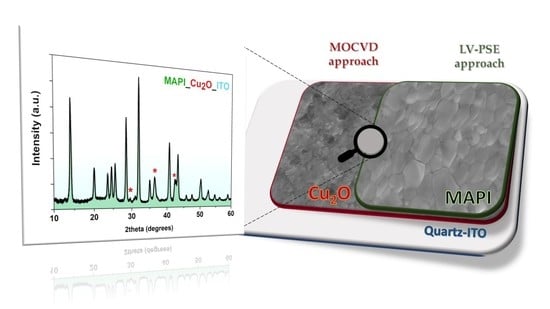
A Low Temperature Growth of Cu2O Thin Films as Hole Transporting Material for Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cu2O Synthesis
2.2. MAPI Deposition
2.3. Characterization
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Jawhari, H.A. A review of recent advances in transparent p-type Cu2O-based thin film transistors. Mater. Sci. Semicond. Process. 2015, 40, 241–252. [Google Scholar] [CrossRef]
- Meyer, B.K.; Polity, A.; Reppin, D.; Becker, M.; Hering, P.; Klar, P.J.; Sander, T.; Reindl, C.; Benz, J.; Eickhoff, M.; et al. Binary copper oxide semiconductors: From materials towards devices. Phys. Status Solidi B 2012, 249, 1487–1509. [Google Scholar] [CrossRef]
- Lupan, O.; Cretu, V.; Postica, V.; Ababii, N.; Polonskyi, O.; Kaidas, V.; Schütt, F.; Mishra, S.K.; Monaico, E.; Tiginyanu, I.; et al. Enhanced ethanol vapour sensing performances of copper oxide. Sens. Actuators B 2016, 224, 434–448. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Mohtar, S.S.; Aziz, F.; Mhamad, S.A.; Aziz, M. Review of various strategies to boost the photocatalytic activity of the cuprous oxide-based photocatalyst. J. Environ. Chem. Eng. 2021, 9, 105138. [Google Scholar] [CrossRef]
- Elfadill, N.G.; Hashim, M.R.; Saron, K.M.A.; Chahrour, K.M.; Qaeed, M.A.; Bououdina, M. Ultraviolet Visible photo-response of p-Cu2O/n-ZnO heterojunction prepared on flexible (PET) substrate. Mater. Chem. Phys. 2015, 156, 54–60. [Google Scholar] [CrossRef]
- Ghamgosar, P.; Rigoni, F.; Shujie You, S.; Dobryden, I.; Kohan, M.G.; Pellegrino, A.L.; Concina, I.; Almqvist, N.; Malandrino, G.; Vomiero, A. ZnO-Cu2O core-shell nanowires as stable and fast response photodetectors. Nano Energy 2018, 51, 308–316. [Google Scholar] [CrossRef]
- Jun Seo, Y.; Arunachalam, M.; Ahn, K.-S.; Hyung Kang, S. Integrating heteromixtured Cu2O/CuO photocathode interface through a hydrogen treatment for photoelectrochemical hydrogen evolution reaction. Appl. Surf. Sci. 2020, 551, 149375. [Google Scholar] [CrossRef]
- Li, C.; Fang, T.; Hu, H.; Wang, Y.; Liu, X.; Zhou, S.; Fu, J.; Wang, W. Synthesis and enhanced bias-free photoelectrochemical water-splitting activity of ferroelectric BaTiO3/Cu2O heterostructures under solar light irradiation. Ceram. Int. 2021, 47, 11379–11386. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Zhuka, S.; Tana, H.R.; Gong, X.; Dalapati, G.K. Palladium nanostructure incorporated cupric oxide thin film with strong optical absorption, compatible charge collection and low recombination loss for low cost solar cell applications. Nano Energy 2018, 46, 158–167. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Sekkat, A.; Bellet, D.; Chichignoud, G.; Kaminski-Cachopo, A.; Muñoz-Rojas, D.; Favre, W. Open-air, low-temperature deposition of phase pure Cu2O thin films as efficient hole-transporting layers for silicon heterojunction solar cells. J. Mater. Chem. A 2021, 9, 15968–15974. [Google Scholar] [CrossRef]
- Kim, S.; Jung, Y.S.; Hong, J.S.; Kim, K.H. Fabrication of copper oxide-based heterojunction thin film solar cells using sputtering. Mol. Cryst. Liq. Cryst. 2018, 677, 10–18. [Google Scholar] [CrossRef]
- Calil, L.; Kazim, S.; Gratzel, M.; Kurias, S.A. Hole-Transport Materials for Perovskite Solar Cells. Angew. Chem. Int. Ed. 2016, 55, 14522–14545. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Chen, Y.; Jia, J.; Zong, X.; Sun, Z.; Wu, Q.; Xue, S. Dopant-Free Hole-Transport Materials Based on 2,4,6-Triarylpyridine for Inverted Planar Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 1672–1683. [Google Scholar] [CrossRef]
- Messmer, C.; Bivour, M.; Schön, J.; Hermle, M. Requirements for Efficient Hole Extraction in Transition Metal Oxide-Based Silicon Heterojunction Solar Cells. J. Appl. Phys. 2018, 124, 085702. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Yue, Y.; Liu, J.; Zhang, W.; Yang, X.; Chen, H.; Bi, E.; Ashraful, I.; Grätzel, M.; et al. Efficient and stable large-area perovskite solar cells with inorganic charge extraction layers. Science 2015, 350, 944–948. [Google Scholar] [CrossRef] [Green Version]
- Markose, K.; Shaji, M.; Bhatia, S.; Nair, P.R.; Saji, K.J.; Antony, A.; Jayaraj, M.K. Novel boron doped p-type Cu2O thin film as hole selective contact in c-Si solar cell. ACS Appl. Mater. Interfaces 2020, 12, 12972–12981. [Google Scholar] [CrossRef]
- Jayathilaka, C.; Kumara, L.S.R.; Ohara, K.; Song, C.; Kohara, S.; Sakata, O.; Siripala, W.; Jayanetti, S. Enhancement of Solar Cell Performance of Electrodeposited Ti/n-Cu2O/p-Cu2O/Au Homojunction Solar Cells by Interface and Surface Modification. Crystals 2020, 10, 609. [Google Scholar] [CrossRef]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Miyano, K. Ammoniated aqueous precursor ink processed copper iodide as hole transport layer for inverted planar perovskite solar cells. Sol. Energy Mater. Sol. Cells 2020, 210, 110486. [Google Scholar] [CrossRef]
- Ye, S.; Sun, W.; Li, Y.; Yan, W.; Peng, H.; Bian, Z.; Liu, Z.; Huang, C. CuSCN-Based Inverted Planar Perovskite Solar Cell with an Average PCE of 15.6%. Nano Lett. 2015, 15, 3723–3728. [Google Scholar] [CrossRef]
- Nitta, R.; Kubota, Y.; Kishi, T.; Yano, T.; Matsushita, N. One-step direct fabrication of phase-pure Cu2O films via the spin-spray technique using a mixed alkaline solution. Mater. Chem. Phys. 2020, 243, 122442. [Google Scholar] [CrossRef]
- Karle, S.; Rogalla, D.; Ludwig, A.; Becker, H.-W.; Wieck, A.D.; Grafen, M.; Devi, A. Synthesis and evaluation of new copper ketoiminate precursors for a facile and additive-free solution-based approach to nanoscale copper oxide thin films. Dalton Trans. 2017, 46, 2670–2679. [Google Scholar] [CrossRef] [PubMed]
- Ibupoto, Z.H.; Khun, K.; Lu, J.; Willander, M. The synthesis of CuO nanoleaves, structural characterization, and their glucose sensing application. Appl. Phys. Lett. 2013, 102, 103701. [Google Scholar] [CrossRef]
- Borkar, R.; Dahake, R.; Rayalu, S.; Bansiwal, A. Copper Oxide Nanograss for Efficient and Stable Photoelectrochemical Hydrogen Production by Water Splitting. J. Electron. Mater. 2018, 47, 1824–1831. [Google Scholar] [CrossRef]
- Lakshmanan, A.; Alex, Z.C.; Meher, S.R. Cu2O thin films grown by magnetron sputtering as solar cell absorber layers. Mater. Sci. Semicond. Process. 2022, 148, 106818. [Google Scholar] [CrossRef]
- Huo, W.; Shi, J.; Mei, Z.; Liu, L.; Li, J.; Gu, L.; Du, Z.; Xue, Q. High-index Cu2O (113) film on faceted MgO (110) by molecular beam epitaxy. J. Cryst. Growth 2015, 420, 32–36. [Google Scholar] [CrossRef]
- Hu, X.; Schuster, J.; Schulz, S.E.; Gessner, T. Surface chemistry of copper metal and copper oxide atomic layer deposition from copper(II) acetylacetonate: A combined first-principles and reactive molecular dynamics study. Phys. Chem. Chem. Phys. 2015, 17, 26892–26902. [Google Scholar] [CrossRef] [Green Version]
- Karapetyan, A.; Reymers, A.; Giorgio, S.; Fauquet, C.; Sajti, L.; Nitsche, S.; Nersesyan, M.; Gevorgyan, V.; Marine, W. Cuprous oxide thin films prepared by thermal oxidation of copper layer. Morphological and optical properties. J. Lumin. 2015, 159, 325–332. [Google Scholar] [CrossRef]
- Kobayashi, H.; Nakamura, T.; Takahashi, N. Preparation of Cu2O films on MgO (1 1 0) substrate by means of halide chemical vapor deposition under atmospheric pressure. Mater. Chem. Phys. 2007, 106, 292–295. [Google Scholar] [CrossRef]
- Condorelli, G.G.; Malandrino, G.; Fragalà, I. Metal-Organic Chemical Vapor Deposition of Copper-Containing Phases: Kinetics and Reaction Mechanisms. Chem. Mater. 1994, 6, 1861–1866. [Google Scholar] [CrossRef]
- Arana-Chavez, D.; Toumayan, E.; Lora, F.; McCaslin, C.; Adomaitis, R.A. Modeling the Transport and Reaction Mechanisms of Copper Oxide CVD. Chem. Vap. Depos. 2010, 16, 336–345. [Google Scholar] [CrossRef]
- Sekkat, A.; Nguyen, V.H.; Masse de La Huerta, C.A.; Rapenne, L.; Bellet, D.; Kaminski-Cachopo, A.; Chichignoud, G.; Muñoz-Rojas, D. Open-air printing of Cu2O thin films with high hole mobility for semitransparent solar harvesters. Commun. Mater. 2021, 2, 78. [Google Scholar] [CrossRef]
- Jürgensen, L.; Höll, D.; Frank, M.; Ludwig, T.; Graf, D.; Schmidt-Verma, A.K.; Raauf, A.; Gessner, I.; Mathur, S. Controlled growth of Cu and CuOx thin films from subvalent copper precursors. Dalton Trans. 2020, 49, 13317–13325. [Google Scholar] [CrossRef] [PubMed]
- Pousaneh, E.; Korb, M.; Dzhagan, V.; Weber, M.; Noll, J.; Mehring, M.; Zahn, D.R.T.; Schulz, S.E.; Lang, H. β-Ketoiminato-based copper(II) complexes as CVD precursors for copper and copper oxide layer formation. Dalton Trans. 2018, 47, 10002–10016. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, G.G.; Malandrino, G.; Fragalà, I. Kinetic Study of MOCVD Fabrication of Copper(I) and Copper(II) Oxide Films. Chem. Vap. Depos. 1999, 5, 21–27. [Google Scholar] [CrossRef]
- Liu, H.; Nguyen, V.H.; Roussel, H.; Gélard, I.; Rapenne, L.; Deschanvres, J.-L.; Jiménez, C.; Muñoz-Rojas, D. The Role of Humidity in Tuning the Texture and Electrical Properties of Cu2O Thin Films Deposited via Aerosol-Assisted CVD. Adv. Mater. Interfaces 2019, 6, 1801364. [Google Scholar] [CrossRef] [Green Version]
- Barreca, D.; Gasparotto, A.; Maccato, C.; Tondello, E.; Lebedev, O.I.; Van Tendeloo, G. CVD of Copper Oxides from a β-Diketonate Diamine Precursor: Tailoring the Nano-Organization. Cryst. Growth Des. 2009, 9, 2471–2480. [Google Scholar] [CrossRef]
- Maruyama, T. Copper oxide thin films prepared by chemical vapor deposition from copper dipivaloylmethanate. Sol. Energy Mater. Sol. Cells 1998, 56, 85–92. [Google Scholar] [CrossRef]
- Lay, E.; Song, Y.-H.; Chiu, Y.-C.; Lin, Y.-M.; Chi, Y. New CVD Precursors Capable of Depositing Copper Metal under Mixed O2/Ar Atmosphere. Inorg. Chem. 2005, 44, 7226–7233. [Google Scholar] [CrossRef]
- Gupta, N.; Singh, R.; Wu, F.; Narayan, J.; McMillen, C.; Alapatt, G.F.; Poole, K.F.; Hwu, S.; Sulejmanovic, D.; Young, M.; et al. Deposition and characterization of nanostructured Cu2O thin-film for potential photovoltaic applications. J. Mater. Res. 2013, 28, 1740–1746. [Google Scholar] [CrossRef]
- Smecca, E.; Valenzano, V.; Valastro, S.; Deretzis, I.; Mannino, G.; Malandrino, G.; Accorsi, G.; Colella, S.; Rizzo, A.; La Magna, A.; et al. Two-step MAPbI3 deposition by Low-Vacuum Proximity Space-Effusion for high-efficiency inverted semitransparent perovskite solar cells. J. Mater. Chem. A 2021, 9, 16456–16469. [Google Scholar] [CrossRef]
- Smecca, E.; Jena, A.K.; Deretzis, I.; Valastro, S.; Sanzaro, S.; Mannino, G.; Bongiorno, C.; La Magna, A.; Miyasaka, T.; Alberti, A. MAPbI3 Deposition by LV-PSE on TiO2 for Photovoltaic Application. Front. Electron. 2021, 2, 726171. [Google Scholar] [CrossRef]
- Alberti, A.; Smecca, E.; Valastro, S.; Deretzis, J.; Mannino, G.; Bongiorno, C.; Fisicaro, G.; La Magna, A. Perovskite Solar Cells from the viewpoint of innovation and sustainability. Phys. Chem. Chem. Phys. 2022, 24, 21549–21566. [Google Scholar] [CrossRef] [PubMed]
- Aithssi, A.; Atourki, L.; Labchir, N.; Ouafi, M.; Abouabassi, K.; Elfanaoui, A.; Ihlal, A.; Bouabid, K. Optical and dielectric properties of electrochemically deposited p-Cu2O films. Mater. Res. Express 2020, 7, 016424. [Google Scholar] [CrossRef]
- Xue, J.; Shen, Q.; Liang, W.; Liu, X.; Bian, L.; Xu, B. Preparation and formation mechanism of smooth and uniform Cu2O thin films by electrodeposition method. Surf. Coat. Technol. 2013, 216, 166–171. [Google Scholar] [CrossRef]
- Chen, A.; Long, H.; Li, X.; Li, Y.; Yang, G.; Lu, P. Controlled growth and characteristics of single-phase Cu2O and CuO films by pulsed laser deposition. Vacuum 2009, 83, 927–930. [Google Scholar] [CrossRef]
- Sawicka-Chudy, P.; Sibinski, M.; Pawelek, R.; Wisz, G.; Cieniek, B.; Potera, P.; Szczepan, P.; Adamiak, S.; Cholewa, M.; Glowa, L. Characteristics of TiO2, Cu2O, and TiO2/Cu2O thin films for application in PV devices. AIP Adv. 2019, 9, 055206. [Google Scholar] [CrossRef] [Green Version]
- Chua, D.; Kim, S.B.; Li, K.; Gordon, R. Low Temperature Chemical Vapor Deposition of Cuprous Oxide Thin Films Using a Copper(I) Amidinate Precursor. ACS Appl. Energy Mater. 2019, 2, 7750–7756. [Google Scholar] [CrossRef]
- Ben Rabeha, M.; Khedmia, N.; Fodhaa, M.A.; Kanzaria, M. The Effect of Thickness on Optical Band Gap and N-type Conductivity of CuInS2 Thin Films Annealed in Air Atmosphere. Energy Procedia 2014, 44, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Sönmezoğlu, S.; Arslan, A.; Serin, T.; Serin, N. The effects of film thickness on the optical properties of TiO2–SnO2 compound thin films. Phys. Scr. 2011, 84, 065602. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, Y.; Peng, X.; Zhong, K.; Lin, Y.; Huang, Z. The Phase Evolution and Physical Properties of Binary Copper Oxide Thin Films Prepared by Reactive Magnetron Sputtering. Materials 2018, 11, 1253. [Google Scholar] [CrossRef]
- Murali, D.S.; Kumar, S.; Choudhary, R.J.; Wadikar, A.D.; Jain, M.K.; Subrahmanyam, A. Synthesis of Cu2O from CuO thin films: Optical and electrical properties. AIP Adv. 2015, 5, 047143. [Google Scholar] [CrossRef]
- Schroder, D.K. Semiconductor Material and Device Characterization, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Resende, J.; Nguyen, V.-S.; Fleischmann, C.; Bottiglieri, L.; Brochen, S.; Vandervorst, W.; Favre, W.; Jiménez, C.; Deschanvres, J.-L.; Nguyen, N.D. Grain-boundary segregation of magnesium in doped cuprous oxide and impact on electrical transport properties. Sci. Rep. 2021, 11, 7788. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lei, H.; Xiong, L.; Li, B.; Chen, Z.; Wen, J.; Yang, G.; Li, G.; Fang, G. Single phase, high hole mobility Cu2O films as an efficient and robust hole transporting layer for organic solar cells. J. Mater. Chem. A 2017, 5, 11055–11062. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, Y.; Liu, X.; Xue, S. Dopant-free Hole-transporting Materials for CH3NH3PbI3 Inverted Perovskite Solar Cells with an Approximate Efficiency of 20%. ACS Appl. Energy Mater. 2021, 4, 5756–5766. [Google Scholar] [CrossRef]
- Huang, J.; Ge, C.; Qin, F.; Zou, Y.; Zhou, Y.; Li, W.-S.; Gao, X. Achieve Better Performance of Inverted Perovskite Solar Cells by Using the Fluorinated Polymer as the Electron Transporting Layer. ACS Appl. Energy Mater. 2022, 5, 2522–2530. [Google Scholar] [CrossRef]
- Baikie, T.; Fang, Y.; Kadro, J.M.; Schreyer, M.; Wei, F.; Mhaisalkar, S.G.; Graetzel, M.; White, T.J. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitized solar cell applications. J. Mater. Chem. A 2013, 1, 5628–5641. [Google Scholar] [CrossRef]
- Udalova, N.N.; Nemygina, E.M.; Zharenova, E.A.; Tutantsev, A.S.; Sudakov, A.A.; Grishko, A.Y.; Belich, N.A.; Goodilin, E.A.; Tarasov, A.B. New Aspects of Copper Electrode Metamorphosis in Perovskite Solar Cells. J. Phys. Chem. C 2020, 124, 24601–24607. [Google Scholar] [CrossRef]








| Sample | Carrier Type | Resistivity (Ω cm) | Carrier Concentration (cm−3) | Mobility (cm2 V−1 s−1) |
|---|---|---|---|---|
| 230 nm | Holes | 2 | 1.5 × 1016 | 5.2 |
| 140 nm | Holes | 73 | 2.0 × 1016 | 4.3 |
| 90 nm | Holes | 32 | 2.8 × 1016 | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrino, A.L.; Lo Presti, F.; Smecca, E.; Valastro, S.; Greco, G.; Di Franco, S.; Roccaforte, F.; Alberti, A.; Malandrino, G. A Low Temperature Growth of Cu2O Thin Films as Hole Transporting Material for Perovskite Solar Cells. Materials 2022, 15, 7790. https://doi.org/10.3390/ma15217790
Pellegrino AL, Lo Presti F, Smecca E, Valastro S, Greco G, Di Franco S, Roccaforte F, Alberti A, Malandrino G. A Low Temperature Growth of Cu2O Thin Films as Hole Transporting Material for Perovskite Solar Cells. Materials. 2022; 15(21):7790. https://doi.org/10.3390/ma15217790
Chicago/Turabian StylePellegrino, Anna L., Francesca Lo Presti, Emanuele Smecca, Salvatore Valastro, Giuseppe Greco, Salvatore Di Franco, Fabrizio Roccaforte, Alessandra Alberti, and Graziella Malandrino. 2022. "A Low Temperature Growth of Cu2O Thin Films as Hole Transporting Material for Perovskite Solar Cells" Materials 15, no. 21: 7790. https://doi.org/10.3390/ma15217790
APA StylePellegrino, A. L., Lo Presti, F., Smecca, E., Valastro, S., Greco, G., Di Franco, S., Roccaforte, F., Alberti, A., & Malandrino, G. (2022). A Low Temperature Growth of Cu2O Thin Films as Hole Transporting Material for Perovskite Solar Cells. Materials, 15(21), 7790. https://doi.org/10.3390/ma15217790