Role of Carbon Phase in the Formation of Foam Glass Porous Structure
Abstract
1. Introduction
- Interaction of batch components;
- Release of gases;
- Formation of pores in a viscous plastic glass mass.
2. Materials and Methods
3. Results and Discussion
3.1. Formation of Carbon Phase
3.2. Influence of Carbon Phase on Glass Foaming
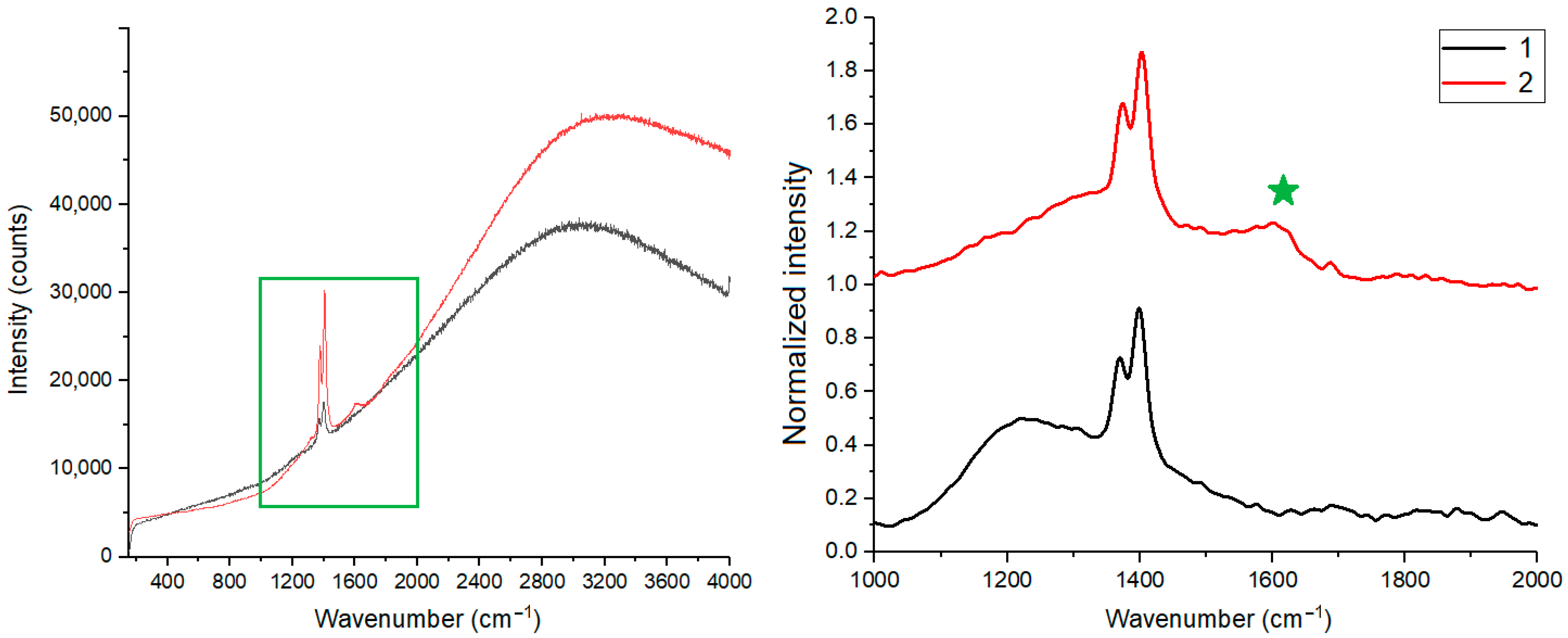
3.3. Structure and Properties of Carbon Phase
- -
- The small size of carbon particles (according to calculations below, their size is no more than 535 nm);
- -
- A presumably amorphous structure of carbon particles due to their size;
- -
- Fusion (immersion) of carbon particles into plastic glass mass during heat treatment.
- -
- Glass particles have a spherical shape;
- -
- All particles are of the same size;
- -
- Liquid foaming film is evenly distributed on the entire surface.
3.4. The Mechanism of the Carbon Phase Action on the Foaming of the Foam Glass Batch
- (a)
- The air enclosed between the glass powder particles;
- (b)
- The gases formed during the thermal decomposition of glycerol.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tuan, B.L.A.; Hwang, C.L.; Lin, K.L.; Chen, Y.Y.; Young, M.P. Development of Lightweight Aggregate from Sewage Sludge and Waste Glass Powder for Concrete. Constr. Build. Mater. 2013, 47, 334–339. [Google Scholar] [CrossRef]
- Huang, C.H.; Wang, S.Y. Application of Water Treatment Sludge in the Manufacturing of Lightweight Aggregate. Constr. Build. Mater. 2013, 43, 174–183. [Google Scholar] [CrossRef]
- Tsai, C.C.; Wang, K.S.; Chiou, I.J. Effect of SiO2-Al2O3-Flux Ratio Change on the Bloating Characteristics of Lightweight Aggregate Material Produced from Recycled Sewage Sludge. J. Hazard. Mater. 2006, 134, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kourti, I.; Cheeseman, C.R. Properties and Microstructure of Lightweight Aggregate Produced from Lignite Coal Fly Ash and Recycled Glass. Resour. Conserv. Recycl. 2010, 54, 769–775. [Google Scholar] [CrossRef]
- Hesky, D.; Aneziris, C.G.; Groß, U.; Horn, A. Water and Waterglass Mixtures for Foam Glass Production. Ceram. Int. 2015, 41, 12604–12613. [Google Scholar] [CrossRef]
- Kazantseva, L.K.; Rashchenko, S.v. Optimization of Porous Heat-Insulating Ceramics Manufacturing from Zeolitic Rocks. Ceram. Int. 2016, 42, 19250–19256. [Google Scholar] [CrossRef]
- Bento, A.C.; Kubaski, E.T.; Sequinel, T.; Pianaro, S.A.; Varela, J.A.; Tebcherani, S.M. Glass Foam of Macroporosity Using Glass Waste and Sodium Hydroxide as the Foaming Agent. Ceram. Int. 2013, 39, 2423–2430. [Google Scholar] [CrossRef]
- Vaisman, Y.I.; Ketov, A.A.; Ketov, Y.A.; Slesarev, M.Y. The Expansion Kinetics of Cellular Glass in the Thermoplastic State under the Hydrated Mechanism of Gas Formation. Glas. Phys. Chem. 2017, 43, 330–334. [Google Scholar] [CrossRef]
- da Silva, R.C.; Kubaski, E.T.; Tenório-Neto, E.T.; Lima-Tenório, M.K.; Tebcherani, S.M. Foam Glass Using Sodium Hydroxide as Foaming Agent: Study on the Reaction Mechanism in Soda-Lime Glass Matrix. J. Non-Cryst. Solids 2019, 511, 177–182. [Google Scholar] [CrossRef]
- Vereshagin, V.I.; Sokolova, S.N. Granulated Foam Glass-Ceramic Material from Zeolitic Rocks. Constr. Build. Mater. 2008, 22, 999–1003. [Google Scholar] [CrossRef]
- Tereshchenko, I.M.; Dormeshkin, O.B.; Kravchuk, A.P.; Zhikh, B.P. Status and Prospects of Development of Production of Glassy Foamed Heat-Insulation Materials. Glass Ceram. 2017, 74, 216–219. [Google Scholar] [CrossRef]
- Davraz, M.; Koru, M.; Akdağ, A.E.; Kılınçarslan, Ş.; Delikanlı, Y.E.; Çabuk, M. An Investigation of Foaming Additives and Usage Rates in the Production of Ultra-Light Foam Glass. J. Therm. Anal. 2022, 147, 3567–3576. [Google Scholar] [CrossRef]
- Goltsman, B.M.; Yatsenko, L.A.; Goltsman, N.S. Production of Foam Glass Materials from Silicate Raw Materials by Hydrate Mechanism. Solid State Phenom. 2020, 299, 293–298. [Google Scholar] [CrossRef]
- Volland, S.; Kazmina, O.; Vereshchagin, V.; Dushkina, M. Recycling of Sand Sludge as a Resource for Lightweight Aggregates. Constr. Build. Mater. 2014, 52, 361–365. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Huang, H.; Meng, X.; Liu, Y.; Tu, S.; Li, B. Novel Glass Ceramic Foams Materials Based on Polishing Porcelain Waste Using the Carbon Ash Waste as Foaming Agent. Constr. Build. Mater. 2016, 125, 1093–1100. [Google Scholar] [CrossRef]
- Wang, H.; Feng, K.; Zhou, Y.; Sun, Q.; Shi, H. Effects of Na2B4O7·5H2O on the Properties of Foam Glass from Waste Glass and Titania-Bearing Blast Furnace Slag. Mater. Lett. 2014, 132, 176–178. [Google Scholar] [CrossRef]
- Hou, L.-J.; Liu, T.-Y.; Lu, A.-X. Red Mud and Fly Ash-Based Ceramic Foams Using Starch and Manganese Dioxide as Foaming Agent. Trans. Nonferrous Met. Soc. China 2017, 27, 591–598. [Google Scholar] [CrossRef]
- Liao, Y.C.; Huang, C.Y. Glass Foam from the Mixture of Reservoir Sediment and Na2CO3. Ceram. Int. 2012, 38, 4415–4420. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Ferreira, D.D.; Andreola, F.; Lancellotti, I.; Barbieri, L.; Ferreira, J.M.F. Environmental Friendly Management of CRT Glass by Foaming with Waste Egg Shells, Calcite or Dolomite. Ceram. Int. 2014, 40, 13371–13379. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Huang, H.; Meng, K.; Hu, K.; Hu, P.; Wang, X.; Zhang, Z.; Meng, X. Novel Glass Ceramic Foams Materials Based on Red Mud. Ceram. Int. 2014, 40, 6677–6683. [Google Scholar] [CrossRef]
- Wei, Y.L.; Cheng, S.H.; Ou, K.T.; Kuo, P.J.; Chung, T.H.; Xie, X.Q. Effect of Calcium Compounds on Lightweight Aggregates Prepared by Firing a Mixture of Coal Fly Ash and Waste Glass. Ceram. Int. 2017, 43, 15573–15579. [Google Scholar] [CrossRef]
- Xi, C.; Zheng, F.; Xu, J.; Yang, W.; Peng, Y.; Li, Y.; Li, P.; Zhen, Q.; Bashir, S.; Liu, J.L. Preparation of Glass-Ceramic Foams Using Extracted Titanium Tailing and Glass Waste as Raw Materials. Constr. Build. Mater. 2018, 190, 896–909. [Google Scholar] [CrossRef]
- Lakov, L.; Toncheva, K.; Staneva, A.; Ilcheva, Z.; Simeonova, T. Composition, synthesis and properties of color architecture building foam glass obtained from waste packing glass. J. Chem. Technol. Metall. 2013, 48, 130–135. [Google Scholar]
- Benhaoua, F.; Ayadi, A.; Stiti, N.; Lamri, Y.; Ratni, H. Elaboration and Characterization of a Cellular Glass Based Cullet Loaded with Carbon Fibers. Mater. Lett. 2015, 160, 278–281. [Google Scholar] [CrossRef]
- Petersen, R.R.; König, J.; Smedskjaer, M.M.; Yue, Y. Effect of Na2CO3 as Foaming Agent on Dynamics and Structure of Foam Glass Melts. J. Non-Cryst. Solids 2014, 400, 1–5. [Google Scholar] [CrossRef]
- Petersen, R.R.; König, J.; Yue, Y. The Viscosity Window of the Silicate Glass Foam Production. J. Non-Cryst. Solids 2017, 456, 49–54. [Google Scholar] [CrossRef]
- Ayadi, A.; Stiti, N.; Boumchedda, K.; Rennai, H.; Lerari, Y. Elaboration and Characterization of Porous Granules Based on Waste Glass. Powder Technol. 2011, 208, 423–426. [Google Scholar] [CrossRef]
- König, J.; Petersen, R.R.; Yue, Y. Influence of the Glass-Calcium Carbonate Mixture’s Characteristics on the Foaming Process and the Properties of the Foam Glass. J. Eur. Ceram. Soc. 2014, 34, 1591–1598. [Google Scholar] [CrossRef]
- Gol’tsman, B.M.; Yatsenko, E.A. Modern Methods for Foaming of Glass and Silicate Raw Materials: Review and Analysis. Theor. Found. Chem. Eng. 2022, 56, 549–558. [Google Scholar] [CrossRef]
- Lee, C.T. Production of Alumino-Borosilicate Foamed Glass Body from Waste LCD Glass. J. Ind. Eng. Chem. 2013, 19, 1916–1925. [Google Scholar] [CrossRef]
- Semukhin, B.S.; Votinov, A.v.; Kazmina, O.v. Properties of Foamglass with Fullerene-Like Mesostructure. Russ. Phys. J. 2020, 63, 710–712. [Google Scholar] [CrossRef]
- Østergaard, M.B.; Petersen, R.R.; König, J.; Yue, Y. Effect of Alkali Phosphate Content on Foaming of CRT Panel Glass Using Mn3O4 and Carbon as Foaming Agents. J. Non-Cryst. Solids 2018, 482, 217–222. [Google Scholar] [CrossRef]
- König, J.; Petersen, R.R.; Yue, Y. Fabrication of Highly Insulating Foam Glass Made from CRT Panel Glass. Ceram. Int. 2015, 41, 9793–9800. [Google Scholar] [CrossRef]
- Hribar, U.; Spreitzer, M.; König, J. Applicability of Water Glass for the Transfer of the Glass-Foaming Process from Controlled to Air Atmosphere. J. Clean. Prod. 2020, 282, 125428. [Google Scholar] [CrossRef]
- Vaisman, I.; Ketov, A.; Ketov, I. Cellular Glass Obtained from Non-Powder Preforms by Foaming with Steam. Ceram. Int. 2016, 42, 15261–15268. [Google Scholar] [CrossRef]
- Yatsenko, E.A.; Gol’tsman, B.M.; Smolii, V.A.; Gol’tsman, N.S.; Yatsenko, L.A. Study on the Possibility of Applying Organic Compounds as Pore-Forming Agents for the Synthesis of Foam Glass. Glass Phys. Chem. 2019, 45, 138–142. [Google Scholar] [CrossRef]
- Vereshchagin, V.I.; Sokolova, S.N. Effect of the technological parameters on the properties of granular porous crystal glass material based on zeolite-bearing rock. Glass Ceram. 2009, 66, 46–49. [Google Scholar] [CrossRef]
- Yatsenko, E.A.; Goltsman, B.M.; Smoliy, V.A.; Kosarev, A.S.; Bezuglov, R.V. Investigation of the Influence of Foaming Agents’ Type and Ratio on the Foaming and Reactionary Abilities of Foamed Slag Glass. Biosci. Biotechnol. Res. Asia 2015, 12, 625–632. [Google Scholar] [CrossRef]
- Sasmal, N.; Garai, M.; Karmakar, B. Preparation and Characterization of Novel Foamed Porous Glass-Ceramics. Mater. Charact. 2015, 103, 90–100. [Google Scholar] [CrossRef]
- Hasheminia, S.; Nemati, A.; Eftekhari Yekta, B.; Alizadeh, P. Preparation and Characterisation of Diopside-Based Glass-Ceramic Foams. Ceram. Int. 2012, 38, 2005–2010. [Google Scholar] [CrossRef]
- Bernardo, E.; Cedro, R.; Florean, M.; Hreglich, S. Reutilization and Stabilization of Wastes by the Production of Glass Foams. Ceram. Int. 2007, 33, 963–968. [Google Scholar] [CrossRef]
- Yot, P.G.; Méar, F.O. Lead Extraction from Waste Funnel Cathode-Ray Tubes Glasses by Reaction with Silicon Carbide and Titanium Nitride. J. Hazard. Mater. 2009, 172, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Fractional Foam Glass Insulation for Railway Construction. RF Patent 2681157, 28 August 2017.
- Way of Foamglass Production. RF Patent 2745544, 9 July 2019.
- Valliyappan, T.; Bakhshi, N.N.; Dalai, A.K. Pyrolysis of glycerol for the production of hydrogen or syn gas. Bioresour. Technol. 2008, 99, 4476–4483. [Google Scholar] [CrossRef]
- Foamglas Industrial Insulation Handbook; Pittsburgh Corning Europe: Waterloo, Belgium, 1992; pp. 73–75.
- Yatsenko, E.A.; Gol’tsman, B.M.; Kosarev, A.S.; Karandashova, N.S.; Smolii, V.A.; Yatsenko, L.A. Synthesis of Foamed Glass Based on Slag and a Glycerol Pore-Forming Mixture. Glass Phys. Chem. 2018, 44, 152–155. [Google Scholar] [CrossRef]
- Karandashova, N.S.; Goltsman, B.M.; Yatsenko, E.A. Analysis of Influence of Foaming Mixture Components on Structure and Properties of Foam Glass. Proc. IOP Conf. Ser. Mater. Sci. Eng. 2017, 262, 012020. [Google Scholar] [CrossRef]
- Yatsenko, E.A.; Goltsman, B.M.; Smolii, V.A.; Kosarev, A.S. Foamed Slag Glass—Eco-Friendly Insulating Material Based on Slag Waste. In Proceedings of the 2015 IEEE 15th International Conference on Environment and Electrical Engineering, EEEIC 2015, Rome, Italy, 10–13 June 2015; pp. 819–823. [Google Scholar] [CrossRef]
- Yatsenko, E.A.; Goltsman, B.M.; Klimova, L.V.; Yatsenko, L.A. Peculiarities of Foam Glass Synthesis from Natural Silica-Containing Raw Materials. J. Anal. Calorim. 2020, 142, 119–127. [Google Scholar] [CrossRef]
- Goltsman, B.M.; Goltsman, N.S. Study of Recycling Processes of Mixed Waste Glass to Obtain Expanded Glass When Using Glycerol Blowing Agent. Ecol. Ind. Russ. 2022, 26, 27–33. [Google Scholar] [CrossRef]
- Gol’tsman, B.M.; Yatsenko, E.A. Influence of Liquid Porophore Distribution on the Formation of the Porous Structure of Foam Glass. Glass Ceram. 2022, 79, 229–233. [Google Scholar] [CrossRef]
- Shill, F. Foam Glass; Stroyizdat: Moscow, Russia, 1965; pp. 43–60. (In Russian) [Google Scholar]
- Malykhin, E.M.; Krivchenko, V.A.; Lopaev, D.V.; Rakhimova, T.V.; Zyryanov, S.M. The Structure of Thin Carbon Films Deposited at 13.5 nm EUV Irradiation. Mosc. Univ. Phys. Bull. 2011, 66, 54–58. [Google Scholar] [CrossRef]
- Gupta, A.; Chen, G.; Joshi, P.; Tadigadapa, S.; Eklund, P.C. Raman Scattering from High-Frequency Phonons in Supported n-Graphene Layer Films. Nano Lett. 2006, 6, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Nakayama, T.; Miyake, A.; Takase, H.; Terashima, S.; Sudo, T.; Watanabe, Y.; Fukuda, Y. Analysis of Carbon Deposition on Multilayer Mirrors by Using Two Different Beamlines. In Proceedings of the Alternative Lithographic Technologies, SPIE, San Jose, CA, USA, 17 March 2009; Volume 7271, p. 72713. [Google Scholar] [CrossRef]
- Petersen, R.R.; König, J.; Iversen, N.; Østergaard, M.B.; Yue, Y. The Foaming Mechanism of Glass Foams Prepared from the Mixture of Mn3O4, Carbon and CRT Panel Glass. Ceram. Int. 2021, 47, 2839–2847. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel Fuel Production by Transesterification of Oils: Review. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]


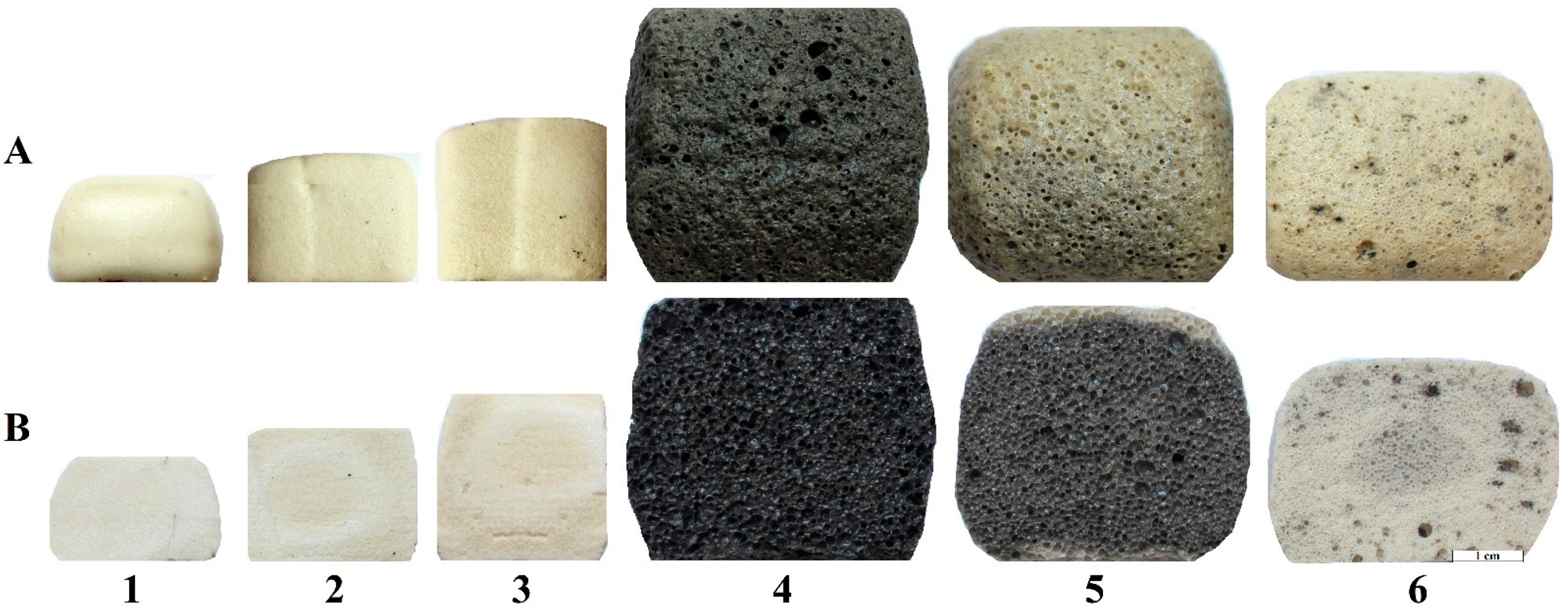
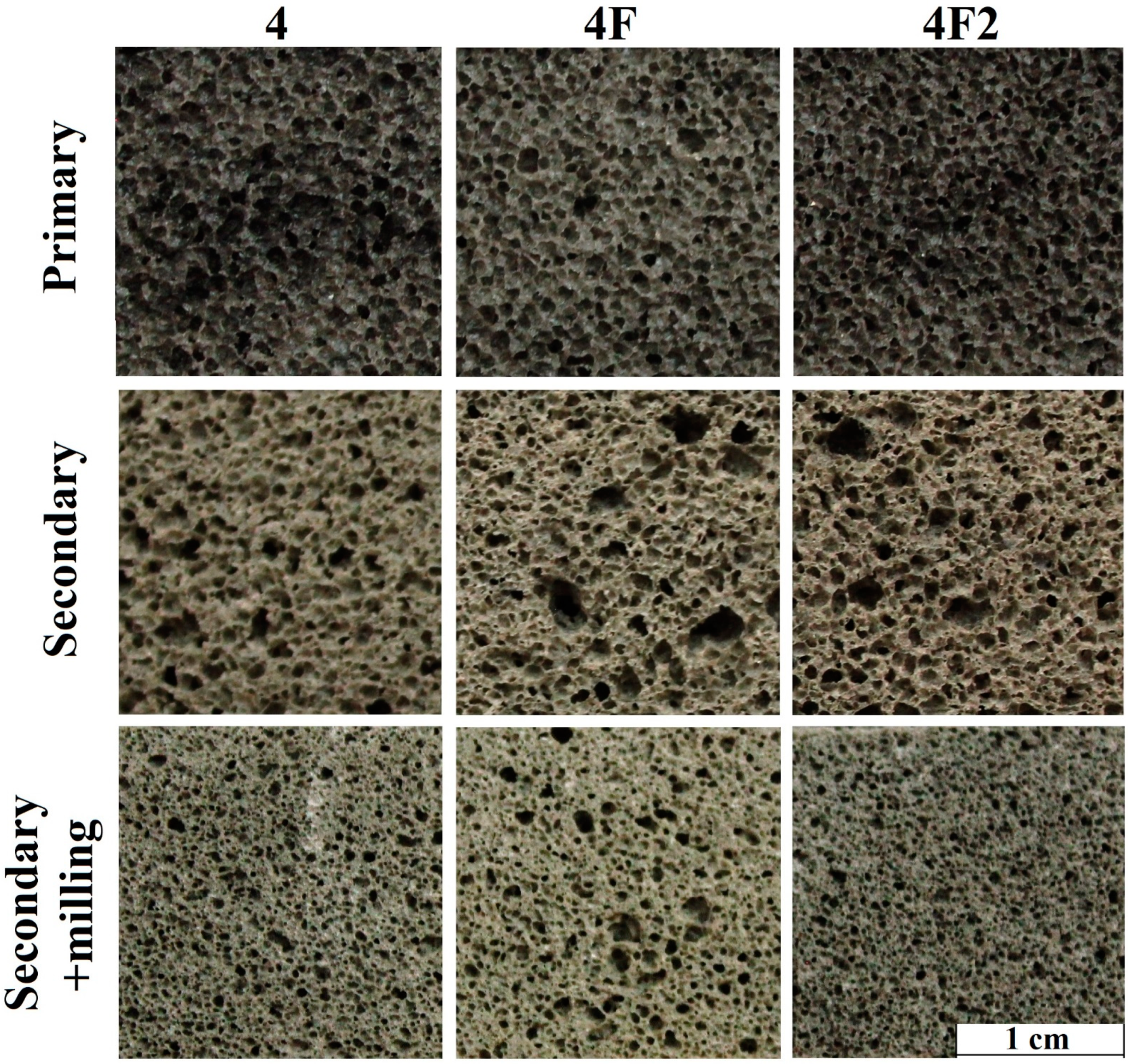
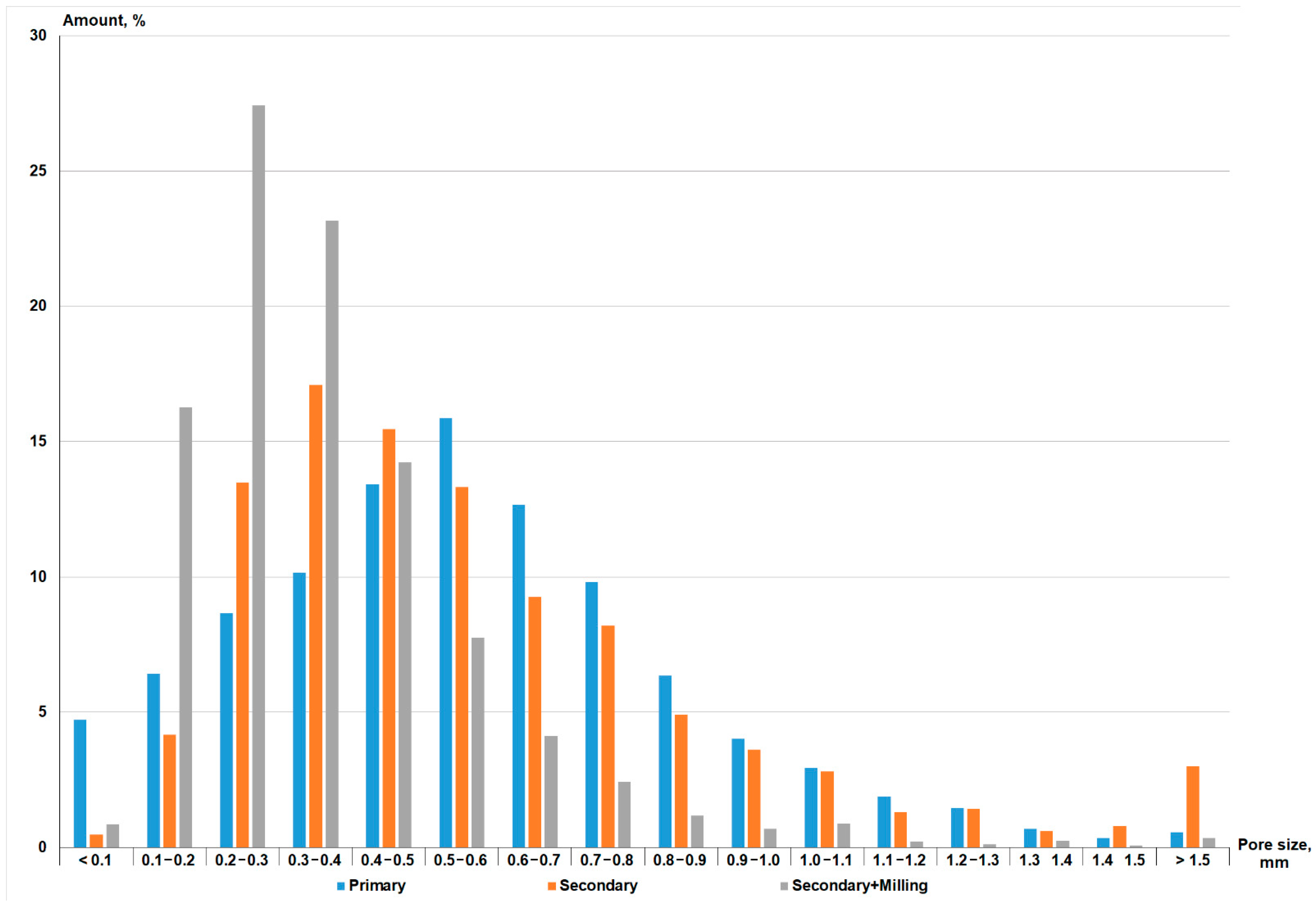

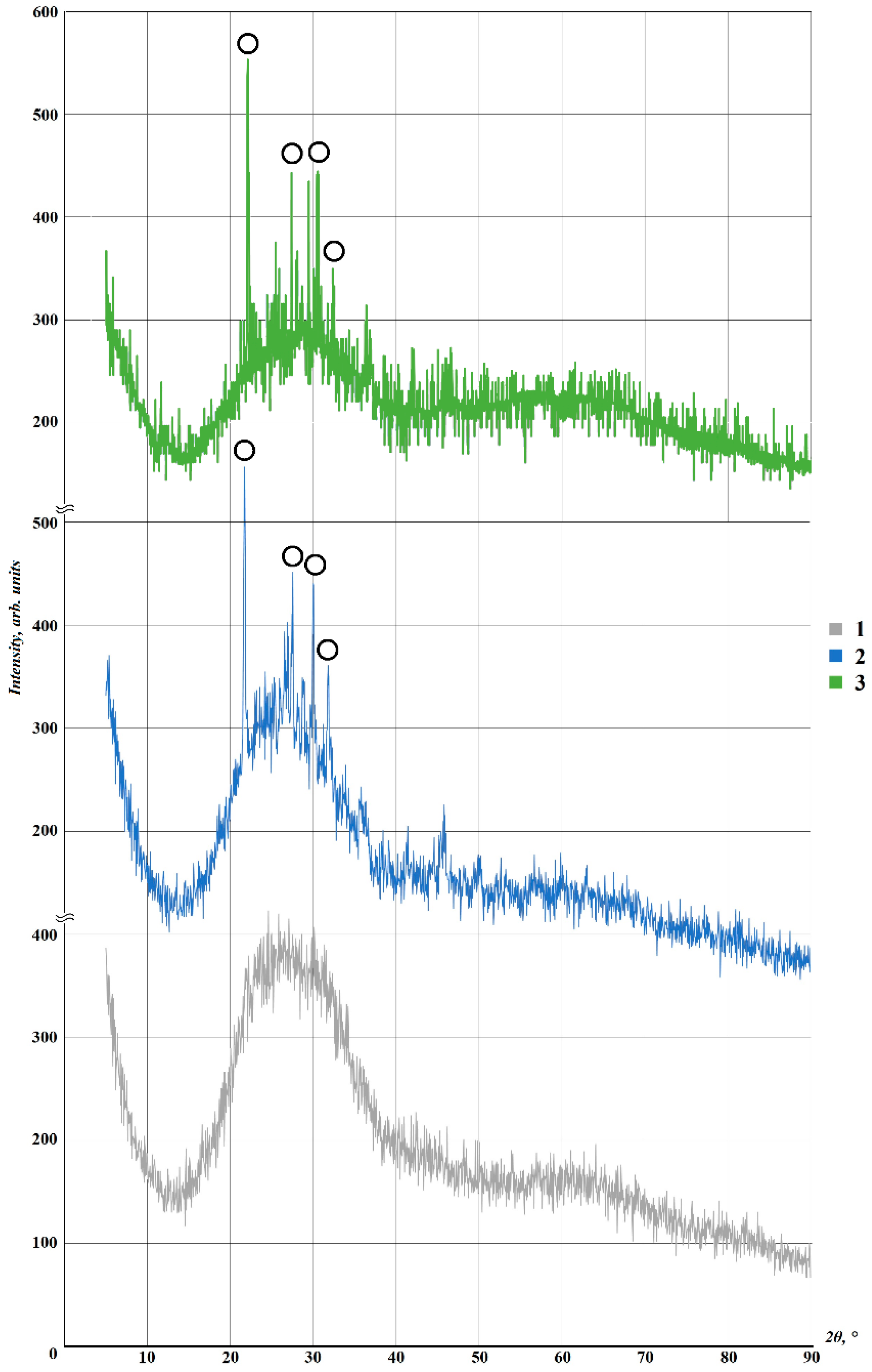

| Composition | Amount of the Component, wt. % | ||
|---|---|---|---|
| Glass Powder | Glycerol | Waterglass | |
| 1 | 99 | 1 | - |
| 2 | 95 | 5 | - |
| 3 | 91 | 9 | - |
| 4 | 90 | 1 | 9 |
| 5 | 90 | 5 | 5 |
| 6 | 90 | 9 | 1 |
| Composition | Amount of the Component, wt. % | |||
|---|---|---|---|---|
| Glass Powder | Glycerol | Waterglass | Water | |
| 4 | 90 | 1 | 9 | - |
| 4F | 90 | 1 | 9 | 3 * |
| 4F2 | 90 | 1 | 6 | 3 |
| Secondary Foaming | Density, kg/m3, of Composition, # | ||
|---|---|---|---|
| 4 | 4F | 4F2 | |
| Original samples | |||
| Before | 161 ± 3 | 157 ± 7 | 156 ± 6 |
| After | 229 ± 21 | 238 ± 18 | 236 ± 20 |
| Milled samples | |||
| Before | 860 ± 13 | 868 ± 14 | 869 ± 14 |
| After | 723 ± 6 | 659 ± 5 | 695 ± 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goltsman, B.M.; Yatsenko, E.A. Role of Carbon Phase in the Formation of Foam Glass Porous Structure. Materials 2022, 15, 7913. https://doi.org/10.3390/ma15227913
Goltsman BM, Yatsenko EA. Role of Carbon Phase in the Formation of Foam Glass Porous Structure. Materials. 2022; 15(22):7913. https://doi.org/10.3390/ma15227913
Chicago/Turabian StyleGoltsman, Boris M., and Elena A. Yatsenko. 2022. "Role of Carbon Phase in the Formation of Foam Glass Porous Structure" Materials 15, no. 22: 7913. https://doi.org/10.3390/ma15227913
APA StyleGoltsman, B. M., & Yatsenko, E. A. (2022). Role of Carbon Phase in the Formation of Foam Glass Porous Structure. Materials, 15(22), 7913. https://doi.org/10.3390/ma15227913







