Structural, Magnetic, and Electrical Properties of CoFe2O4 Nanostructures Synthesized Using Microwave-Assisted Hydrothermal Method
Abstract
1. Introduction
2. Experimental
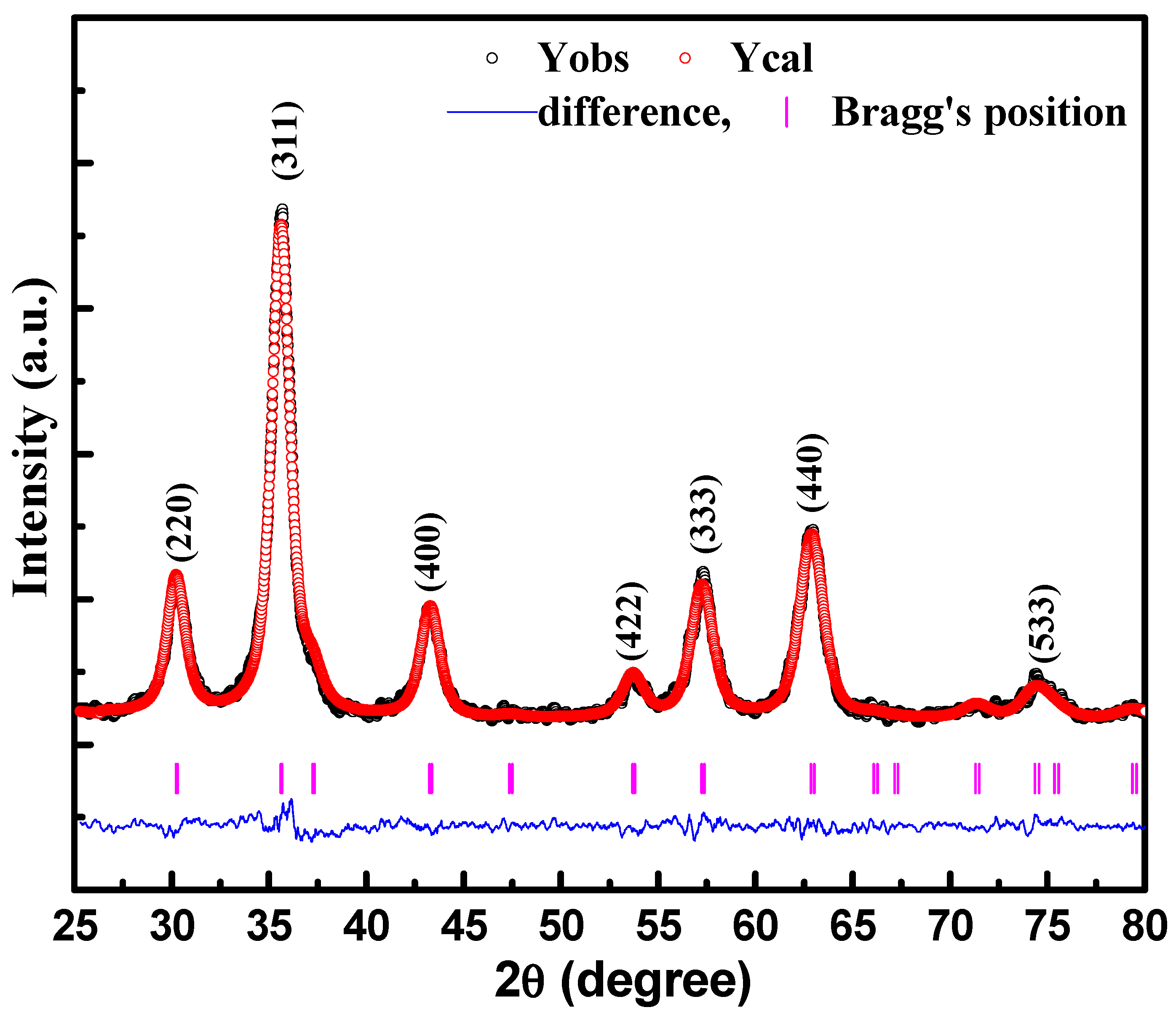
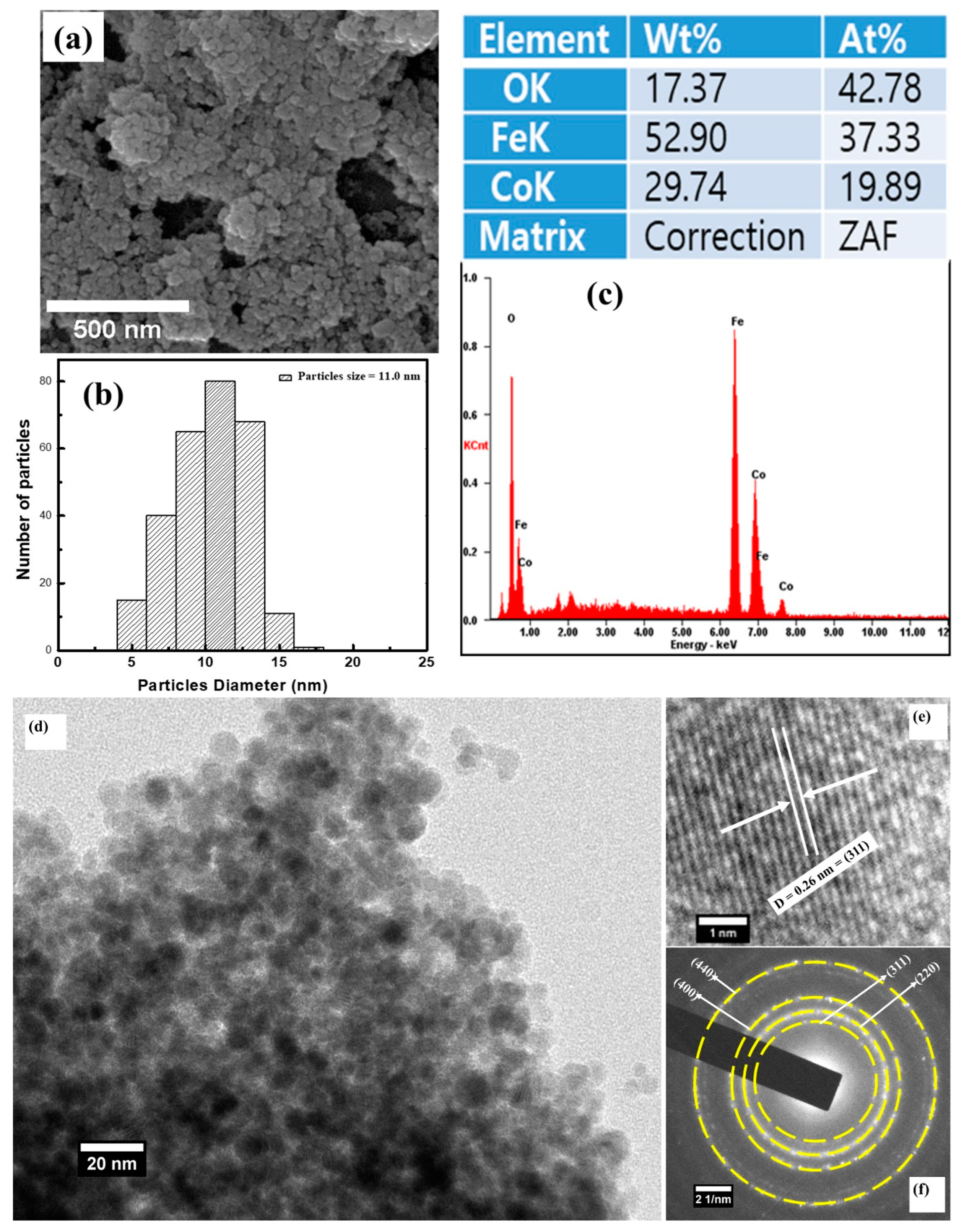
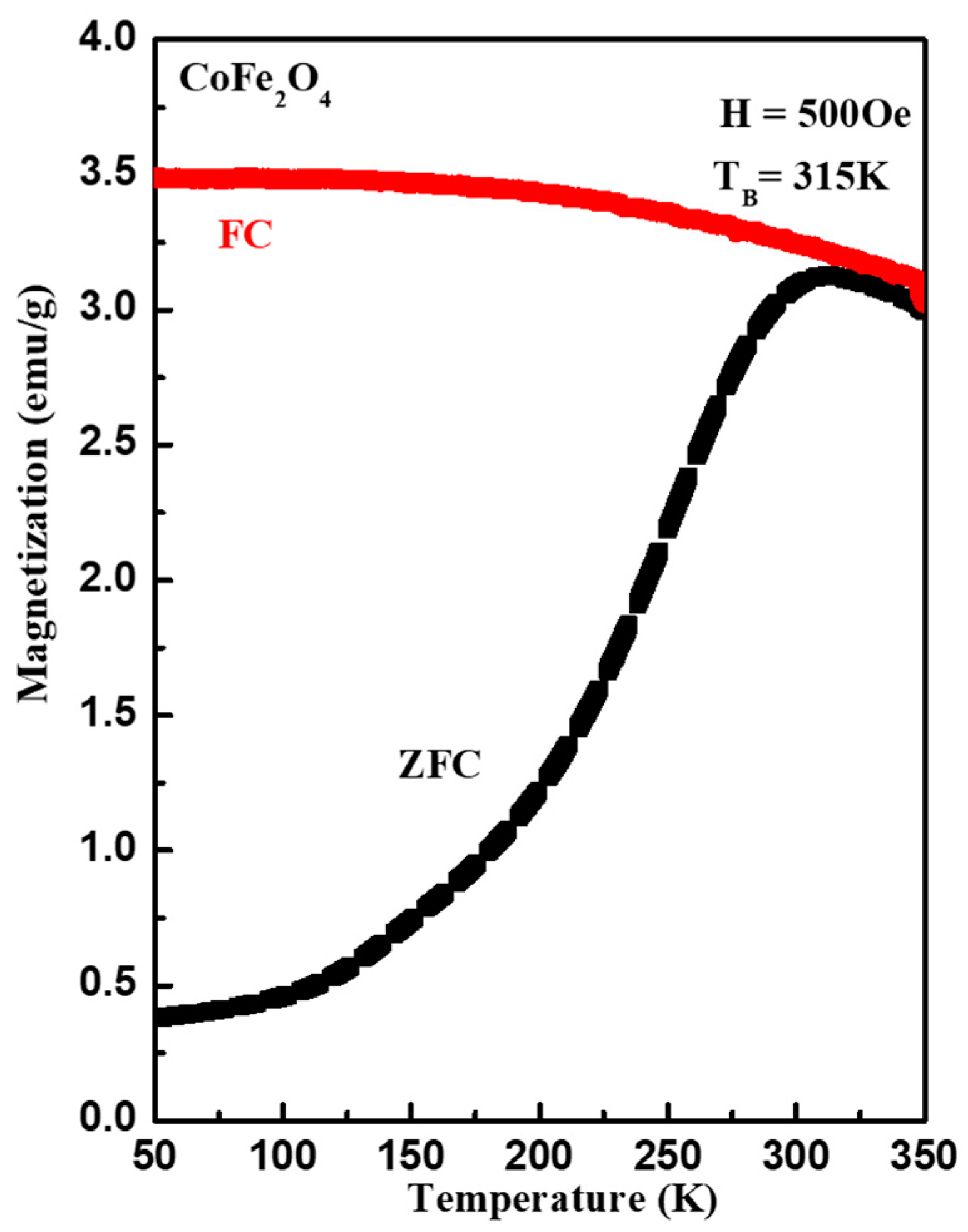
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamed Racik, K.; Anand, S.; Muniyappan, S.; Nandhini, S.; Rameshkumar, S.; Mani, D.; Karuppasamy, P.; Pandian, M.S.; Ramasamy, P. Preparation of CoFe2O4/SiO2 nanocomposite as potential electrode materials for supercapacitors. Inorg. Chem. Commun. 2022, 146, 110036. [Google Scholar] [CrossRef]
- Mathew, T.; Shylesh, S.; Reddy, S.N.; Sebastian, C.P.; Date, S.K.; Rao, B.S.; Kulkarni, S.D. Redistribution of cations amongst different lattice sites in Cu1-xCoxFe2O4 ferrospinels during alkylation: Magnetic study. Catal. Lett. 2004, 93, 155–163. [Google Scholar] [CrossRef]
- Lee, S.J.; Song, S.H.; Lo, C.C.; Aldini, S.T.; Jiles, D.C. Magneto-optic properties of CoFe2-xGaxO4 J. Appl. Phys. 2007, 101, 9–11. [Google Scholar] [CrossRef]
- Nakagomi, F.; Da Silva, S.W.; Garg, V.K.; Oliveira, A.C.; Morais, P.C.; Franco Júnior, A.; Lima, E.C.D. The influence of cobalt population on the structural properties of CoxFe3-xO4 J. Appl. Phys. 2007, 101, 9–11. [Google Scholar] [CrossRef]
- Stewart, S.J.; Figueroa, S.J.A.; Ramallo López, J.M.; Marchetti, S.G.; Bengoa, J.F.; Prado, R.J.; Requejo, F.G. Cationic exchange in nanosized ZnFe2O4 spinel revealed by experimental and simulated near-edge absorption structure. Phys. Rev. B 2007, 75, 073408. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastava, G.; Almutairi, G.; Ahmed, F.; Shaalan, N.M.; Dalela, S.; Kumar, R.; Kumar, A.P.; Alvi, P.A.; Chae, K.H.; et al. Structure and electrochemical properties of La-doped BiFeO3 nanoparticles. J. Electron. Spectros. Relat. Phenom. 2021, 253, 147138. [Google Scholar] [CrossRef]
- Hashim, M.; Alimuddin Kumar, S.; Shirsath, S.E.; Mohammed, E.M.; Chung, H.; Kumar, R. Studies on the activation energy from the ac conductivity measurements of rubber ferrite composites containing manganese zinc ferrite. Phys. B Condens. Matter. 2012, 407, 4097–4103. [Google Scholar] [CrossRef]
- Liu, K.; Baker, S.M.; Tuominen, M.; Russell, T.P.; Schuller, I.K. Tailoring exchange bias with magnetic nanostructures. Phys. Rev. B 2001, 63, 604031–604034. [Google Scholar] [CrossRef]
- Shirsath, S.E.; Liu, X.; Yasukawa, Y.; Li, S.; Morisako, A. Switching of magnetic easy-axis using crystal orientation for large perpendicular coercivity in CoFe2O4 thin film. Sci. Rep. 2016, 6, 30074. [Google Scholar] [CrossRef]
- Park, J.; Joo, J.; Soon, G.K.; Jang, Y.; Hyeon, T. Synthesis of monodisperse spherical nanocrystal. Angew. Chem. Int. Ed. 2007, 46, 4630–4660. [Google Scholar] [CrossRef] [PubMed]
- Anjum, G.; Kumar, R.; Mollah, S.; Shukla, D.K.; Kumar, S.; Lee, C.G. Structural, dielectric, and magnetic properties of La0.8Bi0.2Fe1−xMnxO3 (0.0 ≤ x ≤ 0.4) multiferroics. J. Appl. Phys. 2010, 107, 103916. [Google Scholar] [CrossRef]
- Kumari, A.; Kumari, K.; Aljawfi, R.N.; Alvi, P.A.; Dalela, S.; Ahmad, M.M.; Chawla, A.K.; Kumar, R.; Vij, A.; Kumar, S. Role of La substitution on structural, optical, and multiferroic properties of BiFeO3 nanoparticles. Appl. Nanosci. 2021, 1–20. [Google Scholar] [CrossRef]
- Wang, Z.; Downs, T.; Pischedda, V.; Shetty, R.; Saxena, K.; Zha, S.; Zhao, S.; Schiferl, D.; Waskowska, A. High-pressure X-ray diffraction and Raman spectroscopic studies of the tetragonal spinel CoFe2O4. Phys. Rev. B 2003, 68, 2–7. [Google Scholar]
- Hashim, M.; Alimuddin Kumar, S.; Shirsath, S.E.; Kotnala, R.K.; Chung, H.; Kumar, R. Structural properties and magnetic interactions in Ni0.5Mg0.5Fe2-xCrxO4 (0 ≤ x ≤ 1) ferrite nanoparticles. Powder Technol. 2012, 229, 37–44. [Google Scholar] [CrossRef]
- Kumar, S.; Alimuddin Kumar, R.; Thakur, P.; Chae, K.H.; Angadi, B.; Choi, W.K. Electrical transport, magnetic, and electronic structure studies of Mg0.95Mn0.05Fe2−2xTi2xO4 ± δ (0 ≤ x ≤ 0.5) ferrites. J. Phys. Condens. Matter 2007, 19, 476210. [Google Scholar] [CrossRef]
- Hashim, M.; Shirsath, S.E.; Meena, S.S.; Mane, M.L.; Kumar, S.; Bhatt, P.; Kumar, R.; Prasad, N.K.; Alla, S.K.; Shah, J.; et al. Manganese ferrite prepared using reverse micelle process: Structural and magnetic properties characterization. J. Alloy. Compd. 2015, 642, 70–77. [Google Scholar] [CrossRef]
- Zerr, A.; Miehe, G.; Serghiou, G.; Schwarz, M.; Kroke, E.; Riedel, R.; Fueß, H.; Kroll, P.; Boehler, R. Synthesis of cubic silicon nitride. Nature 1999, 400, 340–342. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kumar, R.; Kumar, S.; Siva Kumar, V.V.; Knobel, M.; Reddy, V.R.; Banerjee, A.; Singh, M. Magnetic study of Mg0.95Mn0.05Fe2O4 ferrite nanoparticles. Solid State Commun. 2007, 141, 203–208. [Google Scholar] [CrossRef]
- Kumar, S.; Farea, A.M.M.; Batoo, K.M.; Lee, C.G.; Koo, B.H.; Yousef, A.; Alimuddi. Mössbauer studies of Co0.5CdxFe2.5−xO4 (0.0 ⩽ x ⩽ 0.5) ferrite. Phys. B Condens. Matter 2008, 403, 3604–3607. [Google Scholar] [CrossRef]
- Bayoumi, W. Structural and electrical properties of zinc-substituted cobalt ferrite. J. Mater. Sci. 2007, 42, 8254–8261. [Google Scholar] [CrossRef]
- Patil, A.N.; Patil, M.G.; Patankar, K.K.; Mathe, V.L.; Mahajan, R.P.; Patil, S.A. Dielectric behaviour and a.c. conductivity in CuxFe3-xO4 ferrite. Bull. Mater. Sci. 2000, 23, 447–452. [Google Scholar] [CrossRef]
- Rane, K.S.; Verenkar VM, S.; Sawant, P.Y. Dielectric behaviour of MgFe2O4 prepared from chemically beneficiated iron ore rejects. Bull. Mater. Sci. 2001, 24, 323–330. [Google Scholar] [CrossRef]
- Ušáková, M.; Lukáč, J.; Dosoudil, R.; Jančárik, V.; Grusková, A.; Ušák, E.; Sláma, J.; Šubrt, J. Influence of Cu2+ ions on structural and magnetic properties of NiZn ferrites. J. Mater. Sci. Mater. Electron. 2007, 18, 1183–1189. [Google Scholar] [CrossRef]
- Pillai, V.; Shah, D.O. Synthesis of high-coercivity cobalt ferrite particles using water-in-oil microemulsions. J. Magn. Magn. Mater. 1996, 163, 243–248. [Google Scholar] [CrossRef]
- Ahn, Y.; Choi, E.J.; Kim, S.; Ok, H.N. Magnetization and Mössbauer study of cobalt ferrite particles from nanophase cobalt iron carbonate. Mater. Lett. 2001, 50, 47–52. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kumar, R.; Kumar, S.; Knobel, M.; Meneses, C.T.; Siva Kumar, V.V.; Reddy, V.R.; Singh, M.; Lee, C.G. Role of interparticle interactions on the magnetic behavior of Mg0.95Mn0.05Fe2O4 ferrite nanoparticles. J. Phys. Condens. Matter 2008, 20, 235214. [Google Scholar] [CrossRef] [PubMed]
- Sedlacik, M.; Pavlinek, V.; Peer, P.; Filip, P. Tailoring the magnetic properties and magnetorheological behavior of spinel nanocrystalline cobalt ferrite by varying annealing temperature. Dalt. Trans. 2014, 43, 6919–6924. [Google Scholar] [CrossRef]
- Kumari, K.; Aljawfi, R.N.; Chawla, A.K.; Kumar, R.; Alvi, P.A.; Alshoaibi, A.; Vij, A.; Ahmed, F.; Abu-samak, M.; Kumar, S. Engineering the optical properties of Cu doped CeO2 NCs for application in white LED. Ceram. Int. 2020, 46, 7482–7488. [Google Scholar] [CrossRef]
- Sahu, J.; Kumar, S.; Vats, V.S.; Alvi, P.A.; Dalela, B.; Kumar, S.; Phase, D.M.; Gupta, M.; Dalela, S. Exploring the Defects and Vacancies with Photoluminescence and XANES Studies of Gd3+-Substituted ZnO Nanoparticles. Part. Part. Syst. Charact. 2022, 39, 2200116. [Google Scholar] [CrossRef]
- Mahhouti, Z.; El Moussaoui, H.; Mahfoud, T.; Hamedoun, M.; El Marssi, M.; Lahmar, A.; El Kenz, A.; Benyoussef, A. Chemical synthesis and magnetic properties of monodisperse cobalt ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 14913–14922. [Google Scholar] [CrossRef]
- Cernea, M.; Galizia, P.; Ciuchi, I.; Aldica, G.; Mihalache, V.; Diamandescu, L.; Galassi, C. CoFe2O4 magnetic ceramic derived from gel and densified by spark plasma sintering. J. Alloys Compd. 2016, 656, 854–862. [Google Scholar] [CrossRef]
- Koops, C.G. On the Dispersion of Resistivity and Dielectric Constant of Some Semiconductors at Audiofrequencies. Phys. Rev. 1951, 83, 121–124. [Google Scholar] [CrossRef]
- Asandulesa, M.; Kostromin, S.; Podshivalov, A.; Tameev, A.; Bronnikov, S. Relaxation processes in a polymer composite for bulk heterojunction: A dielectric spectroscopy study. Polymer 2020, 203, 122785. [Google Scholar] [CrossRef]
- Reddy, P.V.; Rao, T.S. Dielectric behaviour of mixed Li-Ni ferrites at low frequencies. J. Less Common Met. 1982, 86, 255–261. [Google Scholar] [CrossRef]
- Asandulesa, M.; Chibac-Scutaru, A.L.; Culica, M.E.; Melinte, V.; Coseri, S. Cellulose-based films with enhanced load of nitrogen containing heterocycles: The impact on the surface morphology and proton conductivity. Appl. Surf. Sci. 2023, 607, 155077. [Google Scholar] [CrossRef]
- Kumari, A.; Kumari, K.; Ahmed, F.; Alshoaibi, A.; Alvi, P.A.; Dalela, S.; Ahmad, M.M.; Aljawfi, R.N.; Dua, P.; Vij, A.; et al. Influence of Sm doping on structural, ferroelectric, electrical, optical and magnetic properties of BaTiO3. Vacuum 2021, 184, 109872. [Google Scholar] [CrossRef]
- Asandulesa, M.; Kostromin, S.; Tameev, A.; Aleksandrov, A.; Bronnikov, S. Molecular Dynamics and Conductivity of a PTB7:PC71BM Photovoltaic Polymer Blend: A Dielectric Spectroscopy Study. ACS Appl. Polym. Mater. 2021, 3, 4869–4878. [Google Scholar] [CrossRef]


| 200 K | 300 K | 350 K | |
|---|---|---|---|
| MS (emu/g) | 50 | 48 | 46 |
| MR (emu/g) | 16 | 3.7 | 0 |
| HC (Oe) | 755 | 91 | 0 |
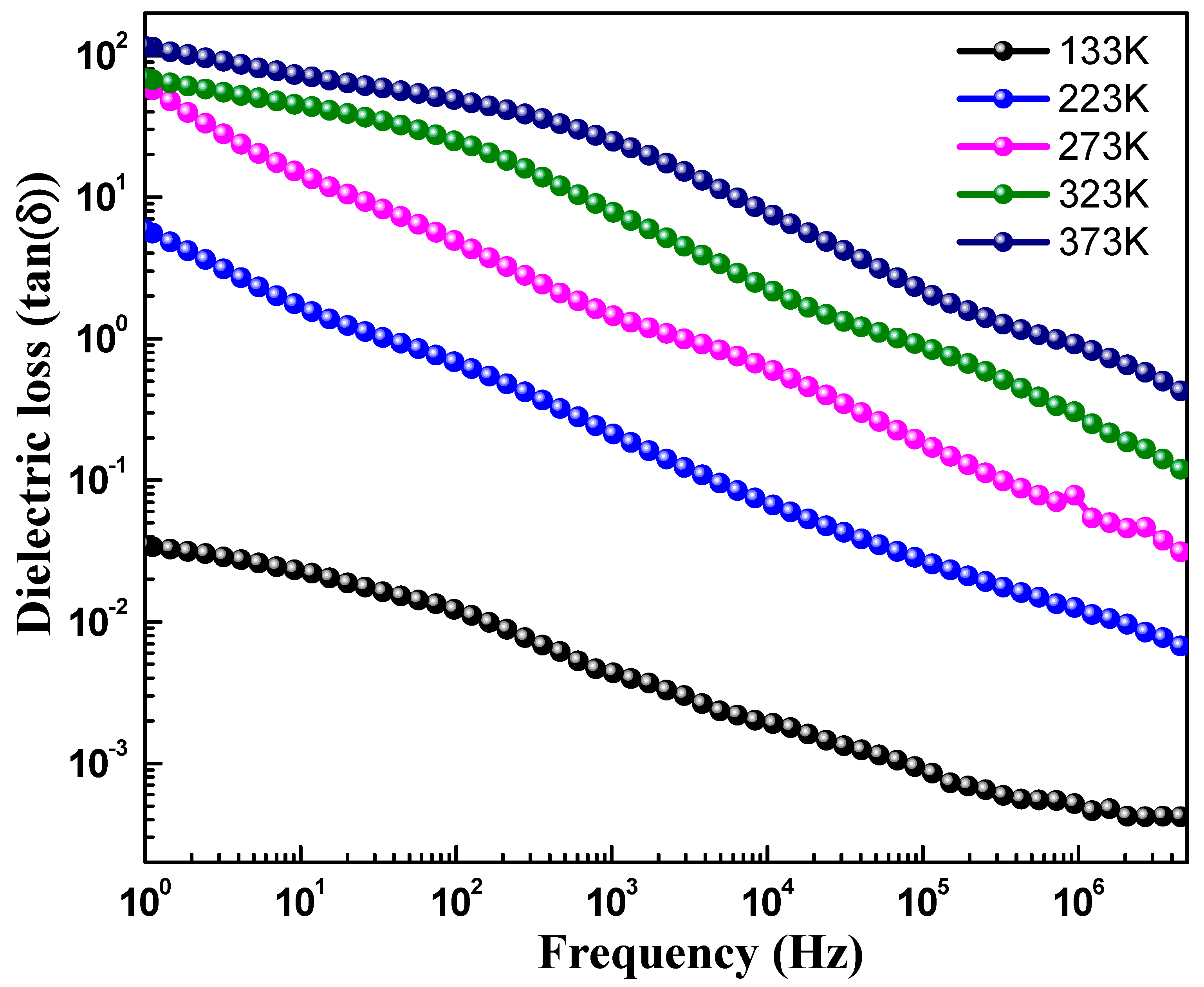
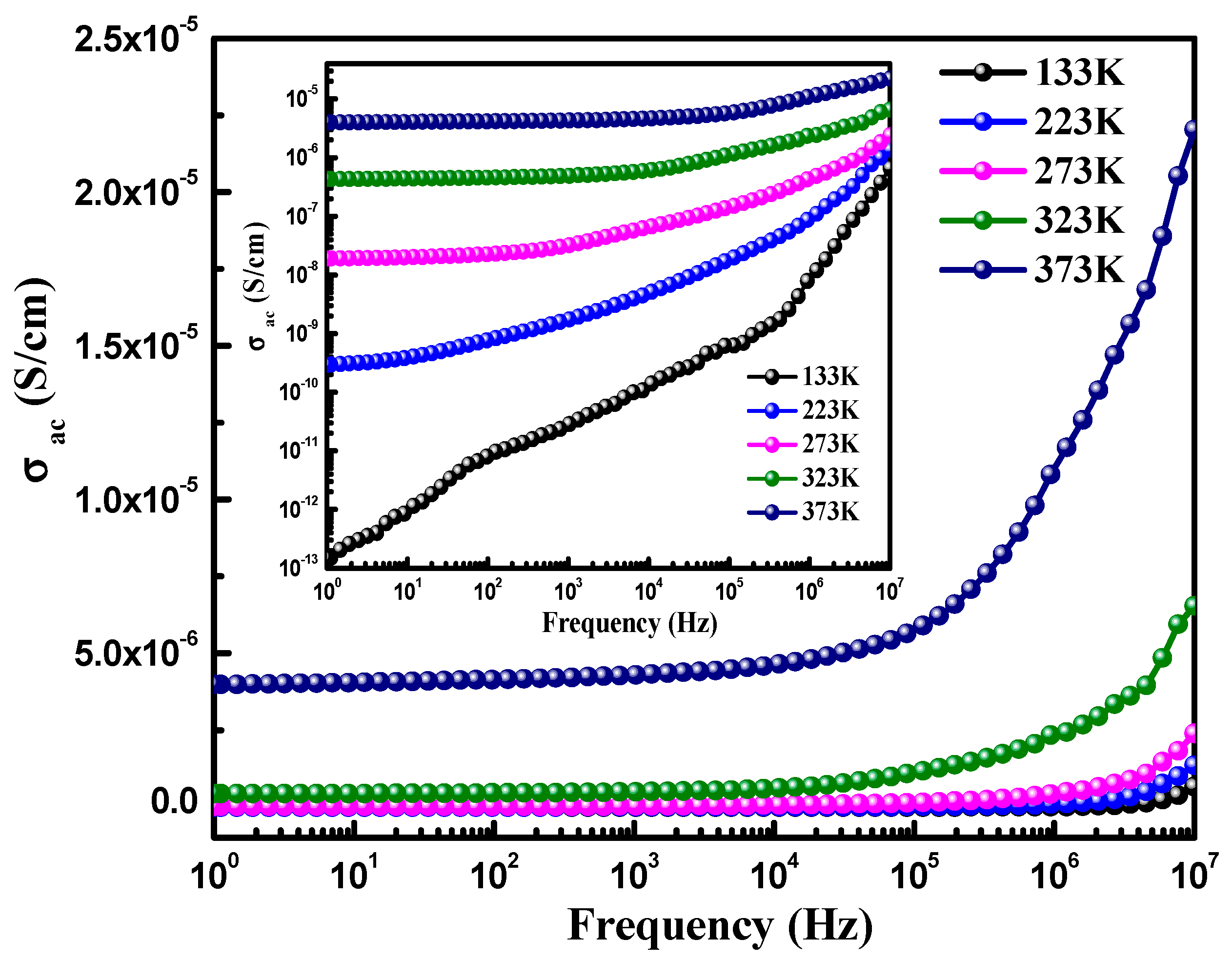
| CoFe2O4 | 133 K | 233 K | 273 K | 323 K | 373 K |
|---|---|---|---|---|---|
| Dielectric constant (ε′) | 11.4 | 11.8 | 12.4 | 14.3 | 20.8 |
| Loss tangent (tanδ) | 4.6 × 10−4 | 1.4 × 10−3 | 5.0 × 10−2 | 0.23 | 0.82 |
| ac conductivity (σac) | 1.2 × 10−8 | 1.1 × 10−7 | 4.8 × 10−7 | 2.4 × 10−6 | 1.2 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Ahmed, F.; Shaalan, N.M.; Kumar, R.; Alshoaibi, A.; Arshi, N.; Dalela, S.; Sayeed, F.; Dwivedi, S.; Kumari, K. Structural, Magnetic, and Electrical Properties of CoFe2O4 Nanostructures Synthesized Using Microwave-Assisted Hydrothermal Method. Materials 2022, 15, 7955. https://doi.org/10.3390/ma15227955
Kumar S, Ahmed F, Shaalan NM, Kumar R, Alshoaibi A, Arshi N, Dalela S, Sayeed F, Dwivedi S, Kumari K. Structural, Magnetic, and Electrical Properties of CoFe2O4 Nanostructures Synthesized Using Microwave-Assisted Hydrothermal Method. Materials. 2022; 15(22):7955. https://doi.org/10.3390/ma15227955
Chicago/Turabian StyleKumar, Shalendra, Faheem Ahmed, Nagih M. Shaalan, Rajesh Kumar, Adil Alshoaibi, Nishat Arshi, Saurabh Dalela, Fatima Sayeed, Sourabh Dwivedi, and Kavita Kumari. 2022. "Structural, Magnetic, and Electrical Properties of CoFe2O4 Nanostructures Synthesized Using Microwave-Assisted Hydrothermal Method" Materials 15, no. 22: 7955. https://doi.org/10.3390/ma15227955
APA StyleKumar, S., Ahmed, F., Shaalan, N. M., Kumar, R., Alshoaibi, A., Arshi, N., Dalela, S., Sayeed, F., Dwivedi, S., & Kumari, K. (2022). Structural, Magnetic, and Electrical Properties of CoFe2O4 Nanostructures Synthesized Using Microwave-Assisted Hydrothermal Method. Materials, 15(22), 7955. https://doi.org/10.3390/ma15227955














