–13 December 2006. [Google Scholar]
- Chen, K.N.; Fan, A.; Tan, C.S.; Reif, R. Bonding parameters of blanket copper wafer bonding. J. Electron. Mater. 2006, 35, 230–234. [Google Scholar] [CrossRef]
- Chen, K.-N.; Fan, A.; Reif, R. Microstructure examination of copper wafer bonding. J. Electron. Mater. 2001, 30, 331–335. [Google Scholar] [CrossRef]
- Chen, K.N.; Tan, C.S.; Fan, A.; Reif, R. Copper bonded layers analysis and effects of copper surface conditions on bonding quality for three-dimensional integration. J. Electron. Mater. 2005, 34, 1464–1467. [Google Scholar] [CrossRef]
- Liu, D.; Chen, P.-C.; Chen, K.-N. A novel low-temperature Cu-Cu direct bonding with Cr wetting layer and Au passivation layer. In Proceedings of the IEEE 70th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 3–30 June 2020. [Google Scholar]
- Chen, P.-C.; Liu, D.; Chen, K.-N. Low-Temperature Wafer-Level Metal Bonding with Gold Thin Film at 100 °C. In Proceedings of the International 3D Systems Integration Conference (3DIC), Sendai, Japan, 8–10 October 2019. [Google Scholar]
- Liu, D.; Chen, P.-C.; Tsai, Y.-C.; Chen, K.-N. Low temperature Cu to Cu direct bonding below 150° C with Au passivation layer. In Proceedings of the International 3D Systems Integration Conference (3DIC), Sendai, Japan, 8–10 October 2019. [Google Scholar]
- Chou, T.-C.; Huang, S.-Y.; Chen, P.-J.; Hu, H.-W.; Liu, D.; Chang, C.-W.; Ni, T.-H.; Chen, C.-J.; Lin, Y.-M.; Chang, T.-C.; et al. Electrical and Reliability Investigation of Cu-to-Cu Bonding with Silver Passivation Layer in 3-D Integration. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 11, 36–42. [Google Scholar] [CrossRef]
- Lin, P.-F.; Tran, D.-P.; Liu, H.-C.; Li, Y.-Y.; Chen, C. Interfacial Characterization of Low-Temperature Cu-to-Cu Direct Bonding with Chemical Mechanical Planarized Nanotwinned Cu Films. Materials 2022, 15, 937. [Google Scholar] [CrossRef]
- Zantye, P.B.; Kumar, A.; Sikder, A. Chemical mechanical planarization for microelectronics applications. Mater. Sci. Eng. R Rep. 2004, 45, 89–220. [Google Scholar] [CrossRef]
- Banerjee, G.; Rhoades, R.L. Chemical mechanical planarization historical review and future direction. ECS Trans. 2008, 13, 1. [Google Scholar] [CrossRef]
- Krishnan, M.; Nalaskowski, J.W.; Cook, L.M. Chemical Mechanical Planarization: Slurry Chemistry, Materials, and Mechanisms. Chem. Rev. 2010, 110, 178–204. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, Y.; Xue, F. Properties of low melting point Sn–Zn–Bi solders. J. Alloy. Compd. 2005, 397, 260–264. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, K.N. Low melting point solders based on Sn, Bi, and In elements. Mater. Today Adv. 2020, 8, 100115. [Google Scholar] [CrossRef]
- Wu, Z.; Cai, J.; Wang, Q.; Wang, J.; Wang, D. Wafer-Level Hermetic Package by Low-Temperature Cu/Sn TLP Bonding with Optimized Sn Thickness. J. Electron. Mater. 2017, 46, 6111–6118. [Google Scholar]
- Liu, H.; Wang, K.; Aasmundtveit, K.; Hoivik, N. Intermetallic compound formation mechanisms for Cu-Sn solid–liquid interdiffusion bonding. J. Electron. Mater. 2012, 41, 2453–2462. [Google Scholar] [CrossRef]
- Luu, T.-T.; Duan, A.; Wang, K.; Aasmundtveit, K.; Hoivik, N. Cu/Sn SLID wafer-level bonding optimization. In Proceedings of the IEEE 63rd Electronic Components and Technology Conference, Las Vegas, NV, USA, 28–31 May 2013. [Google Scholar]
- Tang, Y.-S.; Chen, H.-C.; Kho, Y.-T.; Hsieh, Y.-S.; Chang, Y.-J.; Chen, K.-N. Investigation and Optimization of Ultrathin Buffer Layers Used in Cu/Sn Eutectic Bonding. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 8, 1225–1230. [Google Scholar] [CrossRef]
- Das, S.; Tiwari, A.N.; Kulkarni, A.R. Thermo-compression bonding of alumina ceramics to metal. J. Mater. Sci. 2004, 39, 3345–3355. [Google Scholar] [CrossRef]
- Yuhan, C.; Le, L. Wafer level hermetic packaging based on Cu–Sn isothermal solidification technology. J. Semicond. 2009, 30, 086001. [Google Scholar] [CrossRef]
- Van de Wiel, H.; Vardøy, A.B.; Hayes, G.; Fischer, H.; Lapadatu, A.; Taklo, M. Characterization of hermetic wafer-level Cu-Sn SLID bonding. In Proceedings of the 4th Electronic System-Integration Technology Conference, Amsterdam, The Netherlands, 17–20 September 2012. [Google Scholar]
- Lu, M.-H.; Hsieh, K.-C. Sn-Cu Intermetallic Grain Morphology Related to Sn Layer Thickness. J. Electron. Mater. 2007, 36, 1448–1454. [Google Scholar] [CrossRef]
- Yang, L.; Atanasova, T.; Radisic, A.; Deconinck, J.; West, A.C.; Vereecken, P. Wafer-scale Cu plating uniformity on thin Cu seed layers. Electrochim. Acta 2013, 104, 242–248. [Google Scholar] [CrossRef]
- Takahashi, K.M. Electroplating Copper onto Resistive Barrier Films. J. Electrochem. Soc. 2000, 147, 1414. [Google Scholar] [CrossRef]
- Zhang, R.; Tian, Y.; Hang, C.; Liu, B.; Wang, C. Formation mechanism and orientation of Cu3Sn grains in Cu–Sn intermetallic compound joints. Mater. Lett. 2013, 110, 137–140. [Google Scholar] [CrossRef]
- Chen, Y.; Chung, C.; Yang, C.; Kao, C.R. Single-joint shear strength of micro Cu pillar solder bumps with different amounts of intermetallics. Microelectron. Reliab. 2013, 53, 47–52. [Google Scholar] [CrossRef]
- Kao, C.-W.; Kung, P.-Y.; Chang, C.-C.; Huang, W.-C.; Chang, F.-L.; Kao, C.R. Highly Robust Ti Adhesion Layer during Terminal Reaction in Micro-Bumps. Materials 2022, 15, 4297. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, F.; Lee, S.W.R. Effect of interfacial strength between Cu 6 Sn 5 and Cu 3 Sn intermetallics on the brittle fracture failure of lead-free solder joints with OSP pad finish. In Proceedings of the IEEE 61st Electronic Components and Technology Conference (ECTC), Lake Buena Vista, FL, USA, 31 May–3 June 2011. [Google Scholar]
- Yao, P.; Li, X. Investigation on shear fracture of different strain rates for Cu/Cu3Sn/Cu solder joints derived from Cu–15μm Sn–Cu sandwich structure. J. Mater. Sci. Mater. Electron. 2020, 31, 2862–2876. [Google Scholar] [CrossRef]
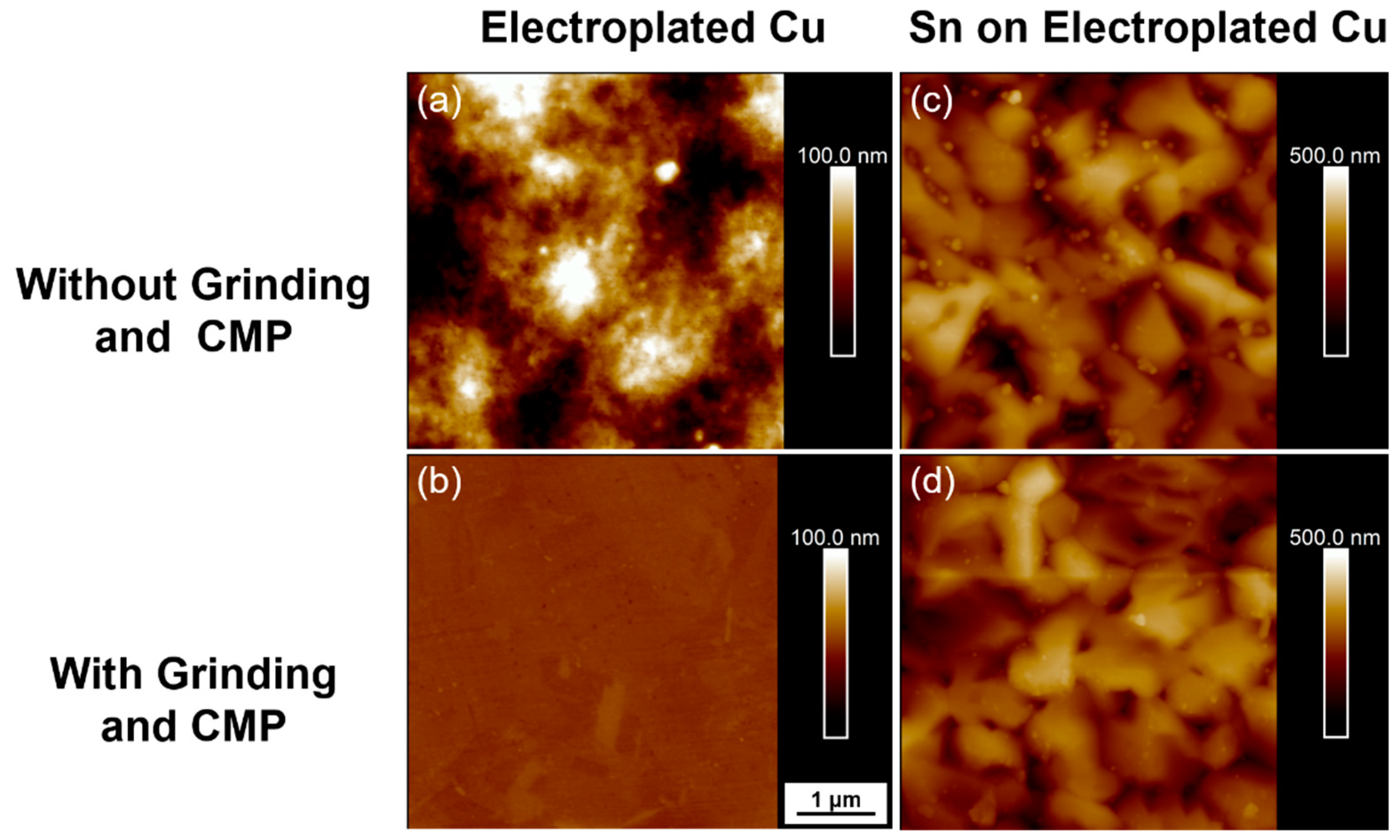


| Rq | Electroplated Cu | Sn on Electroplated Cu |
|---|---|---|
| Without Grinding + CMP | 22.1 nm | 61.1 nm |
| With Grinding + CMP | 1.32 nm | 56.2 nm |
| Maximum Height Difference | Electroplated Cu | Sn on Electroplated Cu |
|---|---|---|
| Without Grinding + CMP | 0.96 μm | 0.84 μm |
| With Grinding + CMP | 0.28 μm | 0.42 μm |
| Sn Thickness | Temperature | Pressure | Time | Porosity | Unbonded Percentage |
|---|---|---|---|---|---|
| 1 μm | 250 °C | 1 MPa | 1 min | 0.9% | 13.2% |
| 220 °C | 1 MPa | 1 min | 1.5% | 20.2% | |
| 800 nm | 250 °C | 2 MPa | 1 min | 1.7% | 22.1% |
| 220 °C | 2 MPa | 1 min | 4.8% | 37.3% |
| Sn Thickness | Temperature | Pressure | Time | Porosity | Unbonded Percentage |
|---|---|---|---|---|---|
| 800 nm | 220 °C | 2 MPa | 1 min | 4.8% → 0.3% | 37.3% → 7.35% |
| 800 nm | 250 °C | 2 MPa → 1 MPa | 1 min | 1.21% | 22.7% |
| 800 nm → 600 nm | 250 °C | 2 MPa | 1 min | 1.25% | 16.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kung, P.-Y.; Huang, W.-L.; Kao, C.-L.; Lin, Y.-S.; Hung, Y.-C.; Kao, C.R. Investigation of Low-Pressure Sn-Passivated Cu-to-Cu Direct Bonding in 3D-Integration. Materials 2022, 15, 7783. https://doi.org/10.3390/ma15217783
Kung P-Y, Huang W-L, Kao C-L, Lin Y-S, Hung Y-C, Kao CR. Investigation of Low-Pressure Sn-Passivated Cu-to-Cu Direct Bonding in 3D-Integration. Materials. 2022; 15(21):7783. https://doi.org/10.3390/ma15217783
Chicago/Turabian StyleKung, Po-Yu, Wei-Lun Huang, Chin-Li Kao, Yung-Sheng Lin, Yun-Ching Hung, and C. R. Kao. 2022. "Investigation of Low-Pressure Sn-Passivated Cu-to-Cu Direct Bonding in 3D-Integration" Materials 15, no. 21: 7783. https://doi.org/10.3390/ma15217783
APA StyleKung, P.-Y., Huang, W.-L., Kao, C.-L., Lin, Y.-S., Hung, Y.-C., & Kao, C. R. (2022). Investigation of Low-Pressure Sn-Passivated Cu-to-Cu Direct Bonding in 3D-Integration. Materials, 15(21), 7783. https://doi.org/10.3390/ma15217783








