Review on Fluorescent Carbon/Graphene Quantum Dots: Promising Material for Energy Storage and Next-Generation Light-Emitting Diodes
Abstract
:1. Introduction
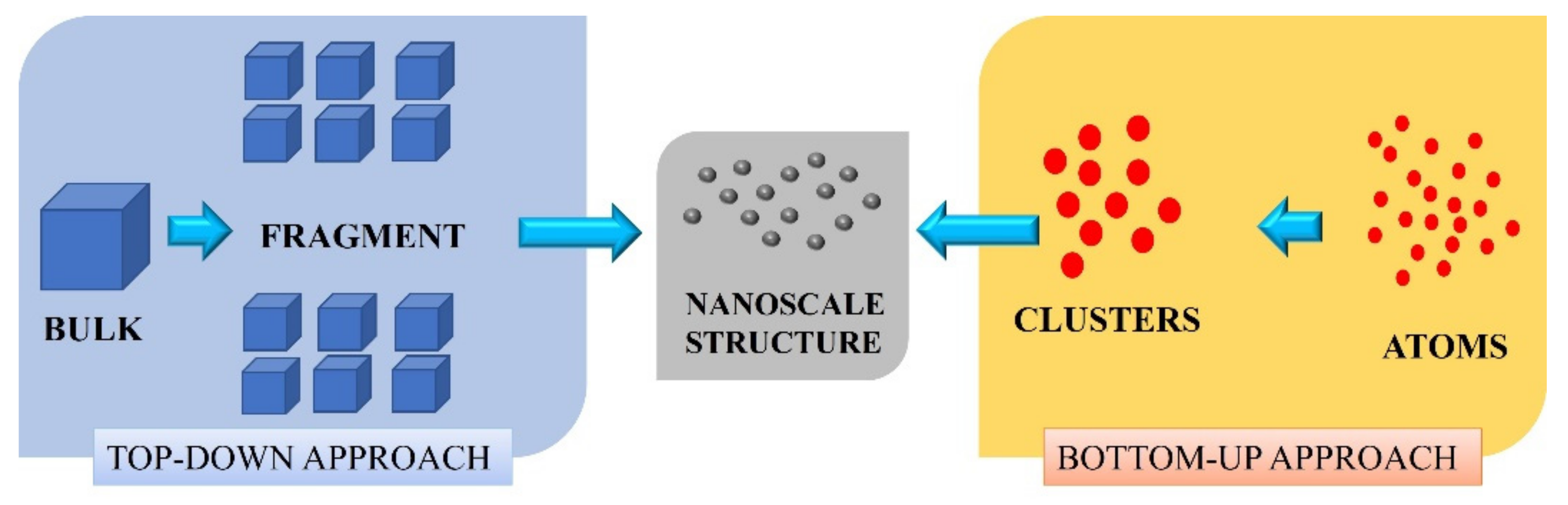
2. Methodology
3. Top-Down Approach
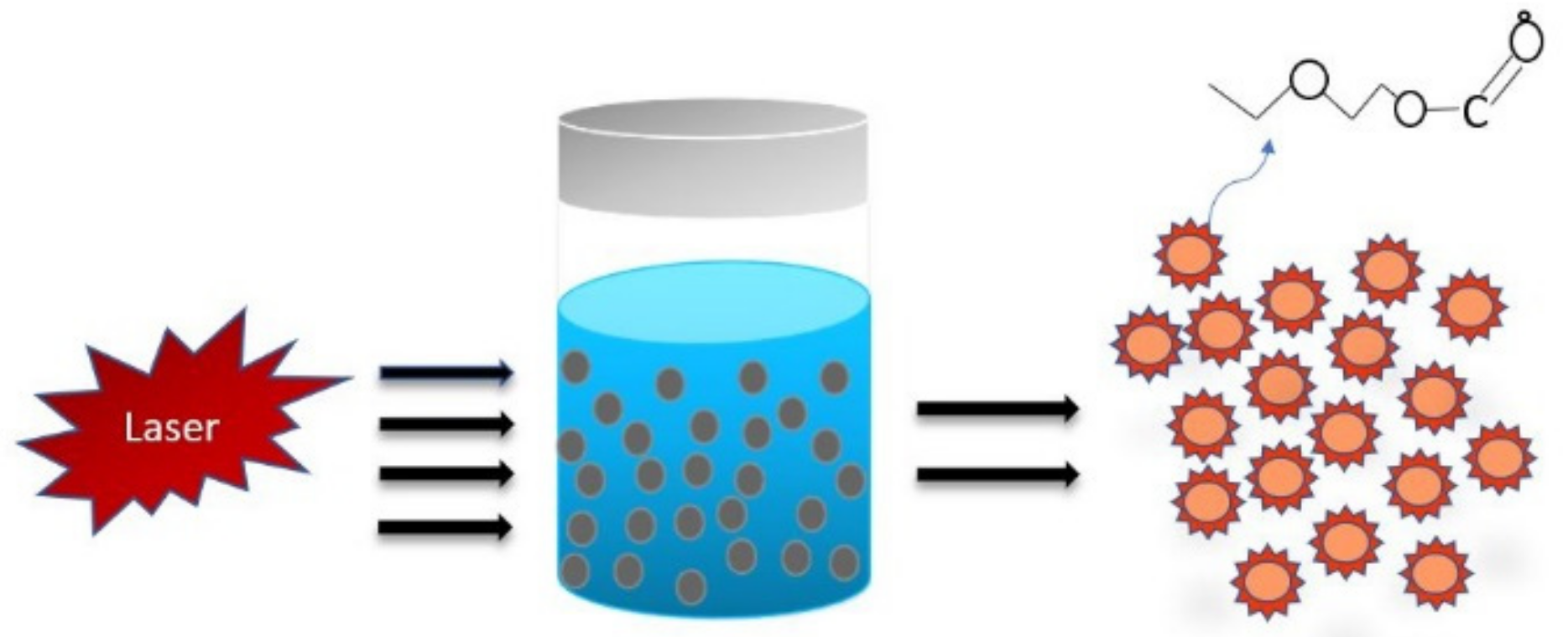
3.1. Laser Ablation
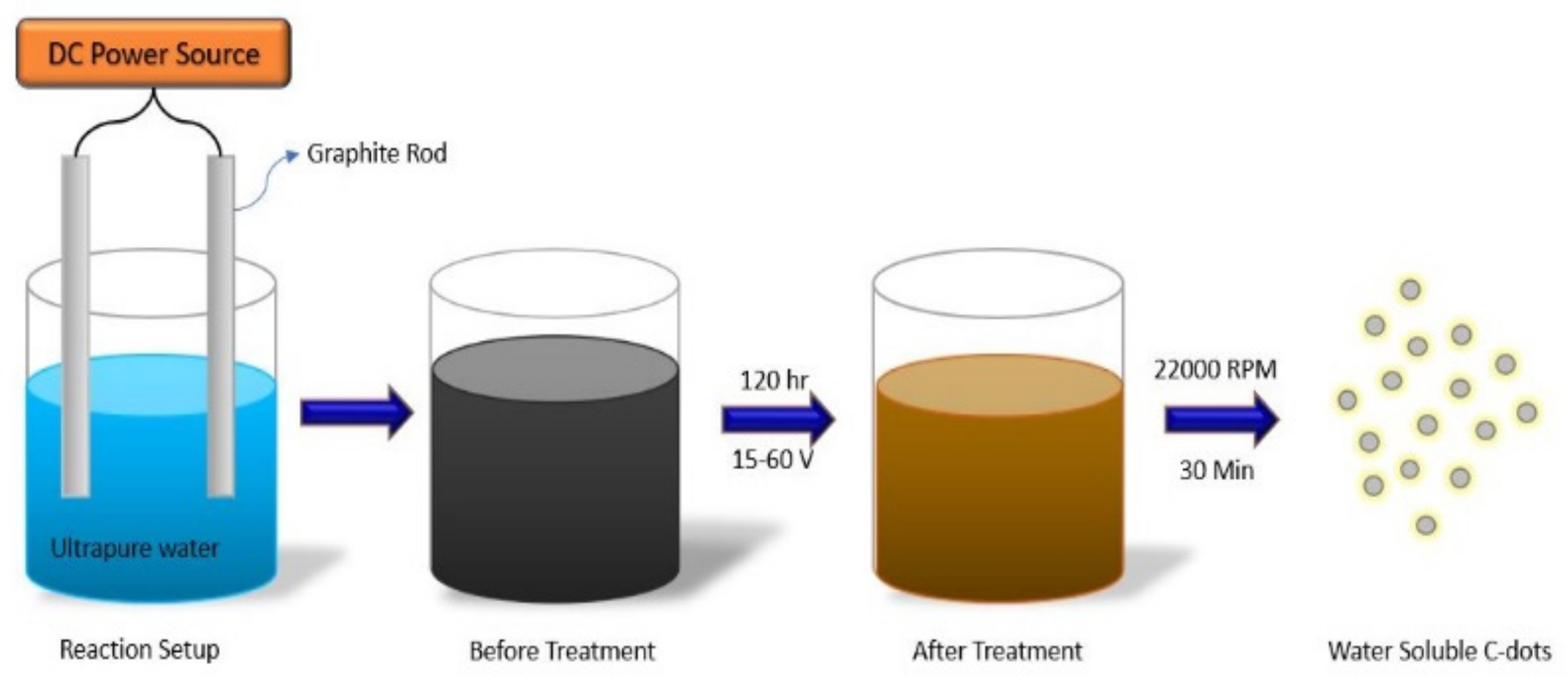
3.2. Electrochemical Methods
3.3. Arc Discharge
4. Bottom-Up Approach
4.1. Microwave-Assisted Technique
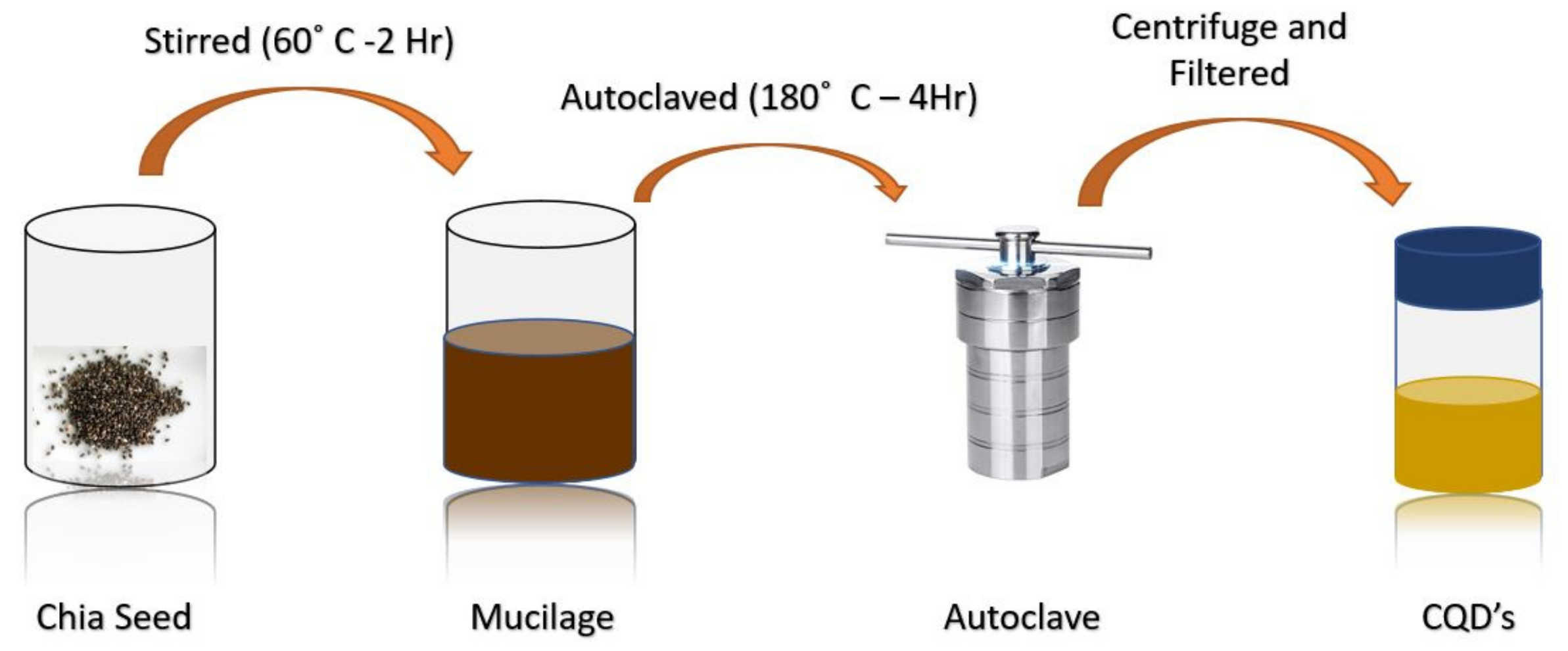
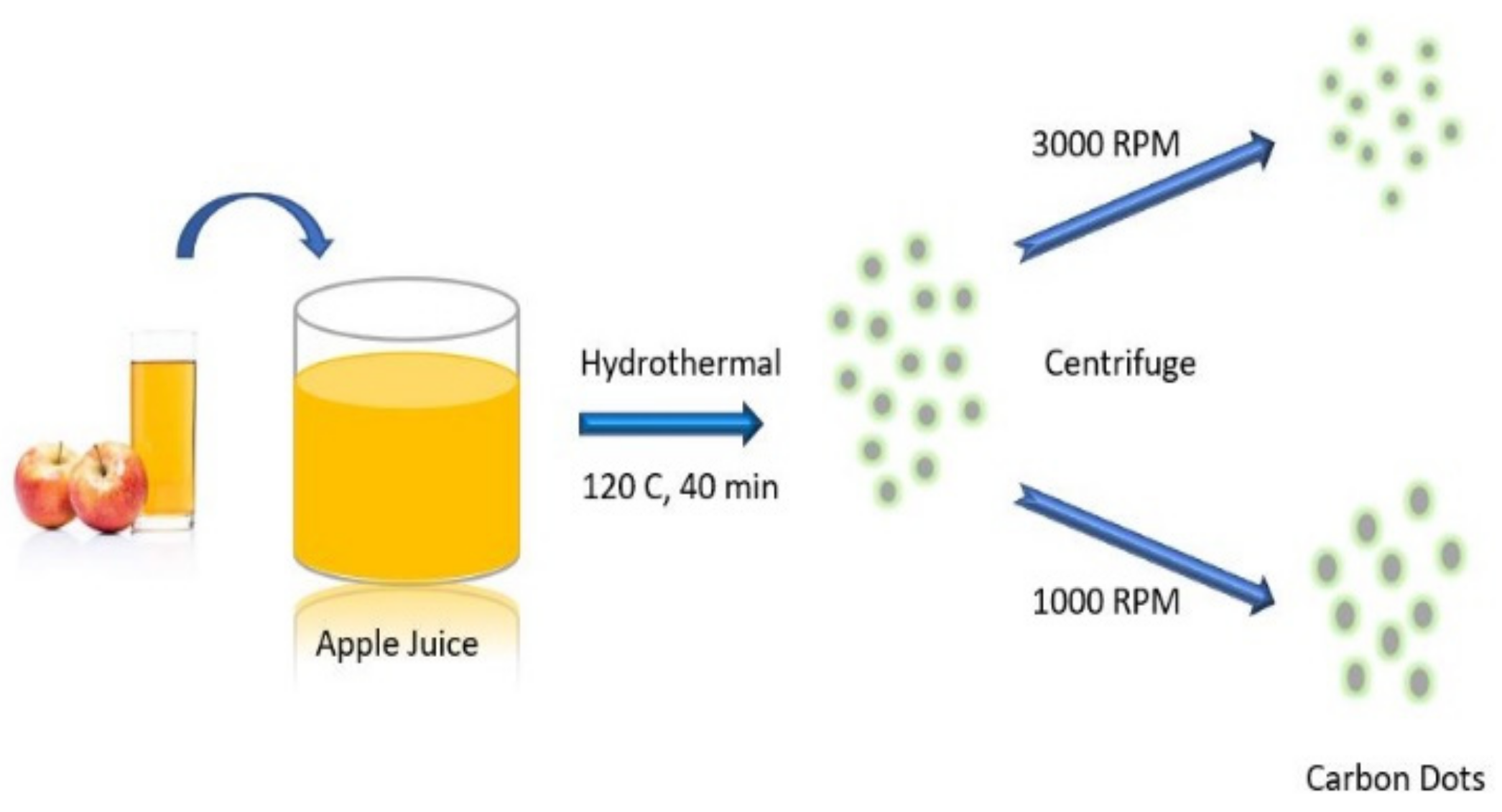
4.2. Hydrothermal/Solvothermal
4.3. Thermal/Combustion Method
4.4. Template Method
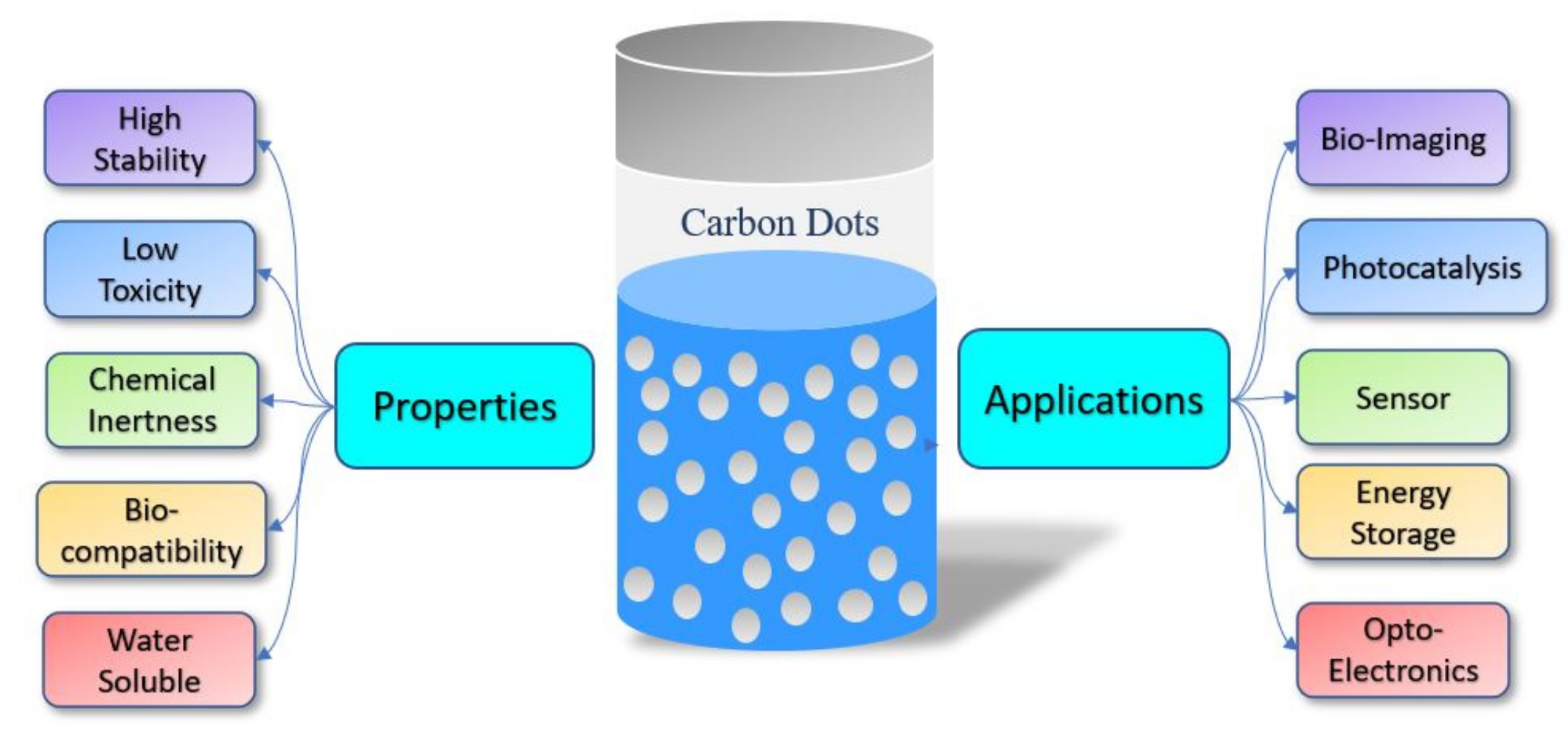
5. Characterisation: Physical and Chemical Properties
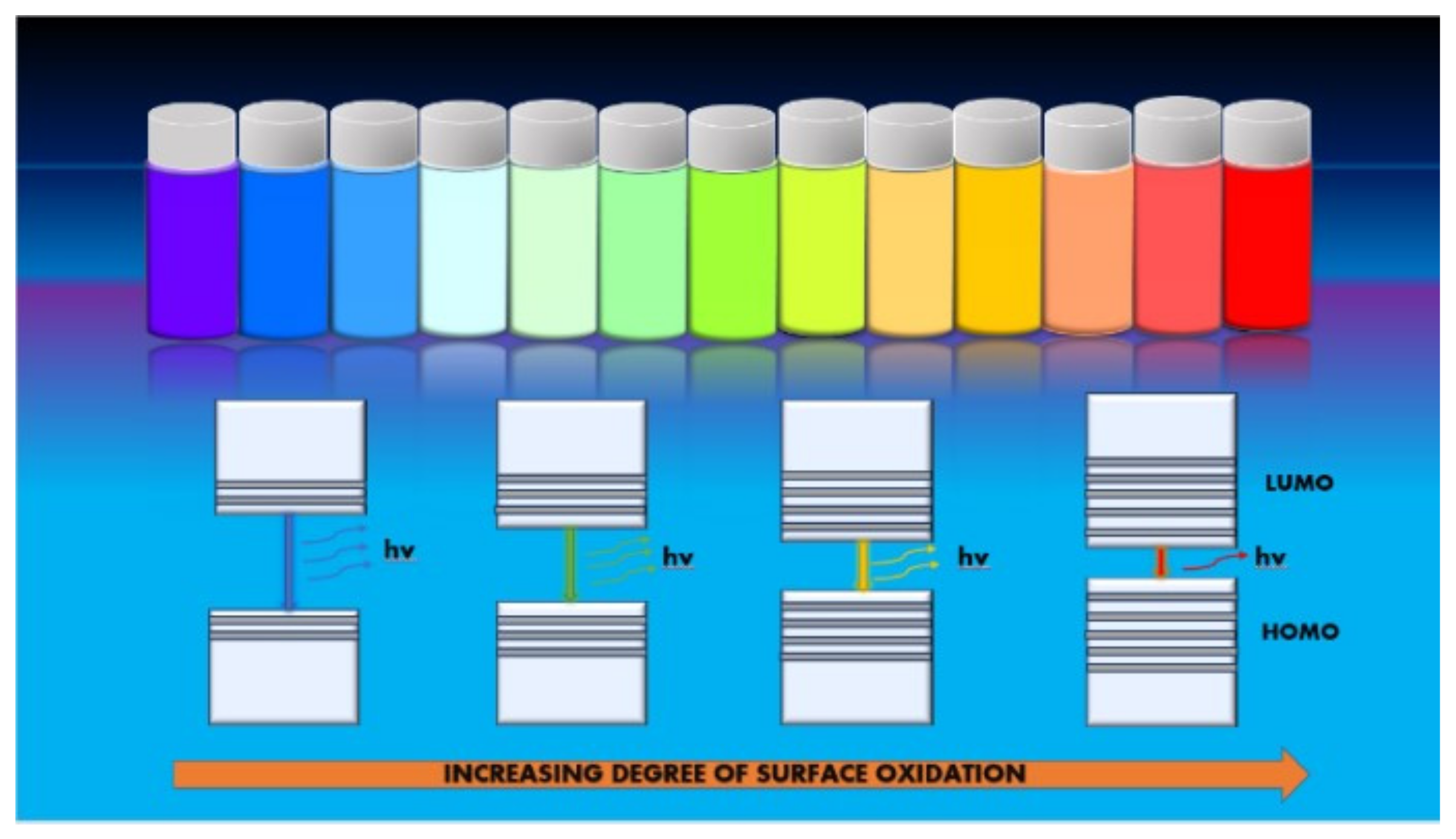
5.1. Optical Properties
5.1.1. UV-Absorption
5.1.2. Photoluminescence
5.1.3. FTIR
5.2. Applications
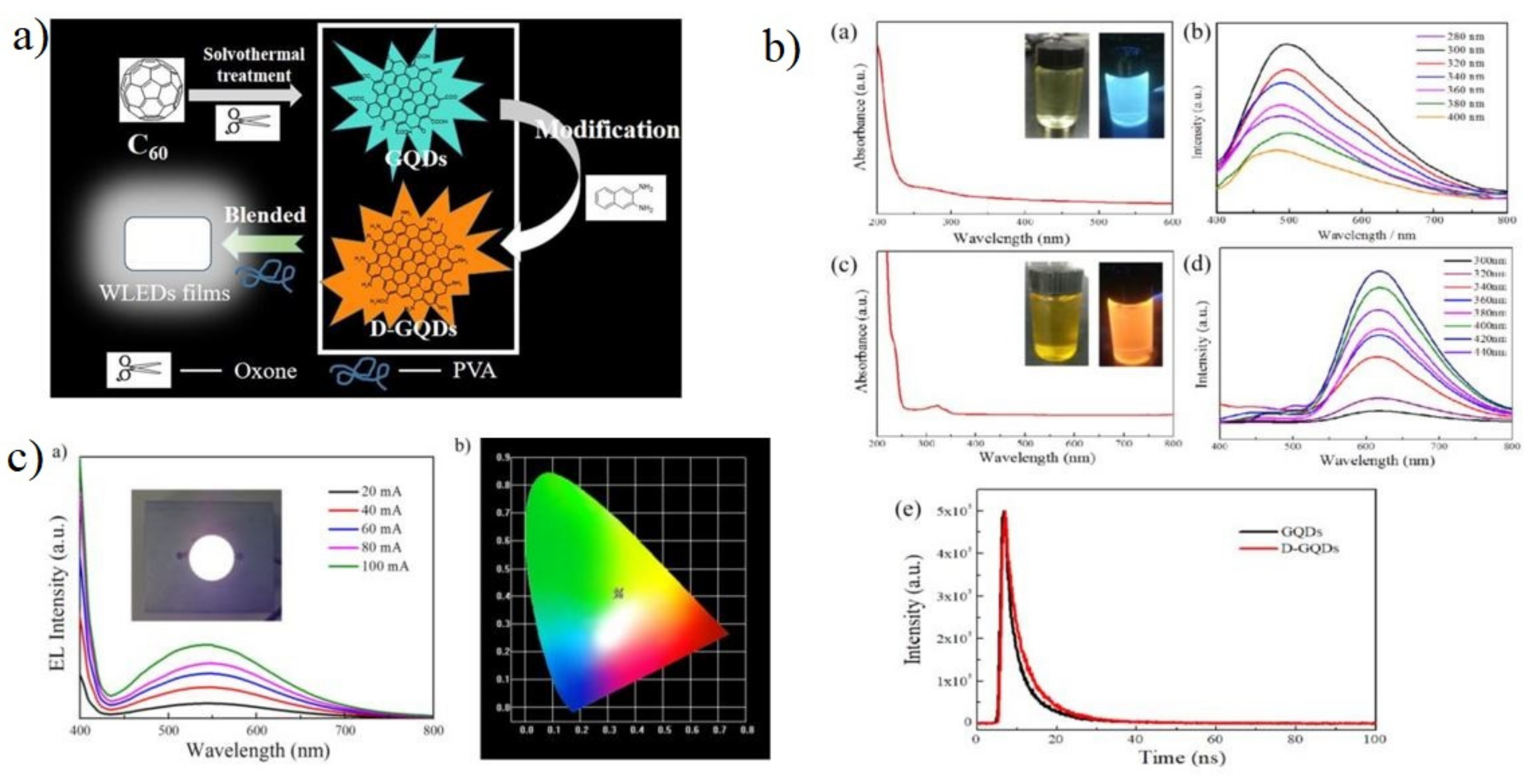
5.2.1. Light Emitting Diode
5.2.2. Energy Storage Application
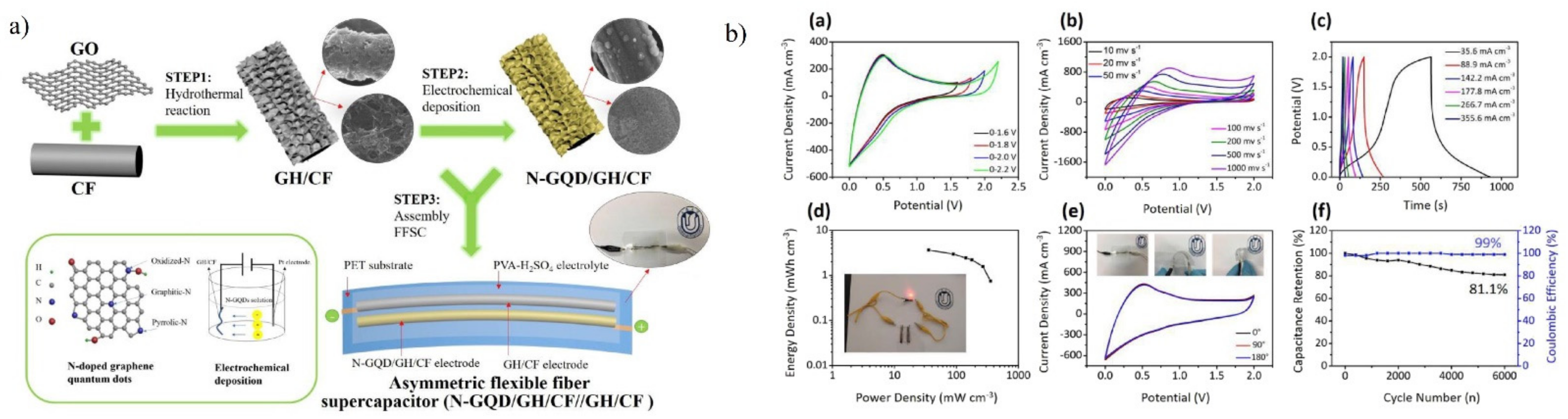
5.2.3. Supercapacitors
5.2.4. Batteries
6. Conclusions and Future Aspects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vahdani, M.R.K.; Ehsanfard, N. Nonlinear Optical Properties of a Slab of CdSe/ZnS Quantum Dot Matrix. Phys. B Condens. Matter 2018, 548, 1–9. [Google Scholar] [CrossRef]
- Ornes, S. Quantum Dots. Proc. Natl. Acad. Sci. USA 2016, 113, 2796–2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.M.; Zarghami, N.; Kouhi, M.; Akbarzadeh, A.; Davaran, S. Quantum Dots: Synthesis, Bioapplications, and Toxicity. Nanoscale Res. Lett. 2012, 7, 19–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshma, V.G.; Mohanan, P.V. Quantum Dots: Applications and Safety Consequences. J. Lumin. 2019, 205, 287–298. [Google Scholar] [CrossRef]
- Hu, Z.M.; Fei, G.T.; De Zhang, L. Synthesis and Tunable Emission of Ga2S3 Quantum Dots. Mater. Lett. 2019, 239, 17–20. [Google Scholar] [CrossRef]
- Chen, F.; Yao, Y.; Lin, H.; Hu, Z.; Hu, W.; Zang, Z.; Tang, X. Synthesis of CuInZnS Quantum Dots for Cell Labelling Applications. Ceram. Int. 2018, 44, S34–S37. [Google Scholar] [CrossRef]
- Pierobon, P.; Cappello, G. Quantum Dots to Tail Single Bio-Molecules inside Living Cells. Adv. Drug Deliv. Rev. 2012, 64, 167–178. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.W.; Sun, W.; Wu, F.G. Fluorescent Quantum Dots for Microbial Imaging. Chin. Chem. Lett. 2018, 29, 1475–1485. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, H.; Wu, S.T.; Dong, Y. Quantum Dot Light Emitting Diodes. Compr. Nanosci. Nanotechnol. 2019, 1–5, 35–56. [Google Scholar] [CrossRef]
- Hoang, V.C.; Dave, K.; Gomes, V.G. Carbon Quantum Dot-Based Composites for Energy Storage and Electrocatalysis: Mechanism, Applications and Future Prospects. Nano Energy 2019, 66, 104093. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, G.; Zhu, Y. Carbon-Based Supercapacitors Produced by Activation of Graphene-Surporting Materials. Science 2015, 1, 211–225. [Google Scholar]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.G.; Yoon, T.; Kim, K.S. Nickel-Based Electrocatalysts for Energy-Related Applications: Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution Reactions. ACS Catal. 2017, 7, 7196–7225. [Google Scholar] [CrossRef]
- Borghei, M.; Lehtonen, J.; Liu, L.; Rojas, O.J. Advanced Biomass-Derived Electrocatalysts for the Oxygen Reduction Reaction. Adv. Mater. 2018, 30, 1703691. [Google Scholar] [CrossRef]
- Hoang, V.C.; Do, V.; Nah, I.W.; Lee, C.; Cho, W.I.; Oh, I.H. Facile Coating of Graphene Interlayer onto Li2S as a High Electrochemical Performance Cathode for Lithium Sulfur Battery. Electrochim. Acta 2016, 210, 1–6. [Google Scholar] [CrossRef]
- Li, L.; Zheng, Y.; Zhang, S.; Yang, J.; Shao, Z.; Guo, Z. Recent Progress on Sodium Ion Batteries: Potential High-Performance Anodes. Energy Environ. Sci. 2018, 11, 2310–2340. [Google Scholar] [CrossRef] [Green Version]
- Xue, B.; Yang, Y.; Sun, Y.; Fan, J.; Li, X.; Zhang, Z. Photoluminescent Lignin Hybridized Carbon Quantum Dots Composites for Bioimaging Applications. Int. J. Biol. Macromol. 2019, 122, 954–961. [Google Scholar] [CrossRef]
- Lim, H.; Liu, Y.; Kim, H.Y.; Son, D.I. Facile Synthesis and Characterization of Carbon Quantum Dots and Photovoltaic Applications. Thin Solid Film. 2018, 660, 672–677. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon Quantum Dots from Natural Resource: A Review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Devi, P.; Rajput, P.; Thakur, A.; Kim, K.H.; Kumar, P. Recent Advances in Carbon Quantum Dot-Based Sensing of Heavy Metals in Water. TrAC—Trends Anal. Chem. 2019, 114, 171–195. [Google Scholar] [CrossRef]
- Jiao, J.; Liu, C.; Li, X.; Liu, J.; Di, D.; Zhang, Y.; Zhao, Q.; Wang, S. Fluorescent Carbon Dot Modified Mesoporous Silica Nanocarriers for Redox-Responsive Controlled Drug Delivery and Bioimaging. J. Colloid Interface Sci. 2016, 483, 343–352. [Google Scholar] [CrossRef]
- Chen, G.; Wu, S.; Hui, L.; Zhao, Y.; Ye, J.; Tan, Z.; Zeng, W.; Tao, Z.; Yang, L.; Zhu, Y. Assembling Carbon Quantum Dots to a Layered Carbon for High-Density Supercapacitor Electrodes. Sci. Rep. 2016, 6, 19028. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Marinovic, A.; Sevilla, M.; Dunn, S.; Titirici, M. Biomass-Derived Carbon Quantum Dot Sensitizers for Solid-State Nanostructured Solar Cells. Angew. Chem. Int. Ed. 2015, 54, 4463–4468. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Yang, J.; Zhou, C.; Mo, Q.; Zhang, Y. Carbon Quantum Dots Modified BiOBr Microspheres with Enhanced Visible Light Photocatalytic Performance. Inorg. Chem. Commun. 2018, 90, 97–100. [Google Scholar] [CrossRef]
- Gyulai, G.; Ouanzi, F.; Bertóti, I.; Mohai, M.; Kolonits, T.; Horváti, K.; Bősze, S. Chemical Structure and in Vitro Cellular Uptake of Luminescent Carbon Quantum Dots Prepared by Solvothermal and Microwave Assisted Techniques. J. Colloid Interface Sci. 2019, 549, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Wang, R.; Li, H.; Shao, J.; Chi, Y.; Lin, X.; Chen, G. Polyamine-Functionalized Carbon Quantum Dots for Chemical Sensing. Carbon N.Y. 2012, 50, 2810–2815. [Google Scholar] [CrossRef]
- Javed, M.; Saqib, A.N.S.; Ata-ur-Rehman; Ali, B.; Faizan, M.; Anang, D.A.; Iqbal, Z.; Abbas, S.M. Carbon Quantum Dots from Glucose Oxidation as a Highly Competent Anode Material for Lithium and Sodium-Ion Batteries. Electrochim. Acta 2019, 297, 250–257. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Tian, Y.; Li, L.; Li, J.; Qiu, T.; Zou, G.; Hou, H.; Ji, X. Honeycomb Hard Carbon Derived from Carbon Quantum Dots as Anode Material for K-Ion Batteries. Mater. Chem. Phys. 2019, 229, 303–309. [Google Scholar] [CrossRef]
- Li, L.; Wu, G.; Yang, G.; Peng, J.; Zhao, J.; Zhu, J.J. Focusing on Luminescent Graphene Quantum Dots: Current Status and Future Perspectives. Nanoscale 2013, 5, 4015–4039. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Sun, J.; Tian, S.; Yang, S.; Ding, S.; Ding, G.; Xie, X.; Jiang, M. Processable Aqueous Dispersions of Graphene Stabilized by Graphene Quantum Dots. Chem. Mater. 2015, 27, 218–226. [Google Scholar] [CrossRef]
- Hao, Y.; Dong, X.; Wang, X.; Ma, H.; Zhang, X. Incorporation of Graphene Nanodots and Oxygen Defects Triggers Robust Coupling between Solar Energy and Reactive Oxygen. J. Mater. Chem. A 2017, 5, 5426–5435. [Google Scholar] [CrossRef]
- Li, X.; Rui, M.; Song, J.; Shen, Z.; Zeng, H. Carbon and Graphene Quantum Dots for Optoelectronic and Energy Devices: A Review. Adv. Funct. Mater. 2015, 25, 4929–4947. [Google Scholar] [CrossRef]
- Wu, W.; Cao, J.; Zhong, M.; Wu, H.; Zhang, F.; Zhang, J.; Guo, S. Separating Graphene Quantum Dots by Lateral Size through Gel Column Chromatography. RSC Adv. 2019, 9, 18898–18901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Hee Shin, D.; Oh Kim, C.; Seok Kang, S.; Sin Joo, S.; Choi, S.H.; Won Hwang, S.; Sone, C. Size-Dependence of Raman Scattering from Graphene Quantum Dots: Interplay between Shape and Thickness. Appl. Phys. Lett. 2013, 102, 053108. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Ludmerczki, R.; Mura, S.; Carbonaro, C.M.; Mandity, I.M.; Carraro, M.; Senes, N.; Garroni, S.; Granozzi, G.; Calvillo, L.; Marras, S.; et al. Carbon Dots from Citric Acid and its Intermediates Formed by Thermal Decomposition. Chem. Eur. J. 2019, 25, 11963. [Google Scholar] [CrossRef] [PubMed]
- Phang, S.J.; Tan, L.L. Recent Advances in Carbon Quantum Dot (CQD)-Based Two Dimensional Materials for Photocatalytic Applications. Catal. Sci. Technol. 2019, 9, 5882–5905. [Google Scholar] [CrossRef]
- Li, K.; Liu, W.; Ni, Y.; Li, D.; Lin, D.; Su, Z.; Wei, G. Technical Synthesis and Biomedical Applications of Graphene Quantum Dots. J. Mater. Chem. B 2017, 5, 4811–4826. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, V.; Lee, N.Y. Green synthesis of carbon quantum dots and their environmental applications. Environ. Res. 2022, 212, 113283. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Z.; Shen, X.; Wang, L. One-Pot Synthesis of Orange Emissive Carbon Quantum Dots for All-Type High Color Rendering Index White Light-Emitting Diodes. ACS Sustain. Chem. Eng. 2022, 10, 8289–8296. [Google Scholar] [CrossRef]
- Saikia, M.; Das, T.; Saikia, B.K. A novel rapid synthesis of highly stable silver nanoparticle/carbon quantum dot nanocomposites derived from low-grade coal feedstock. New J. Chem. 2022, 46, 309–321. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.L.; Niu, K.Y.; Sun, J.; Yang, J.; Zhao, N.Q.; Du, X.W. One-Step Synthesis of Fluorescent Carbon Nanoparticles by Laser Irradiation. J. Mater. Chem. 2009, 19, 484–488. [Google Scholar] [CrossRef]
- Liu, H.; Ye, T.; Mao, C. Fluorescent Carbon Nanoparticles Derived from Candle Soot. Angew. Chem. Int. Ed. 2007, 46, 6473–6475. [Google Scholar] [CrossRef]
- Yan, X.; Li, B.; Li, L.S. Colloidal Graphene Quantum Dots with Well-Defined Structures. Acc. Chem. Res. 2013, 46, 2254–2262. [Google Scholar] [CrossRef] [PubMed]
- Bottini, M.; Balasubramanian, C.; Dawson, M.I.; Bergamaschi, A.; Bellucci, S.; Mustelin, T. Isolation and Characterization of Fluorescent Nanoparticles from Pristine and Oxidized Electric Arc-Produced Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.L.; Zhang, Z.L.; Huang, B.H.; Peng, J.; Zhang, M.; Pang, D.W. Facile Preparation of Low Cytotoxicity Fluorescent Carbon Nanocrystals by Electrooxidation of Graphite. Chem. Commun. 2008, 41, 5116–5118. [Google Scholar] [CrossRef]
- Kuzmin, P.G.; Shafeev, G.A.; Bukin, V.V.; Garnov, S.V.; Farcau, C.; Carles, R.; Warot-Fontrose, B.; Guieu, V.; Viau, G. Silicon Nanoparticles Produced by Femtosecond Laser Ablation in Ethanol: Size Control, Structural Characterization, and Optical Properties. J. Phys. Chem. C 2010, 114, 15266–15273. [Google Scholar] [CrossRef]
- Doñate-Buendia, C.; Torres-Mendieta, R.; Pyatenko, A.; Falomir, E.; Fernández-Alonso, M.; Mínguez-Vega, G. Fabrication by Laser Irradiation in a Continuous Flow Jet of Carbon Quantum Dots for Fluorescence Imaging. ACS Omega 2018, 3, 2735–2742. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Shimizu, Y.; Pyatenko, A.; Kawaguchi, K.; Koshizaki, N. Preparation of Carbon Quantum Dots with Tunable Photoluminescence by Rapid Laser Passivation in Ordinary Organic Solvents. Chem. Commun. 2011, 47, 932–934. [Google Scholar] [CrossRef]
- Singh, A.; Mohapatra, P.K.; Kalyanasundaram, D.; Kumar, S. Self-Functionalized Ultrastable Water Suspension of Luminescent Carbon Quantum Dots. Mater. Chem. Phys. 2019, 225, 23–27. [Google Scholar] [CrossRef]
- Thongpool, V.; Asanithi, P.; Limsuwan, P. Synthesis of Carbon Particles Using Laser Ablation in Ethanol. Procedia Eng. 2012, 32, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A Review on Syntheses, Properties, Characterization and Bioanalytical Applications of Fluorescent Carbon Dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Lu, Q.; Li, H.; Zhang, Y.; Yao, S. Large Scale Preparation of Graphene Quantum Dots from Graphite Oxide in Pure Water via One-Step Electrochemical Tailoring. RSC Adv. 2015, 5, 29704–29707. [Google Scholar] [CrossRef]
- Ahirwar, S.; Mallick, S.; Bahadur, D. Electrochemical Method to Prepare Graphene Quantum Dots and Graphene Oxide Quantum Dots. ACS Omega 2017, 2, 8343–8353. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Lu, Q.; Mi, N.; Li, H.; Liu, M.; Xu, M.; Tan, L.; Xie, Q.; Zhang, Y.; Yao, S. Electrochemical Synthesis of Carbon Nanodots Directly from Alcohols. Chem. A Eur. J. 2014, 20, 4993–4999. [Google Scholar] [CrossRef]
- Bao, L.; Zhang, Z.L.; Tian, Z.Q.; Zhang, L.; Liu, C.; Lin, Y.; Qi, B.; Pang, D.W. Electrochemical Tuning of Luminescent Carbon Nanodots: From Preparation to Luminescence Mechanism. Adv. Mater. 2011, 23, 5801–5806. [Google Scholar] [CrossRef]
- Ming, H.; Ma, Z.; Liu, Y.; Pan, K.; Yu, H.; Wang, F.; Kang, Z. Large Scale Electrochemical Synthesis of High Quality Carbon Nanodots and Their Photocatalytic Property. Dalt. Trans. 2012, 41, 9526–9531. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, Y.; Yu, Y.; Yan, Z.; Chen, J. Carbon Quantum Dots: Synthesis, Characterization, and Assessment of Cytocompatibility. J. Mater. Sci. Mater. Med. 2015, 26, 213. [Google Scholar] [CrossRef]
- Roy, P.; Chen, P.C.; Periasamy, A.P.; Chen, Y.N.; Chang, H.T. Photoluminescent Carbon Nanodots: Synthesis, Physicochemical Properties and Analytical Applications. Mater. Today 2015, 18, 447–458. [Google Scholar] [CrossRef]
- Hernández-Tabares, L.; Carrillo-Barroso, E.; Darias-González, J.G.; Desdín-García, L.F.; Castillo-Torres, R.J.; Arteche-Díaz, J.; Ramos-Aruca, M. Arc Current Control for a Carbon Nanoparticle Synthesis Station. Rev. Cuba. Fis. 2011, 28, 1–76. [Google Scholar]
- Chao-Mujica, F.J.; Garcia-Hernández, L.; Camacho-López, S.; Camacho-López, M.; Camacho-López, M.A.; Reyes Contreras, D.; Pérez-Rodríguez, A.; Peña-Caravaca, J.P.; Páez-Rodríguez, A.; Darias-Gonzalez, J.G.; et al. Carbon Quantum Dots by Submerged Arc Discharge in Water: Synthesis, Characterization, and Mechanism of Formation. J. Appl. Phys. 2021, 129, 163301. [Google Scholar] [CrossRef]
- Hernández-Tabares, L.; Darias-González, J.G.; Arteche-Díaz, J.; Carrillo-Barroso, E.; Ledo-Pereda, L.M.; Desdín-García, L.F. Automated System for the Synthesis of Nanostructures via Arc-Discharge in Liquids. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 035002. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Chen, M.; Chen, W.; Zhou, X. Microwave-Assisted Synthesis of Xylan-Derived Carbon Quantum Dots for Tetracycline Sensing. Opt. Mater. 2018, 85, 329–336. [Google Scholar] [CrossRef]
- Yang, Z.C.; Wang, M.; Yong, A.M.; Wong, S.Y.; Zhang, X.H.; Tan, H.; Chang, A.Y.; Li, X.; Wang, J. Intrinsically Fluorescent Carbon Dots with Tunable Emission Derived from Hydrothermal Treatment of Glucose in the Presence of Monopotassium Phosphate. Chem. Commun. 2011, 47, 11615–11617. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, L.; Li, B.; Song, Y.; Zhao, X.; Zhang, G.; Zhang, S.; Lu, S.; Zhang, J.; Wang, H.; et al. Investigation of Photoluminescence Mechanism of Graphene Quantum Dots and Evaluation of Their Assembly into Polymer Dots. Carbon N.Y. 2014, 77, 462–472. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, W. Hydrothermal Synthesis of Highly Fluorescent Nitrogen-Doped Carbon Quantum Dots with Good Biocompatibility and the Application for Sensing Ellagic Acid. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 240, 118580. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, P.; Liu, C.; Bai, T.; Li, W.; Dai, L.; Liu, W. Highly Luminescent Carbon Nanodots by Microwave-Assisted Pyrolysis. Chem. Commun. 2012, 48, 7955–7957. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Li, Y.; Wang, Z.; Yang, F.; Yang, X. Microwave Synthesis of Fluorescent Carbon Nanoparticles with Electrochemiluminescence Properties. Chem. Commun. 2009, 34, 5118–5120. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; El-Tohamy, M.F.; Oraby, H.F. CA 19-9 Pancreatic Tumor Marker Fluorescence Immunosensing Detection via Immobilized Carbon Quantum Dots Conjugated Gold Nanocomposite. Int. J. Mol. Sci. 2018, 19, 1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Li, Y.; Cheng, L.; Cao, Z.; Liu, W. Water-Soluble and Phosphorus-Containing Carbon Dots with Strong Green Fluorescence for Cell Labeling. J. Mater. Chem. B 2014, 2, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Gu, W.; Ye, L.; Guo, C.; Su, S.; Xu, P.; Xue, M. Microwave-Assisted Polyol Synthesis of Carbon Nitride Dots from Folic Acid for Cell Imaging. Int. J. Nanomed. 2014, 9, 5071–5078. [Google Scholar] [CrossRef] [Green Version]
- Gong, N.; Wang, H.; Li, S.; Deng, Y.; Chen, X.; Ye, L.; Gu, W. Microwave-Assisted Polyol Synthesis of Gadolinium-Doped Green Luminescent Carbon Dots as a Bimodal Nanoprobe. Langmuir 2014, 30, 10933–10939. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, D.; Han, S.; Hu, P.; Liu, R. Bottom-up fabrication of photoluminescent carbon dots with uniform morphology via a soft–hard template approach. Chem. Commun. 2013, 49, 4920–4922. [Google Scholar] [CrossRef]
- Jaiswal, A.; Sankar Ghosh, S.; Chattopadhyay, A. One Step Synthesis of C-Dots by Microwave Mediated Caramelization of Poly(Ethylene Glycol). Chem. Commun. 2012, 48, 407–409. [Google Scholar] [CrossRef]
- Eskalen, H.; Uruş, S.; Cömertpay, S.; Kurt, A.H.; Özgan, Ş. Microwave-Assisted Ultra-Fast Synthesis of Carbon Quantum Dots from Linter: Fluorescence Cancer Imaging and Human Cell Growth Inhibition Properties. Ind. Crops Prod. 2020, 147, 112209. [Google Scholar] [CrossRef]
- Jeong, G.; Lee, J.M.; Lee, J.A.; Praneerad, J.; Choi, C.A.; Supchocksoonthorn, P.; Roy, A.K.; Chae, W.S.; Paoprasert, P.; Yeo, M.K.; et al. Microwave-Assisted Synthesis of Multifunctional Fluorescent Carbon Quantum Dots from A4/B2 Polyamidation Monomer Sets. Appl. Surf. Sci. 2021, 542, 148471. [Google Scholar] [CrossRef]
- Yu, T.; Wang, H.; Guo, C.; Zhai, Y.; Yang, J.; Yuan, J. A Rapid Microwave Synthesis of Green-Emissive Carbon Dots with Solid-State Fluorescence and PH-Sensitive Properties. R. Soc. Open Sci. 2018, 5, 180245. [Google Scholar] [CrossRef] [Green Version]
- Dager, A.; Baliyan, A.; Kurosu, S.; Maekawa, T.; Tachibana, M. Ultrafast Synthesis of Carbon Quantum Dots from Fenugreek Seeds Using Microwave Plasma Enhanced Decomposition: Application of C-QDs to Grow Fluorescent Protein Crystals. Sci. Rep. 2020, 10, 12333. [Google Scholar] [CrossRef]
- Rabenau, A. The Role of Hydrothermal Synthesis in Preparative Chemistry. Angew. Chem. Int. Ed. Engl. 1985, 24, 1026–1040. [Google Scholar] [CrossRef]
- Li, Y.B.; Bando, Y.; Golberg, D.; Liu, Z.W. Ga-Filled Single-Crystalline MgO Nanotube: Wide-Temperature Range Nanothermometer. Appl. Phys. Lett. 2003, 83, 999–1001. [Google Scholar] [CrossRef]
- Yu, S.H. Hydrothermal/Solvothermal Processing of Advanced Ceramic Materials. J. Ceram. Soc. Jpn. 2001, 109, S65–S75. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.Y.; Jia, P.Y.; Chen, D.S.; Wang, L.N. Hydrothermal Synthesis of N-Doped Carbon Quantum Dots and Their Application in Ion-Detection and Cell-Imaging. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2021, 248, 119282. [Google Scholar] [CrossRef] [PubMed]
- Marouzi, S.; Darroudi, M.; Hekmat, A.; Sadri, K.; Kazemi Oskuee, R. One-Pot Hydrothermal Synthesis of Carbon Quantum Dots from Salvia Hispanica L. Seeds and Investigation of Their Biodistribution, and Cytotoxicity Effects. J. Environ. Chem. Eng. 2021, 9, 105461. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, J.; Wang, Y.; Wang, J.; Yang, Y.; Liu, X.; Chen, Y. One-Step Hydrothermal Synthesis of Fluorescence Carbon Quantum Dots with High Product Yield and Quantum Yield. Nanotechnology 2019, 30, 085406. [Google Scholar] [CrossRef] [Green Version]
- Holá, K.; Sudolská, M.; Kalytchuk, S.; Nachtigallová, D.; Rogach, A.L.; Otyepka, M.; Zbořil, R. Graphitic Nitrogen Triggers Red Fluorescence in Carbon Dots. ACS Nano 2017, 11, 12402–12410. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Q.; Chen, D.; Liu, Z.; Zheng, X.; Xu, A.; Yang, S.; Ding, G. Facile and Highly Effective Synthesis of Controllable Lattice Sulfur-Doped Graphene Quantum Dots via Hydrothermal Treatment of Durian. ACS Appl. Mater. Interfaces 2018, 10, 5750–5759. [Google Scholar] [CrossRef]
- Borna, S.; Sabzi, R.E.; Pirsa, S. Synthesis of Carbon Quantum Dots from Apple Juice and Graphite: Investigation of Fluorescence and Structural Properties and Use as an Electrochemical Sensor for Measuring Letrozole. J. Mater. Sci. Mater. Electron. 2021, 32, 10866–10879. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front. Chem. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Li, S.; Zhou, S.; Li, Y.; Li, X.; Zhu, J.; Fan, L.; Yang, S. Exceptionally High Payload of the IR780 Iodide on Folic Acid-Functionalized Graphene Quantum Dots for Targeted Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 22332–22341. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Arora, R.; Dhiman, H.; Pahwa, R. Carbon Quantum Dots: Synthesis, Characterization and Biomedical Applications. Turk. J. Pharm. Sci. 2018, 15, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Stepanidenko, E.A.; Skurlov, I.D.; Khavlyuk, P.D.; Onishchuk, D.A.; Koroleva, A.V.; Zhizhin, E.V.; Arefina, I.A.; Kurdyukov, D.A.; Eurov, D.A.; Golubev, V.G.; et al. Carbon Dots with an Emission in the Near Infrared Produced from Organic Dyes in Porous Silica Microsphere Templates. Nanomaterials 2022, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Zhu, Y.; Yang, X.; Shen, J.; Li, C. Synthesis of Photoluminescent Carbogenic Dots Using Mesoporous Silica Spheres as Nanoreactors. Chem. Commun. 2011, 47, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Lee, G.; Do, S.; Joo, T.; Rhee, S.W. Size-Controlled Soft-Template Synthesis of Carbon Nanodots toward Versatile Photoactive Materials. Small 2014, 10, 506–513. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, C.-Y.; Liu, Y. A Novel One-Step Approach to Synthesize Fluorescent Carbon Nanoparticles. EurJIC 2010, 28, 4411–4414. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Du, P.; Zhou, Y.; Zhang, L.; Li, D.; Tan, H.; Zhao, Z.; Xie, Z.; Sun, Z. Highly luminescent S, N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts. Nanoscale 2013, 5, 12272–12277. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon Quantum Dots: Synthesis, Properties and Applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.C.; Shown, I.; Wei, H.Y.; Chang, Y.C.; Du, H.Y.; Lin, Y.G.; Tseng, C.A.; Wang, C.H.; Chen, L.C.; Lin, Y.C.; et al. Graphene Oxide as a Promising Photocatalyst for CO2 to Methanol Conversion. Nanoscale 2013, 5, 262–268. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, S.; Wang, G.; Mo, R.; He, P.; Sun, J.; Di, Z.; Yuan, N.; Ding, J.; Ding, G.; et al. Negative Induction Effect of Graphite N on Graphene Quantum Dots: Tunable Band Gap Photoluminescence. J. Mater. Chem. C 2015, 3, 8810–8816. [Google Scholar] [CrossRef]
- He, S.; Turnbull, M.J.; Nie, Y.; Sun, X.; Ding, Z. Band Structures of Blue Luminescent Nitrogen-Doped Graphene Quantum Dots by Synchrotron-Based XPS. Surf. Sci. 2018, 676, 51–55. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The Photoluminescence Mechanism in Carbon Dots (Graphene Quantum Dots, Carbon Nanodots, and Polymer Dots): Current State and Future Perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.B.; Wei, J.S.; Xiong, H.M. Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef]
- Wang, X.; Qu, K.; Xu, B.; Ren, J.; Qu, X. Multicolor Luminescent Carbon Nanoparticles: Synthesis, Supramolecular Assembly with Porphyrin, Intrinsic Peroxidase-like Catalytic Activity and Applications. Nano Res. 2011, 4, 908–920. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, X.; Ruan, H.; Yin, K.; Li, H. Production of Yellow-Emitting Carbon Quantum Dots from Fullerene Carbon Soot. Sci. China Mater. 2017, 60, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Dong, L.; Xiong, R.; Hu, A. Practical Access to Bandgap-like N-Doped Carbon Dots with Dual Emission Unzipped from PAN@PMMA Core-Shell Nanoparticles. J. Mater. Chem. C 2013, 1, 7731–7735. [Google Scholar] [CrossRef]
- Dsouza, S.D.; Buerkle, M.; Brunet, P.; Maddi, C.; Padmanaban, D.B.; Morelli, A.; Payam, A.F.; Maguire, P.; Mariotti, D.; Svrcek, V. The Importance of Surface States in N-Doped Carbon Quantum Dots. Carbon N.Y. 2021, 183, 1–11. [Google Scholar] [CrossRef]
- Dimos, K. Tuning Carbon Dots’ Optoelectronic Properties with Polymers. Polymers 2018, 10, 1312. [Google Scholar] [CrossRef] [Green Version]
- Sadrolhosseini, A.R.; Krishnan, G.; Safie, S.; Beygisangchin, M.; Rashid, S.A.; Harun, S.W. Enhancement of the Fluorescence Property of Carbon Quantum Dots Based on Laser Ablated Gold Nanoparticles to Evaluate Pyrene: Publisher’s Note. Opt. Mater. Express 2020, 10, 2705. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Panneerselvam, P.; Marieeswaran, M. A Green Synthetic Route for the Surface-Passivation of Carbon Dots as an Effective Multifunctional Fluorescent Sensor for the Recognition and Detection of Toxic Metal Ions from Aqueous Solution. Anal. Methods 2019, 11, 490–506. [Google Scholar] [CrossRef]
- Li, L.; Dong, T. Photoluminescence Tuning in Carbon Dots: Surface Passivation or/and Functionalization, Heteroatom Doping. J. Mater. Chem. C 2018, 6, 7944–7970. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly Green-Photoluminescent Graphene Quantum Dots for Bioimaging Applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal Route for Cutting Graphene Sheets into Blue-Luminescent Graphene Quantum Dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef]
- Xu, T.; Wan, Z.; Tang, H.; Zhao, C.; Lv, S.; Chen, Y.; Chen, L.; Qiao, Q.; Huang, W. Carbon Quantum Dot Additive Engineering for Efficient and Stable Carbon-Based Perovskite Solar Cells. J. Alloy. Compd. 2021, 859, 157784. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene Oxide as a Chemically Tunable Platform for Optical Applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Cote, L.J.; Cruz-Silva, R.; Huang, J. Flash Reduction and Patterning of Graphite Oxide and Its Polymer Composite. J. Am. Chem. Soc. 2009, 131, 11027–11032. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.T.; Li, S.S.; Lai, W.J.; Yeh, Y.C.; Chen, H.A.; Chen, I.S.; Chen, L.C.; Chen, K.H.; Nemoto, T.; Isoda, S.; et al. Tunable Photoluminescence from Graphene Oxide. Angew. Chem. Int. Ed. 2012, 51, 6662–6666. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.H.; Kim, D.H.; Jun, G.H.; Hong, S.H.; Jeon, S. Tuning the Photoluminescence of Graphene Quantum Dots through the Charge Transfer Effect of Functional Groups. ACS Nano 2013, 7, 1239–1245. [Google Scholar] [CrossRef]
- Qian, Z.; Ma, J.; Shan, X.; Shao, L.; Zhou, J.; Chen, J.; Feng, H. Surface Functionalization of Graphene Quantum Dots with Small Organic Molecules from Photoluminescence Modulation to Bioimaging Applications: An Experimental and Theoretical Investigation. RSC Adv. 2013, 3, 14571–14579. [Google Scholar] [CrossRef]
- Du, Y.; Guo, S. Chemically Doped Fluorescent Carbon and Graphene Quantum Dots for Bioimaging, Sensor, Catalytic and Photoelectronic Applications. Nanoscale 2016, 8, 2532–2543. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Xiong, S.; Wu, X.; Xu, T.; Zhu, X.; Gan, X.; Guo, J.; Shen, J.; Sun, L.; Chu, P.K. Mechanism of Photoluminescence from Chemically Derived Graphene Oxide: Role of Chemical Reduction. Adv. Opt. Mater. 2013, 1, 926–932. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, M.; Huang, H.; Liu, Y.; Kang, Z. Advances, Challenges and Promises of Carbon Dots. Inorg. Chem. Front. 2017, 4, 1963–1986. [Google Scholar] [CrossRef]
- Lai, S.; Jin, Y.; Shi, L.; Zhou, R.; Zhou, Y.; An, D. Mechanisms behind Excitation- and Concentration-Dependent Multicolor Photoluminescence in Graphene Quantum Dots. Nanoscale 2020, 12, 591–601. [Google Scholar] [CrossRef]
- Sabet, M.; Mahdavi, K. Green Synthesis of High Photoluminescence Nitrogen-Doped Carbon Quantum Dots from Grass via a Simple Hydrothermal Method for Removing Organic and Inorganic Water Pollutions. Appl. Surf. Sci. 2019, 463, 283–291. [Google Scholar] [CrossRef]
- Yuan, F.; Li, S.; Fan, Z.; Meng, X.; Fan, L.; Yang, S. Shining Carbon Dots: Synthesis and Biomedical and Optoelectronic Applications. Nano Today 2016, 11, 565–586. [Google Scholar] [CrossRef]
- Malinauskas, T.; Getautis, V.; Tomkeviciene, A.; Simokaitiene, J. E Ffi Cient “Warm-White” OLEDs Based on the Phosphorescent Bis- Cyclometalated Iridium(III) Complex. J. Phys. Chem. C 2014, 118, 11271–11278. [Google Scholar]
- Yuan, F.; Yuan, T.; Sui, L.; Wang, Z.; Xi, Z.; Li, Y.; Li, X.; Fan, L.; Tan, Z.; Chen, A.; et al. Engineering Triangular Carbon Quantum Dots with Unprecedented Narrow Bandwidth Emission for Multicolored LEDs. Nat. Commun. 2018, 9, 2249. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Meng, T.; Yuan, T.; Ni, R.; Li, Y.; Li, X.; Zhang, Y.; Tan, Z.; Lei, S.; et al. Red Phosphorescent Carbon Quantum Dot Organic Framework-Based Electroluminescent Light-Emitting Diodes Exceeding 5% External Quantum Efficiency. J. Am. Chem. Soc. 2021, 143, 18941–18951. [Google Scholar] [CrossRef]
- Wang, B.; Song, H.; Tang, Z.; Yang, B.; Lu, S. Ethanol-Derived White Emissive Carbon Dots: The Formation Process Investigation and Multi-Color/White LEDs Preparation. Nano Res. 2022, 15, 942–949. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, F.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. 53% Efficient Red Emissive Carbon Quantum Dots for High Color Rendering and Stable Warm White-Light-Emitting Diodes. Adv. Mater. 2017, 29, 1702910. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.; Yin, L.; Liu, Y.; Guo, H.; Lai, J.; Han, Y.; Li, G.; Li, M.; Zhang, J.; et al. Full-Color Fluorescent Carbon Quantum Dots. Sci. Adv. 2020, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Lu, D.; Wang, G.G.; Huangfu, J.; Wu, Q.B.; Wang, X.F.; Liu, L.F.; Ye, D.M.; Yan, B.; Han, J. Highly Efficient Orange Emissive Graphene Quantum Dots Prepared by Acid-Free Method for White LEDs. ACS Sustain. Chem. Eng. 2020, 8, 6657–6666. [Google Scholar] [CrossRef]
- Rao, L.; Zhang, Q.; Wen, M.; Mao, Z.; Wei, H.; Chang, H.J.; Niu, X. Solvent Regulation Synthesis of Single-Component White Emission Carbon Quantum Dots for White Light-Emitting Diodes. Nanotechnol. Rev. 2021, 10, 465–477. [Google Scholar] [CrossRef]
- Yin, L.; Zhou, J.; Li, W.; Zhang, J.; Wang, L. Yellow Fluorescent Graphene Quantum Dots as a Phosphor for White Tunable Light-Emitting Diodes. RSC Adv. 2019, 9, 9301–9307. [Google Scholar] [CrossRef] [Green Version]
- Lagonegro, P.; Giovanella, U.; Pasini, M. Carbon Dots as a Sustainable New Platform for Organic Light Emitting Diode. Coatings 2021, 11, 5. [Google Scholar] [CrossRef]
- Shi, L.; Yang, J.H.; Zeng, H.B.; Chen, Y.M.; Yang, S.C.; Wu, C.; Zeng, H.; Yoshihito, O.; Zhang, Q. Carbon Dots with High Fluorescence Quantum Yield: The Fluorescence Originates from Organic Fluorophores. Nanoscale 2016, 8, 14374–14378. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Yang, B. Bioimaging Based on Fluorescent Carbon Dots. RSC Adv. 2014, 4, 27184–27200. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Y.H.; Liu, C.Y.; Ma, D.G. White Light-Emitting Devices Based on Carbon Dots’ Electroluminescence. Chem. Commun. 2011, 47, 3502–3504. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wang, Y.; Kalytchuk, S.; Kershaw, S.V.; Wang, Y.; Wang, P.; Zhang, T.; Zhao, Y.; Zhang, H.; et al. Color-Switchable Electroluminescence of Carbon Dot Light-Emitting Diodes. ACS Nano 2013, 7, 11234–11241. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Li, Y.; Deng, Z.; Lu, T.; Ma, Z.; Zuo, P.; Dai, L.; Wang, L.; Jia, H.; et al. Realization of High-Luminous-Efficiency InGaN Light-Emitting Diodes in the “Green Gap” Range. Sci. Rep. 2015, 5, 10883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawabe, Y.; Abe, J. Electron Mobility Measurement Using Exciplex-Type Organic Light-Emitting Diodes. Appl. Phys. Lett. 2002, 81, 493–495. [Google Scholar] [CrossRef]
- Minh, H.L.; O’Brien, D.; Faulkner, G.; Zeng, L.; Lee, K.; Jung, D.; Oh, Y.; Won, E.T. 100-Mb/s NRZ Visible Light Communications Using a Postequalized White LED. IEEE Photonics Technol. Lett. 2009, 21, 1063–1065. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, B.; Tanyi, E.K.; Cheng, L.J. Red-Emitting Carbon Dots for MicroLED Application. Opt. InfoBase Conf. Pap. 2020, F181, 1–2. [Google Scholar] [CrossRef]
- Dai, W.; Lei, Y.; Xu, M.; Zhao, P.; Zhang, Z.; Zhou, J. Rare-Earth Free Self-Activated Graphene Quantum Dots and Copper-Cysteamine Phosphors for Enhanced White Light-Emitting-Diodes under Single Excitation. Sci. Rep. 2017, 7, 12872. [Google Scholar] [CrossRef] [Green Version]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. And Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lu, X.; Liu, B.; Chen, D.; Tong, Y.; Shen, G. Flexible Energy-Storage Devices: Design Consideration and Recent Progress. Adv. Mater. 2014, 26, 4763–4782. [Google Scholar] [CrossRef]
- Jing, M.; Wang, C.; Hou, H.; Wu, Z.; Zhu, Y.; Yang, Y.; Jia, X.; Zhang, Y.; Ji, X. Ultrafine Nickel Oxide Quantum Dots Enbedded with Few-Layer Exfoliative Graphene for an Asymmetric Supercapacitor: Enhanced Capacitances by Alternating Voltage. J. Power Sources 2015, 298, 241–248. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, S.; Wan, J.; Tang, H.; Chang, L.; He, L.; Zhao, H.; Gao, Y.; Tang, Z. Three-Dimensional Graphene/Metal Oxide Nanoparticle Hybrids for High-Performance Capacitive Deionization of Saline Water. Adv. Mater. 2013, 25, 6270–6276. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.A.; An, N.; Yang, Y.Y.; Li, Z.M.; Wu, H.Y. Facile Synthesis of MnO2/CNTs Composite for Supercapacitor Electrodes with Long Cycle Stability. J. Phys. Chem. C 2014, 118, 22865–22872. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, B.; Wang, J.; Tian, J.; Sun, Y.; Zhang, X.; Yang, H. All-Solid-State Asymmetric Supercapacitors Based on ZnO Quantum Dots/Carbon/CNT and Porous N-Doped Carbon/CNT Electrodes Derived from a Single ZIF-8/CNT Template. J. Mater. Chem. A 2016, 4, 10282–10293. [Google Scholar] [CrossRef]
- Nano, A.; Xu, X.; Li, S.; Zhang, H.; Shen, Y.; Zakeeruddin, S.M.; Grätzel, M.; Cheng, Y.-B.; Wang, M. Subscriber Access Provided by SELCUK UNIV A Power Pack Based on Organometallic Perovskite Solar Cell and Supercapacitor. ACS Nano 2015, 9, 1782–1787. [Google Scholar]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A Review on Recent Advances in Hybrid Supercapacitors: Design, Fabrication and Applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, B.; Feng, L.; Zheng, J.; You, B.; Chen, J.; Zhao, X.; Zhang, C.; Jiang, S.; He, S. Progress in the use of organic potassium salts for the synthesis of porous carbon nanomaterials: Microstructure engineering for advanced supercapacitors. Nanoscale 2022, 14, 8216–8244. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Wei, L.; Deng, W.; Li, S.; Wu, Z.; Cai, J.; Luo, J. Sandwich-like chitosan porous carbon Spheres/MXene composite with high specific capacitance and rate performance for supercapacitors. J. Bioresour. Bioprod. 2022, 7, 63–72. [Google Scholar] [CrossRef]
- Chen, Z.; Wen, J.; Yan, C.; Rice, L.; Sohn, H.; Shen, M.; Cai, M.; Dunn, B.; Lu, Y. High-Performance Supercapacitors Based on Hierarchically Porous Graphite Particles. Adv. Energy Mater. 2011, 1, 551–556. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathi, S.K. Review of Supercapacitors: Materials and Devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Becker, H.I. US2800616 Low Voltage Electrolytic Capacitor. U.S. Patent 2,800,616, 14 April 1954. [Google Scholar]
- Du, Y.; Xiao, P.; Yuan, J.; Chen, J. Research Progress of Graphene-Based Materials on Flexible Supercapacitors. Coatings 2020, 10, 892. [Google Scholar] [CrossRef]
- Strauss, V.; Marsh, K.; Kowal, M.D.; El-Kady, M.; Kaner, R.B. A Simple Route to Porous Graphene from Carbon Nanodots for Supercapacitor Applications. Adv. Mater. 2018, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Song, J.; Yang, H.; Gan, S.; Zhang, Q.; Han, D.; Ivaska, A.; Niu, L. One-Step Synthesis of Graphene/SnO2 Nanocomposites and Its Application in Electrochemical Supercapacitors. Nanotechnology 2009, 20, 455602. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.I.; Sunarso, J.; Wong, B.T.; Lin, H.; Yu, A.; Jia, B. Towards Enhanced Energy Density of Graphene-Based Supercapacitors: Current Status, Approaches, and Future Directions. J. Power Sources 2018, 396, 182–206. [Google Scholar] [CrossRef]
- Davies, A.; Audette, P.; Farrow, B.; Hassan, F.; Chen, Z.; Choi, J.Y.; Yu, A. Graphene-Based Flexible Supercapacitors: Pulse-Electropolymerization of Polypyrrole on Free-Standing Graphene Films. J. Phys. Chem. C 2011, 115, 17612–17620. [Google Scholar] [CrossRef]
- Li, Z.; Wei, J.; Ren, J.; Wu, X.; Wang, L.; Pan, D.; Wu, M. Hierarchical Construction of High-Performance All-Carbon Flexible Fiber Supercapacitors with Graphene Hydrogel and Nitrogen-Doped Graphene Quantum Dots. Carbon N.Y. 2019, 154, 410–419. [Google Scholar] [CrossRef]
- Luo, P.; Guan, X.; Yu, Y.; Li, X.; Yan, F. Hydrothermal Synthesis of Graphene Quantum Dots Supported on Three-Dimensional Graphene for Supercapacitors. Nanomaterials 2019, 9, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, B.; Lang, J.; Guo, R.; Zhang, X.; Yan, X. Engineering the Electrochemical Capacitive Properties of Microsupercapacitors Based on Graphene Quantum Dots/MnO2 Using Ionic Liquid Gel Electrolytes. ACS Appl. Mater. Interfaces 2015, 7, 25378–25389. [Google Scholar] [CrossRef]
- Liu, W.W.; Feng, Y.Q.; Yan, X.B.; Chen, J.T.; Xue, Q.J. Superior Micro-Supercapacitors Based on Graphene Quantum Dots. Adv. Funct. Mater. 2013, 23, 4111–4122. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Wang, L.; Bu, F.; Wei, J.; Pan, D.; Wu, M. Hierarchical 3D All-Carbon Composite Structure Modified with N-Doped Graphene Quantum Dots for High-Performance Flexible Supercapacitors. Small 2018, 14, 1801498. [Google Scholar] [CrossRef]
- Qing, Y.; Jiang, Y.; Lin, H.; Wang, L.; Liu, A.; Cao, Y.; Sheng, R.; Guo, Y.; Fan, C.; Zhang, S.; et al. Boosting the Supercapacitor Performance of Activated Carbon by Constructing Overall Conductive Networks Using Graphene Quantum Dots. J. Mater. Chem. A 2019, 7, 6021–6027. [Google Scholar] [CrossRef]
- Yin, X.; Zhi, C.; Sun, W.; Lv, L.P.; Wang, Y. Multilayer NiO@Co3O4@graphene Quantum Dots Hollow Spheres for High-Performance Lithium-Ion Batteries and Supercapacitors. J. Mater. Chem. A 2019, 7, 7800–7814. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, J.; Qing, Y.; Fan, C.; Wang, L.; Huang, Y.; Sheng, R.; Guo, Y.; Wang, T.; Pan, Y.; et al. Construction of Hierarchical Porous Carbon Nanosheets from Template-Assisted Assembly of Coal-Based Graphene Quantum Dots for High Performance Supercapacitor Electrodes. Mater. Today Energy 2017, 6, 36–45. [Google Scholar] [CrossRef]
- Islam, M.S.; Deng, Y.; Tong, L.; Roy, A.K.; Faisal, S.N.; Hassan, M.; Minett, A.I.; Gomes, V.G. In-Situ Direct Grafting of Graphene Quantum Dots onto Carbon Fibre by Low Temperature Chemical Synthesis for High Performance Flexible Fabric Supercapacitor. Mater. Today Commun. 2017, 10, 112–119. [Google Scholar] [CrossRef]
- Tian, W.; Zhu, J.; Dong, Y.; Zhao, J.; Li, J.; Guo, N.; Lin, H.; Zhang, S.; Jia, D. Micelle-Induced Assembly of Graphene Quantum Dots into Conductive Porous Carbon for High Rate Supercapacitor Electrodes at High Mass Loadings. Carbon N.Y. 2020, 161, 89–96. [Google Scholar] [CrossRef]
- Li, Z.; Bu, F.; Wei, J.; Yao, W.; Wang, L.; Chen, Z.; Pan, D.; Wu, M. Boosting the Energy Storage Densities of Supercapacitors by Incorporating N-Doped Graphene Quantum Dots into Cubic Porous Carbon. Nanoscale 2018, 10, 22871–22883. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Song, H.; Chen, X.; Zhou, J.; Hong, S.; Huang, M. Graphene Quantum Dots as the Electrolyte for Solid State Supercapacitors. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Kamruzzaman, M.; Ali, M.O.; Emon, M.S.A.; Khatun, H.; Ali, M. Recent advances in lithium-ion battery materials for improved electrochemical performance: A review. Results Eng. 2022, 15, 100472. [Google Scholar] [CrossRef]
- Liu, C.; Sun, J.; Zheng, P.; Jiang, L.; Liu, H.; Chai, J.; Liu, Q.; Liu, Z.; Zheng, Y.; Rui, X. Recent advances of non-lithium metal anode materials for solid-state lithium-ion batteries. J. Mater. Chem. A. 2022, 10, 16761–16778. [Google Scholar] [CrossRef]
- Ruiyi, L.; Yuanyuan, J.; Xiaoyan, Z.; Zaijun, L.; Zhiguo, G.; Guangli, W.; Junkang, L. Significantly Enhanced Electrochemical Performance of Lithium Titanate Anode for Lithium Ion Battery by the Hybrid of Nitrogen and Sulfur Co-Doped Graphene Quantum Dots. Electrochim. Acta 2015, 178, 303–311. [Google Scholar] [CrossRef]
- Wu, M.; Chen, H.; Lv, L.P.; Wang, Y. Graphene Quantum Dots Modification of Yolk-Shell Co3O4@CuO Microspheres for Boosted Lithium Storage Performance. Chem. Eng. J. 2019, 373, 985–994. [Google Scholar] [CrossRef]
- Yin, X.; Chen, H.; Zhi, C.; Sun, W.; Lv, L.P.; Wang, Y. Functionalized Graphene Quantum Dot Modification of Yolk–Shell NiO Microspheres for Superior Lithium Storage. Small 2018, 14, 1800589. [Google Scholar] [CrossRef]
- Chen, Z.; Hou, J.; Liu, Q.; Zhou, Q.; Liu, H.; Xu, C. Graphene Quantum Dots Modified Nanoporous SiAl Composite as an Advanced Anode for Lithium Storage. Electrochim. Acta 2019, 318, 228–235. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, C.; Fan, L.; Zhang, N.; Sun, K. Iron Fluoride Vertical Nanosheets Array Modified with Graphene Quantum Dots as Long-Life Cathode for Lithium Ion Batteries. Chem. Eng. J. 2019, 371, 245–251. [Google Scholar] [CrossRef]
- Vijaya Kumar Saroja, A.P.; Garapati, M.S.; ShyiamalaDevi, R.; Kamaraj, M.; Ramaprabhu, S. Facile Synthesis of Heteroatom Doped and Undoped Graphene Quantum Dots as Active Materials for Reversible Lithium and Sodium Ions Storage. Appl. Surf. Sci. 2020, 504, 144430. [Google Scholar] [CrossRef]
- Chao, D.; Zhu, C.; Xia, X.; Liu, J.; Zhang, X.; Wang, J.; Liang, P.; Lin, J.; Zhang, H.; Shen, Z.X.; et al. Graphene Quantum Dots Coated VO2 Arrays for Highly Durable Electrodes for Li and Na Ion Batteries. Nano Lett. 2015, 15, 565–573. [Google Scholar] [CrossRef]
- Noori, A.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Towards Establishing Standard Performance Metrics for Batteries, Supercapacitors and Beyond. Chem. Soc. Rev. 2019, 48, 1272–1341. [Google Scholar] [CrossRef]
- Thangaraj, B.; Chuangchote, S.; Wongyao, N.; Solomon, P.R.; Roongraung, K.; Chaiworn, W.; Surareungchai, W. Flexible Sodium-Ion Batteries Using Electrodes from Samanea Saman Tree Lea—Derived Carbon Quantum Dots Decorated with SnO2 and NaVO3. Clean Energy 2021, 5, 354–374. [Google Scholar] [CrossRef]
- Liu, S.; Cao, X.; Zhang, Y.; Wang, K.; Su, Q.; Chen, J.; He, Q.; Liang, S.; Cao, G.; Pan, A. Carbon Quantum Dot Modified Na3V2(PO4)2F3as a High-Performance Cathode Material for Sodium-Ion Batteries. J. Mater. Chem. A 2020, 8, 18872–18879. [Google Scholar] [CrossRef]
- Song, T.B.; Huang, Z.H.; Niu, X.Q.; Liu, J.; Wei, J.S.; Chen, X.B.; Xiong, H.M. Applications of Carbon Dots in Next-Generation Lithium-Ion Batteries. ChemNanoMat 2020, 6, 1421–1436. [Google Scholar] [CrossRef]
- Hong, W.; Zhang, Y.; Yang, L.; Tian, Y.; Ge, P.; Hu, J.; Wei, W.; Zou, G.; Hou, H.; Ji, X. Carbon Quantum Dot Micelles Tailored Hollow Carbon Anode for Fast Potassium and Sodium Storage. Nano Energy 2019, 65, 104038. [Google Scholar] [CrossRef]
- Prasath, A.; Athika, M.; Duraisamy, E.; Selva Sharma, A.; Sankar Devi, V.; Elumalai, P. Carbon Quantum Dot-Anchored Bismuth Oxide Composites as Potential Electrode for Lithium-Ion Battery and Supercapacitor Applications. ACS Omega 2019, 4, 4943–4954. [Google Scholar] [CrossRef]
- Kumar, Y.R.; Deshmukh, K.; Sadasivuni, K.K.; Pasha, S.K.K. Graphene Quantum Dot Based Materials for Sensing, Bio-Imaging and Energy Storage Applications: A Review. RSC Adv. 2020, 10, 23861–23898. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X.; Yuan, C.; Lou, X.W. Recent Advances in Metal Oxide-Based Electrode Architecture Design for Electrochemical Energy Storage. Adv. Mater. 2012, 24, 5166–5180. [Google Scholar] [CrossRef]
- Gu, S.; Christensen, T.; Hsieh, C.T.; Mallick, B.C.; Gandomi, Y.A.; Li, J.; Chang, J.K. Improved Lithium Storage Capacity and High Rate Capability of Nitrogen-Doped Graphite-like Electrode Materials Prepared from Thermal Pyrolysis of Graphene Quantum Dots. Electrochim. Acta 2020, 354, 136642. [Google Scholar] [CrossRef]
- Hou, B.H.; Wang, Y.Y.; Ning, Q.L.; Fan, C.Y.; Xi, X.T.; Yang, X.; Wang, J.; Zhang, J.P.; Wang, X.; Wu, X.L. An FeP@C Nanoarray Vertically Grown on Graphene Nanosheets: An Ultrastable Li-Ion Battery Anode with Pseudocapacitance-Boosted Electrochemical Kinetics. Nanoscale 2019, 11, 1304–1312. [Google Scholar] [CrossRef]
- Balogun, M.S.; Luo, Y.; Lyu, F.; Wang, F.; Yang, H.; Li, H.; Liang, C.; Huang, M.; Huang, Y.; Tong, Y.X. Carbon Quantum Dot Surface-Engineered VO2 Interwoven Nanowires: A Flexible Cathode Material for Lithium and Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 9733–9744. [Google Scholar] [CrossRef] [PubMed]
- Lijuan, K.; Yongqiang, Y.; Ruiyi, L.; Zaijun, L. Phenylalanine-Functionalized Graphene Quantum Dot-Silicon Nanoparticle Composite as an Anode Material for Lithium Ion Batteries with Largely Enhanced Electrochemical Performance. Electrochim. Acta 2016, 198, 144–155. [Google Scholar] [CrossRef]








| Battery and Method | Precursor | Material | Product | Cycle Stability | Specific Capacity (mA hg−1) | Ref |
|---|---|---|---|---|---|---|
| Na-ion(Hydrothermal) | Graphene oxide | GF aided VO2@GQD nanoarray as working electrode | GQD with size 5 nm | 88% 1500 cycles | 306 at 1/3 C | [188] |
| Na-ion(Hydrothermal) | Graphene oxide and melamine | Nitrogen doped GQDs as an anode | N-GQD | 66% after 500 cycles | 215 at 50 | [187] |
| Li-ion(Hydrothermal) | Citric acid andL-cysteine | Lithium Titanate/N,S-GQDs as an anode | N,S GQD with size 4 to 7 nm | 96.9% 2000 cycle | 169 at 0.1 C | [182] |
| Li-ion(Hydrothermal) | Graphene oxide | GF aided VO 2@GQD nanoarray asworking electrode | GQD | 94%1500 cycle | - | [188] |
| Li-ion(Hydrothermal) | Pyrene | NiO/GQDs-COOH electrode | GQDs-COOH | 1081 mA hg−1 250 cycles | 1334 at 100 | [184] |
| Li-ion(Hydrothermal) | Citric acid | GQDs based SiAl(NP-SiAl/GQDs)anode for lithium storage | GQD | 52.6% 120 cycles | 2507 at 200 | [185] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaurav, A.; Jain, A.; Tripathi, S.K. Review on Fluorescent Carbon/Graphene Quantum Dots: Promising Material for Energy Storage and Next-Generation Light-Emitting Diodes. Materials 2022, 15, 7888. https://doi.org/10.3390/ma15227888
Gaurav A, Jain A, Tripathi SK. Review on Fluorescent Carbon/Graphene Quantum Dots: Promising Material for Energy Storage and Next-Generation Light-Emitting Diodes. Materials. 2022; 15(22):7888. https://doi.org/10.3390/ma15227888
Chicago/Turabian StyleGaurav, Ashish, Amrita Jain, and Santosh Kumar Tripathi. 2022. "Review on Fluorescent Carbon/Graphene Quantum Dots: Promising Material for Energy Storage and Next-Generation Light-Emitting Diodes" Materials 15, no. 22: 7888. https://doi.org/10.3390/ma15227888
APA StyleGaurav, A., Jain, A., & Tripathi, S. K. (2022). Review on Fluorescent Carbon/Graphene Quantum Dots: Promising Material for Energy Storage and Next-Generation Light-Emitting Diodes. Materials, 15(22), 7888. https://doi.org/10.3390/ma15227888










