Synthesis of Rare-Earth-Doped Strontium Tungstate Phosphor at Room Temperature and Applied Flexible Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Crystalline SrWO4 and SrWO4:RE3+ by Co-Precipitation
2.2. Fabricated Flexible Color Composite
2.3. Characterization
3. Results & Discussion
3.1. Characteristics of SrWO4 and Single Doped SrWO4
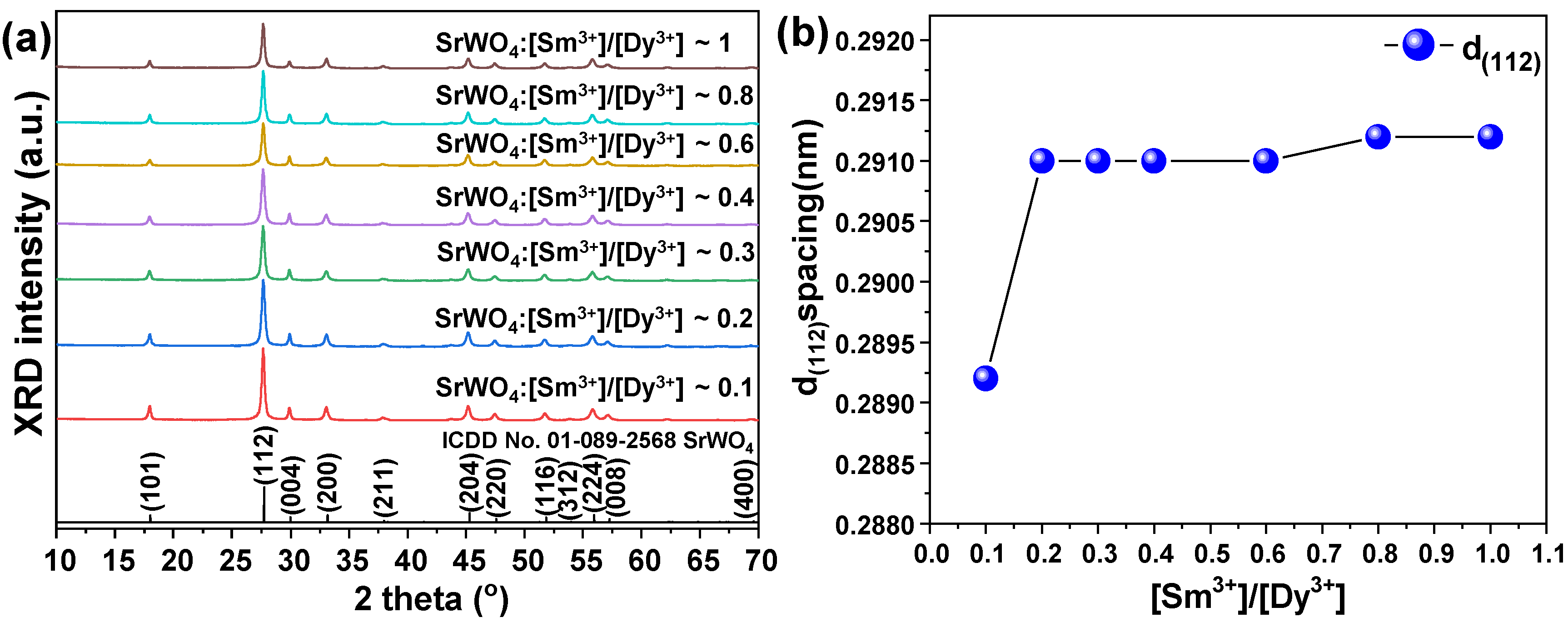
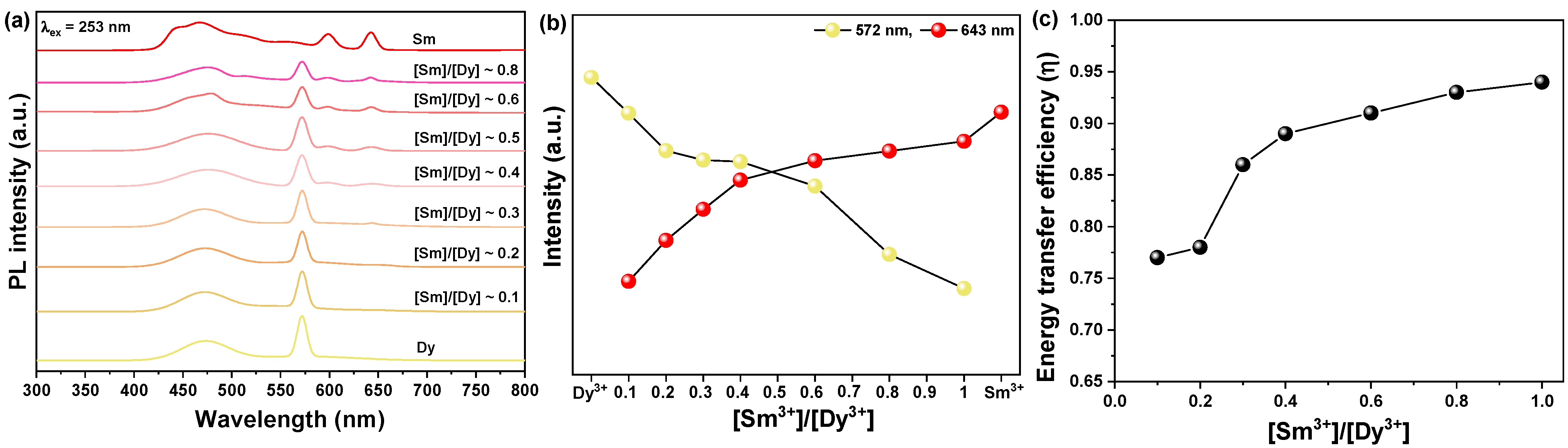
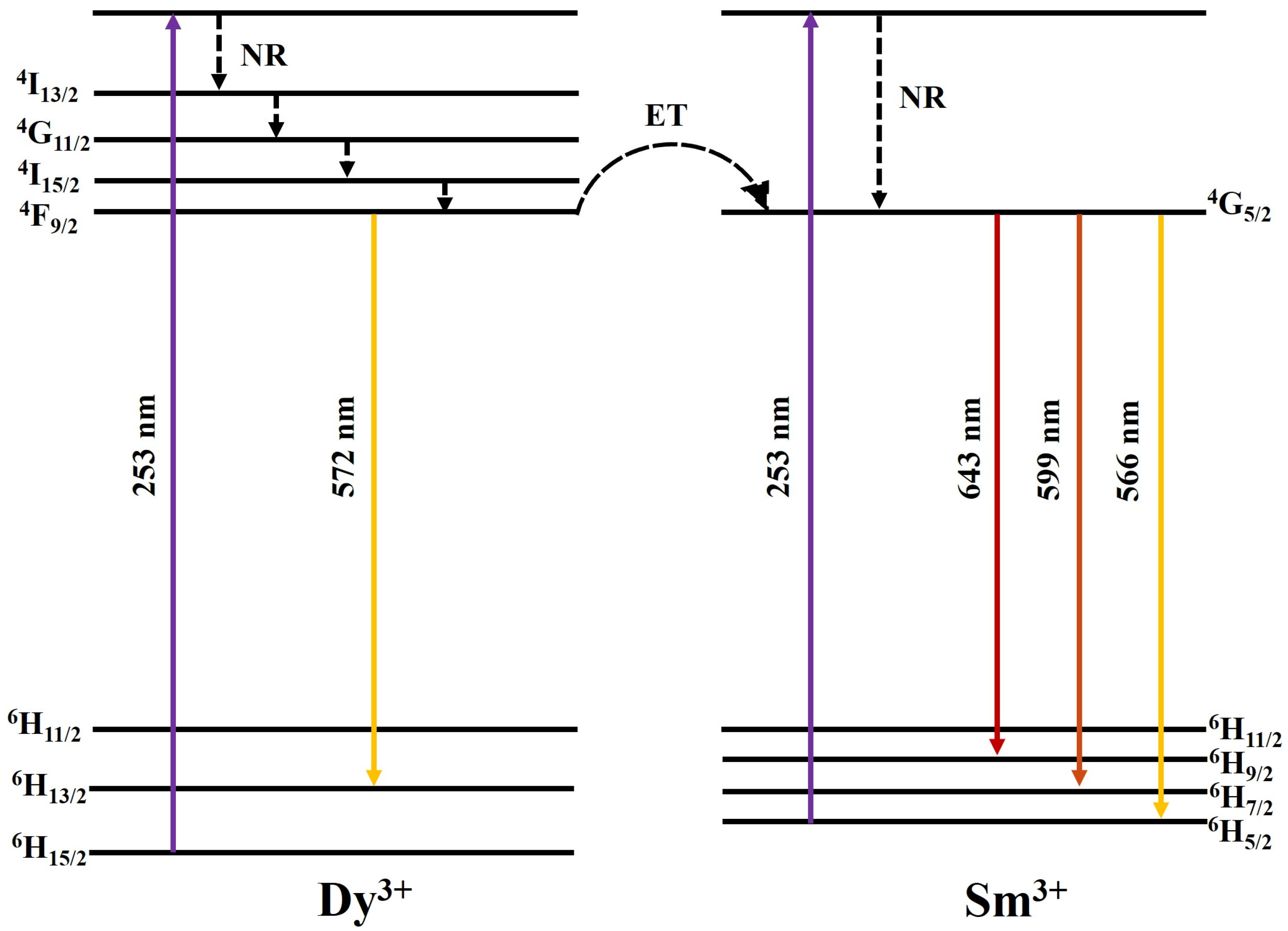
3.2. Characteristics of [Sm3+]/[Dy3+] Co-Doped SrWO4
3.3. Application in a Flexible Composite LED Filter
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Waisawy, S.; George, A.F.; Jadwisienczak, W.M.; Rahman, F. Preparation of balanced trichromatic white phosphors for solid-state white lighting. Luminescence 2017, 32, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.N.; Kim, K.D.; Anoop, G.; Kim, G.S.; Yoo, J.S. Design of highly efficient phosphor-converted white light-emitting diodes with color rendering indices (R1–R15) ≥ 95 for artificial lighting. Sci. Rep. 2019, 9, 16848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wlodarczyk, D.; Bulyk, L.; Berkowski, M.; Glowacki, M.; Kosyl, K.M.; Kaczmarek, S.M.; Kowalski, Z.; Wittlin, A.; Przybylinska, H.; Zhydachevskyy, Y.; et al. High-Pressure Low-Temperature Optical Studies of BaWO4:Ce, Na Crystals. Inorg. Chem. 2019, 58, 5617. [Google Scholar] [CrossRef] [PubMed]
- Dirany, N.; McRae, E.; Arab, M. Morphological and structural investigation of SrWO4 microcrystals in relationship with the electrical impedance properties. CrystEngComm 2017, 19, 5008–5021. [Google Scholar] [CrossRef]
- Koukou, V.; Martini, N.; Valais, I.; Bakas, A.; Kalyvas, N.; Lavdas, E.; Fountos, G.; Kandarakis, I.; Michail, C. Resolution Properties of a Calcium Tungstate (CaWO4) Screen Coupled to a CMOS Imaging Detector. J. Phys. Conf. Ser. 2017, 931, 12027. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Sun, X.; Li, X.; He, J.; Wang, B. Synthesis and Luminescence of BaWO4:Ln3+ (Ln = Eu, Tb, and Dy) Powders. J. Electron. Mater. 2014, 43, 3534–3538. [Google Scholar] [CrossRef]
- Huang, J.; Lu, W.; Yue, D.; Wang, Y.; Wang, Z.; Jin, L.; Zhang, L.; Li, Z. Controllable synthesis of multi-morphological SrWO4:Ln3+ (Ln = Eu, Tb) hierarchical structures and their luminescence properties. CrystEngComm 2019, 21, 6482–6490. [Google Scholar] [CrossRef]
- Tian, B.; Chen, B.; Tian, Y.; Sun, J.; Li, X.; Zhang, J.; Zhong, H.; Cheng, L.; Hua, R. Concentration and temperature quenching mechanisms of Dy3+ luminescence in BaGd2ZnO5 phosphors. J. Phys. Chem. Solids 2012, 73, 1314–1319. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of crystal spectra of rare-earth ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Yu, M.; Xu, X.; Zhang, W.; Chen, X.; Zhang, P.; Huang, Y. The effect of Sm3+ co-doping on the luminescence properties of Ca2·85Li0·15(PO4)1·85(SO4)0.15: Dy3+ white-emitting phosphors. J. Alloys Compd. 2020, 817, 152761. [Google Scholar] [CrossRef]
- Jung, J.; Kim, J.; Shim, Y.; Hwang, D.; Son, C.S. Structure and Photoluminescence Properties of Rare-Earth (Dy3+, Tb3+, Sm3+)-Doped BaWO4 Phosphors Synthesized via Co-Precipitation for Anti-Counterfeiting. Materials 2020, 13, 4165. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Jung, J. Calcium Tungstate Doped with Rare Earth Ions Synthesized at Low Temperatures for Photoactive Composite and Anti-Counterfeiting Applications. Crystals 2021, 11, 1214. [Google Scholar] [CrossRef]
- Tan, X.; Wang, Y.; Zhang, M. Solvothermal synthesis, luminescence and energy transfer of Dy3+ and Sm3+ doped NaLa(WO4)2 nanocubes. J. Photochem. Photobiol. A Chem. 2018, 353, 65–70. [Google Scholar] [CrossRef]
- Sun, X.; Huang, Z.; Fu, X.; Xu, L.; Liu, K.; Yuan, H. Generation of warm white light by doping Sm3+ in Ca3TeO6:Dy3+ fluorescent powders. Ceram. Int. 2020, 46, 14252–14256. [Google Scholar] [CrossRef]
- Van Orman, J.A.; Grove, T.L.; Shimizu, N. Rare earth element diffusion in diopside: Influence of temperature, pressure, and ionic radius, and an elastic model for diffusion in silicates. Contrib. Mineral. Petrol. 2001, 141, 687–703. [Google Scholar] [CrossRef]
- Diaz-Torres, L.A.; De la Rosa, E.; Salas, P.; Romero, V.H.; Angeles-Chávez, C. Efficient photoluminescence of Dy3+ at low concentrations in nanocrystalline ZrO2. J. Solid State Chem. 2008, 181, 75–80. [Google Scholar] [CrossRef]
- Yasaka, P.; Kaewkhao, J. White emission materials from glass doped with rare Earth ions: A review. AIP Conf. Proc. 2016, 1719, 020002. [Google Scholar] [CrossRef] [Green Version]
- Selvalakshmi, T.; Sellaiyan, S.; Uedono, A.; Chandra Bose, A. Investigation of defect related photoluminescence property of multicolour emitting Gd2O3:Dy3+ phosphor. RSC Adv. 2014, 4, 34257. [Google Scholar] [CrossRef]
- Stojadinović, S.; Vasilić, R. Orange–red photoluminescence of Nb2O5:Eu3+, Sm3+ coatings formed by plasma electrolytic oxidation of niobium. J. Alloys Compd. 2016, 685, 881–889. [Google Scholar] [CrossRef]
- Gupta, S.K.; Pathak, N.; Kadam, R.M. An efficient gel-combustion synthesis of visible light emitting barium zirconate perovskite nanoceramics: Probing the photoluminescence of Sm3+ and Eu3+ doped BaZrO3. J. Lumin. 2016, 169, 106–114. [Google Scholar] [CrossRef]
- Yang, L.; Mi, X.; Su, J.; Zhang, X.; Bai, Z.; Wang, N.; Lin, J. Tunable luminescence and energy transfer properties in YVO4:Bi3+, Ln3+ phosphors prepared by microwave sintering method. J. Mater. Sci. Mater. Electron. 2018, 29, 7941. [Google Scholar] [CrossRef]
- Ayvacikli, M.; Kaynar, Ü.H.; Karabulut, Y.; Canimoglu, A.; Bakr, M.; Akca, S.; Can, N. Synthesis and photoluminescence characteristics of Dy incorporated MoO3 phosphor: Suppression concentration quenching. Appl. Radiat. Isot. 2020, 164, 109321. [Google Scholar] [CrossRef] [PubMed]






| SrWO4 Up-Conversion Phosphor Synthesis | ||||
|---|---|---|---|---|
| Reagents | (CH3CO2)Sr | Na2WO4·2H2O | Dy(NO3)3·xH2O | Sm(NO3)3·6H2O |
| Molecular weight (g/mol) | 205.93 | 329.85 | 348.51 | 444.47 |
| Used mole (mmol) | 1 | 1 | 0.25 | 0.025~0.25 |
| [Dy3+]/[Sm3+] Ratio | ||||
| Reagents | (CH3CO2)Sr | Na2WO4·2H2O | Dy(NO3)3·xH2O | Sm(NO3)3·6H2O |
| Used mole (mmol) | 1 | 1 | 0.25 | 0.025 |
| 1 | 1 | 0.25 | 0.05 | |
| 1 | 1 | 0.25 | 0.075 | |
| 1 | 1 | 0.25 | 0.1 | |
| 1 | 1 | 0.25 | 0.15 | |
| 1 | 1 | 0.25 | 0.2 | |
| 1 | 1 | 0.25 | 0.25 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wi, J.-H.; Jung, J.-Y.; Park, S.-G. Synthesis of Rare-Earth-Doped Strontium Tungstate Phosphor at Room Temperature and Applied Flexible Composite. Materials 2022, 15, 8922. https://doi.org/10.3390/ma15248922
Wi J-H, Jung J-Y, Park S-G. Synthesis of Rare-Earth-Doped Strontium Tungstate Phosphor at Room Temperature and Applied Flexible Composite. Materials. 2022; 15(24):8922. https://doi.org/10.3390/ma15248922
Chicago/Turabian StyleWi, Jung-Hyun, Jae-Yong Jung, and Sang-Geon Park. 2022. "Synthesis of Rare-Earth-Doped Strontium Tungstate Phosphor at Room Temperature and Applied Flexible Composite" Materials 15, no. 24: 8922. https://doi.org/10.3390/ma15248922





