Realization of Microfluidic Preconcentrator for N-Pentane Traces Impurities from the Gaseous Media
Abstract
1. Introduction
2. Experimental
2.1. Materials
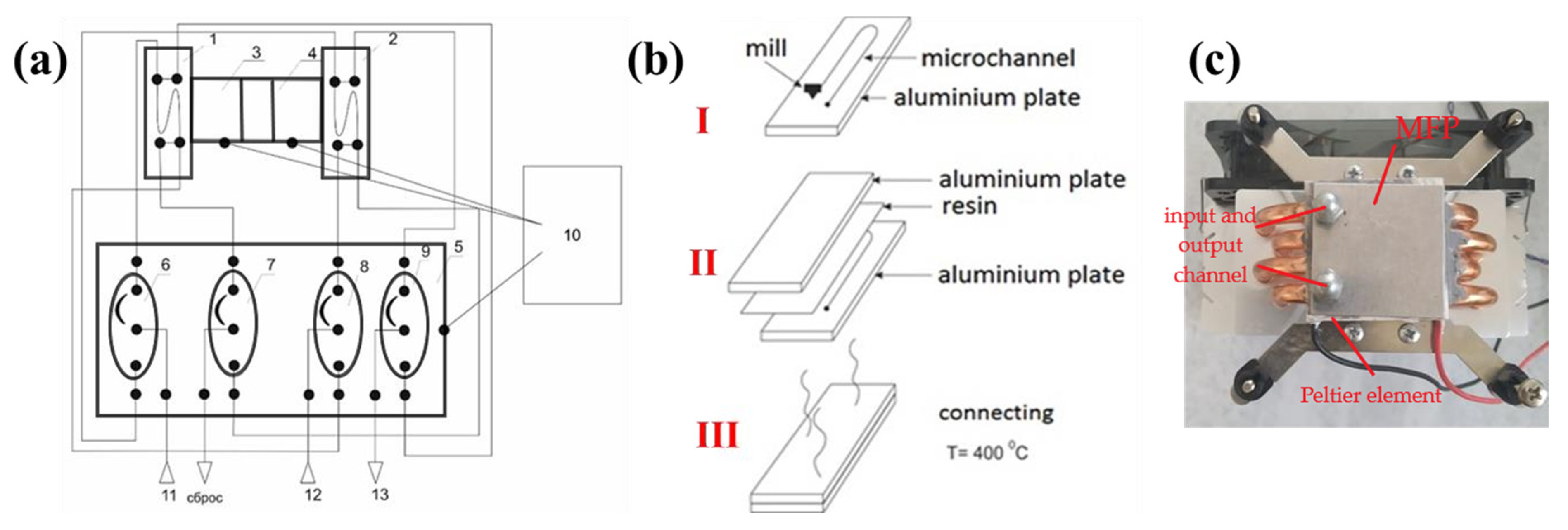
2.2. Device Design for the Microextraction, Fabrication, and Operation Principle
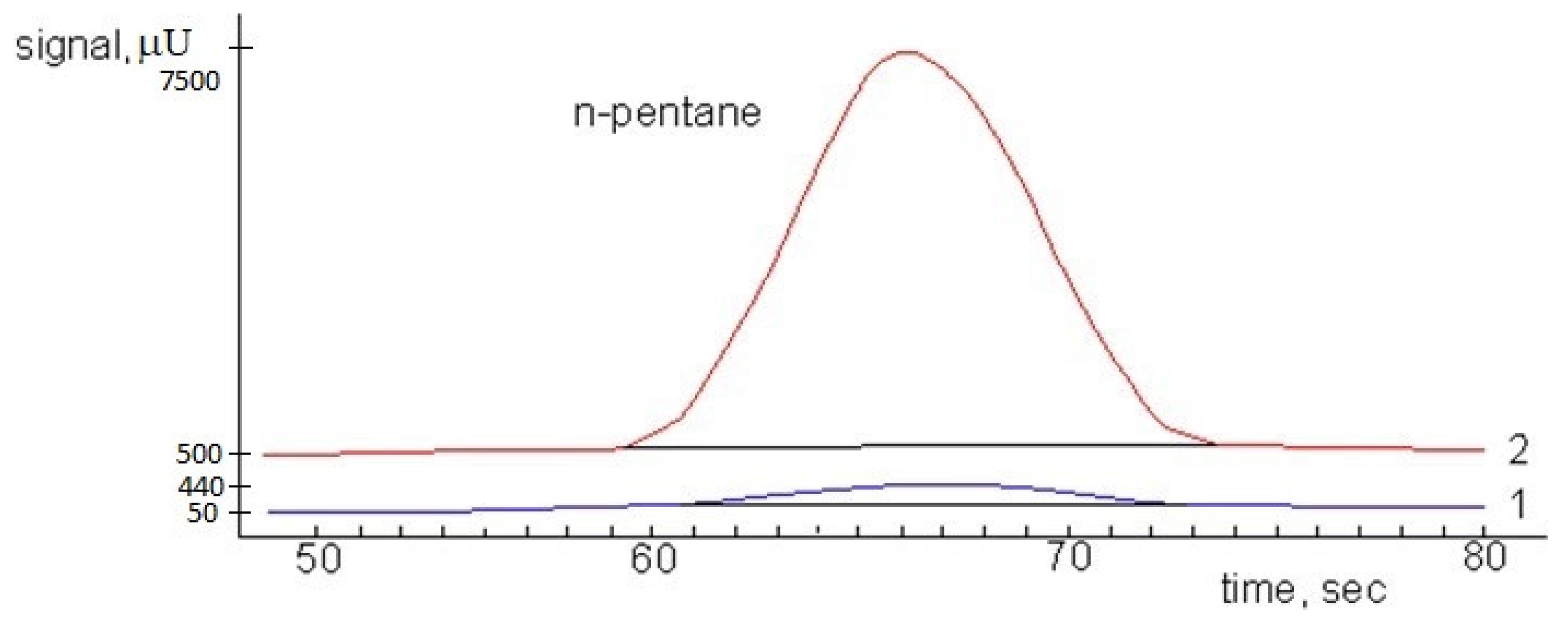
2.3. Conditions for Concentration and GC Analysis
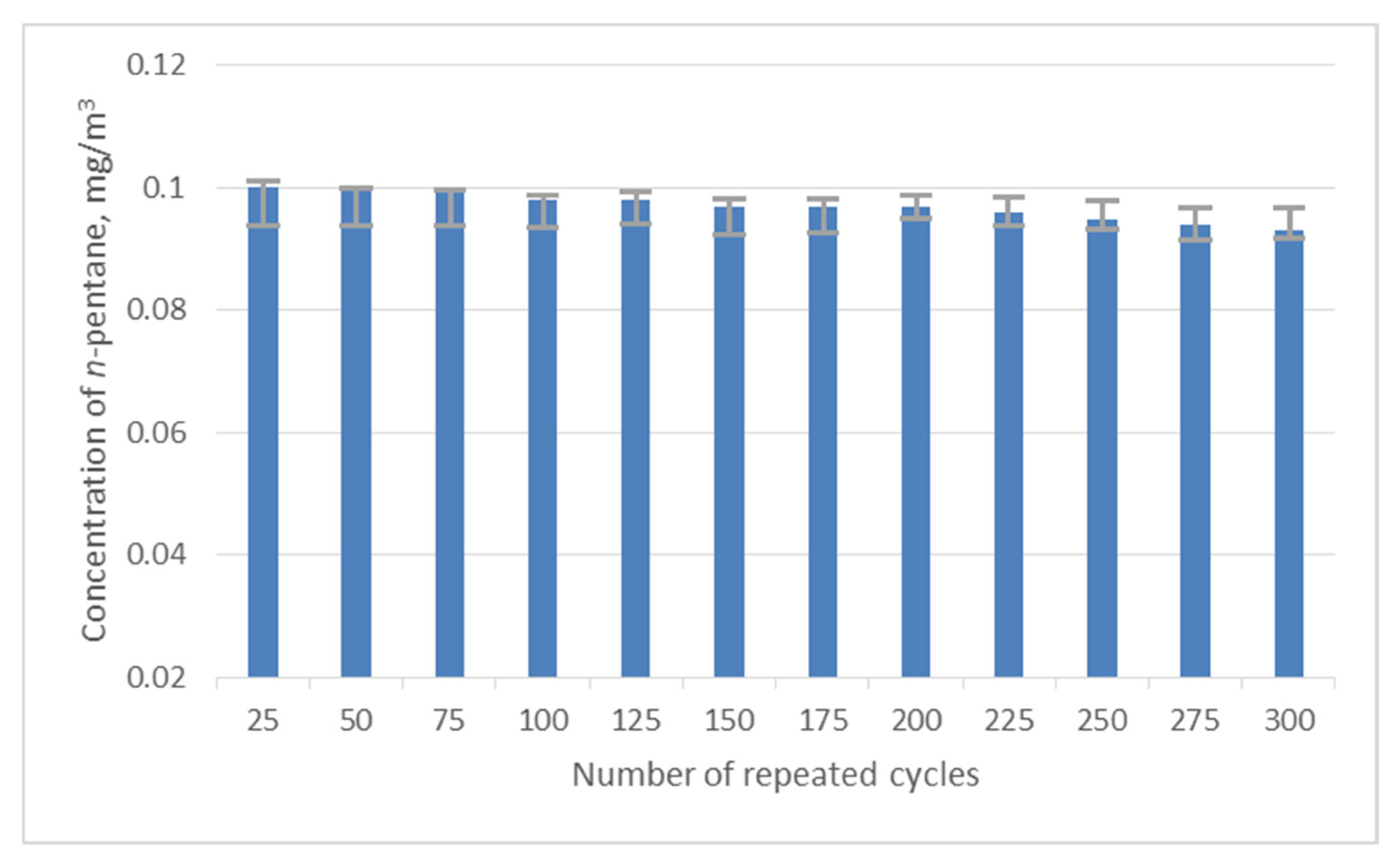
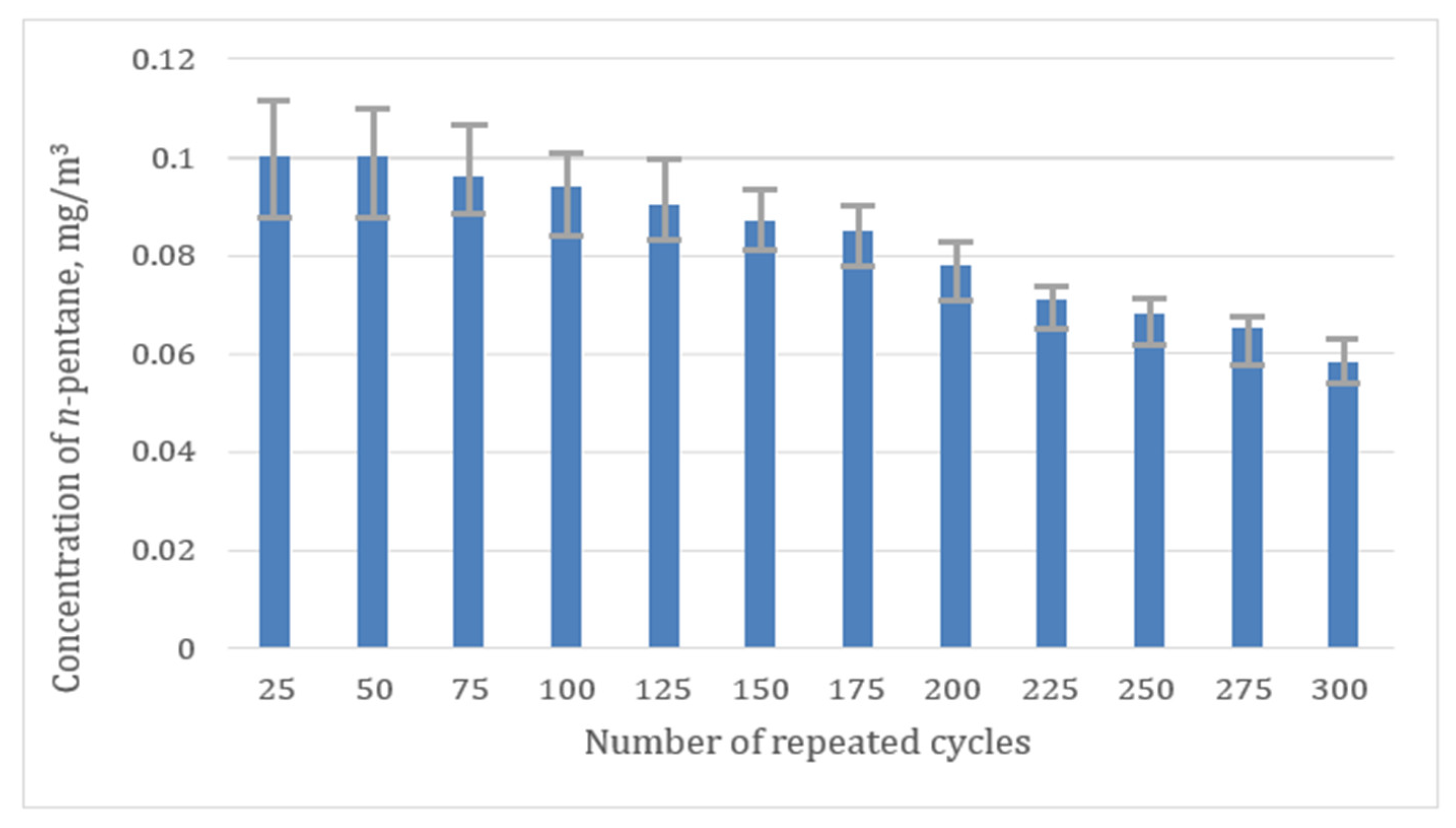
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cikach, F.S.; Dweik, R.A. Cardiovascular biomarkers in exhaled breath. Prog. Cardiovasc. Dis. 2012, 55, 34. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.W.; Akbar, S.; Bhansali, S.; Daniele, M.; Erb, P.D.; Johnson, K.; Liu, C.C.; Miller, D.; Oralkan, O.; Hesketh, P.J. Editors’ Choice—Critical Review—A critical review of solid-state gas sensors. J. Electrochem. Soc. 2020, 167, 037570. [Google Scholar] [CrossRef]
- Siegal, M.P.; Mowry, C.D.; Pfeifer, K.B.; Gallis, D.F.S. Detecting trihalomethanes using nanoporous-carbon coated surface-acoustic-wave sensors. J. Electrochem. Soc. 2015, 162, 114. [Google Scholar] [CrossRef]
- Lemoyne, M.; van Gossum, A.; Kurian, R.; Ostro, M.; Axler, J.; Jeejeebhoy, K.N. Breath pentane analysis as an index of lipid peroxidation: A functional test of vitamin E status. Am. J. Clin. Nutr. 1987, 47, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Sagnik, D.; Mrinal, P. Review—Non-invasive monitoring of human health by exhaled breath analysis: A comprehensive review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar]
- Horrillo, M.C.; Getino, J.; Arés, L.; Robia, J.I.; Gutiérrez, I.S.F.J. Measurements of VOCs with a semiconductor electronic nose. J. Electrochem. Soc. 1998, 145, 2486. [Google Scholar] [CrossRef]
- Masikini, M.; Chowdhury, M.; Nemraoui, O. Review—Metal oxides: Application in exhaled breath acetone chemiresistive sensors. J. Electrochem. Soc. 2020, 167, 037537. [Google Scholar] [CrossRef]
- Akita, T.; Otsuka, Y.; Hayase, M. Microfluidic device for in situ observation of bottom-up copper electrodeposition in a TSV-Like structure. J. Electrochem. Soc. 2020, 167, 162515. [Google Scholar] [CrossRef]
- Rodsud, S.; Limbut, W. A simple electrochemical sensor based on graphene nanoplatelets modified glassy carbon electrode (GrNPs/GCE) for highly sensitive detection of yohimbine (YOH). J. Electrochem. Soc. 2019, 166, B771. [Google Scholar] [CrossRef]
- Hatami, E.; Ashraf, N.; Zavar, M.H.A. On-chip electrochemical sensing of propylparaben as an endocrine disruptor model using a disposable gold platform modified with phosphomolybdate doped polypyrrole and nanodiamond. J. Electrochem. Soc. 2019, 166, B1379. [Google Scholar] [CrossRef]
- Zhang, F.; Co, A.C. Rapid product analysis for the electroreduction of CO2 on heterogeneous and homogeneous catalysts using a rotating ring detector. J. Electrochem. Soc. 2020, 167, 046517. [Google Scholar] [CrossRef]
- Platonov, I.A.; Kolesnichenko, I.N.; Lange, P.K. Chromatographic-desorption method for preparing calibration gas mixtures of volatile organic compounds. Meas. Tech. 2017, 59, 1330. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, M.J.; Pawliszyn, J. Solid-phase microextraction. a solvent-free alternative for sample preparation. Anal. Chem. 1994, 66, 844A. [Google Scholar] [CrossRef]
- Sidelnikov, N.V.; Nikolaeva, O.A.; Platonov, I.A.; Parmon, V.N. Gas chromatography of the future: Columns whose time has come. Adv. Chem. 2016, 85, 1033. [Google Scholar] [CrossRef]
- Kumar, S.; Pavelyev, V.; Tripathi, N.; Platonov, V.; Sharma, P.; Ahmad, R.; Mishra, P.; Khosla, A. Recent advances in the development of carbon nanotubes based flexible sensors. J. Electrochem. Soc. 2020, 167, 047506. [Google Scholar] [CrossRef]
- Lourenco, C.; Turner, C. Breath analysis in disease diagnosis: Methodological considerations and applications. Metabolites 2014, 4, 465. [Google Scholar] [CrossRef]
- Platonov, I.A.; Platonov, V.I.; Arutyunov, Y.I. Planar microchromatographic columns for gas chromatography. J. Anal. Chem. 2016, 71, 907–911. [Google Scholar] [CrossRef]
- Platonov, I.; Rodinkov, O.V.; Gorbacheva, A.R.; Moskvin, L.N.; Kolesnichenko, I.N. Methods and devices for the preparation of standard gas mixtures. J. Anal. Chem. 2018, 73, 109. [Google Scholar] [CrossRef]
- Platonov, I.; Kolesnichenko, I.N.; Lange, P.K. Using chromatography–desorption method of manufacturing gas mixtures for analytical instruments calibration. J. Phys. Conf. Ser. 2018, 1015, 1. [Google Scholar] [CrossRef]
- Slominska, M.; Konieczka, P.; Namiesnik, J. Standard gas mixtures—Indispensable reference materials in the analysis of gaseous media. Trends Anal. Chem. 2010, 29, 419. [Google Scholar] [CrossRef]
- Fijalo, C.; Dymerski, T.; Gebicki, J.; Namiesnik, J. Devices for the production of reference gas mixtures. Crit. Rev. Anal. Chem. 2016, 46, 361. [Google Scholar] [CrossRef] [PubMed]
- Platonov, I.A.; Platonov, V.I.; Ledyaev, M.E.; Voron, S.V. The use of a microthermal desorber for the concentration of trace amounts of hydrocarbons in the air. Sorbtsionnye Khromatograficheskie Protsessy 2021, 21, 805–811. [Google Scholar]
- Ellison, S.L.R.; Williams, A. Guide Quantifying Uncertainty in Analytical Measurement, 3rd ed.; Eurachem/Citac: London, UK, 2000; pp. 1–126. [Google Scholar]
- ISO/IEC GUIDE 98-3:2008; Uncertainty of Measurement—Part 3: Guide to the Expression of Uncertainty in Measurement. Declared 2008-10-01. International Organization for Standardization: Geneva, Switzerland, 2008; 130p.
- Kayutkina, N.I.; Platonov, I.A.; Bulanova, A.V. Sample pretreatment and chromatographic determination of benz[a]pyrene in wastewater. Russ. J. Appl. Chem. 2004, 77, 1379. [Google Scholar] [CrossRef]
- Skog, K.M.; Xiong, F.; Kawashima, H.; Doyle, E.; Soto, R.; Gentner, D.R. Compact, automated, inexpensive, and field-deployable vacuum-outlet gas chromatograph for trace-oncentration gas-phase organic compounds. Anal. Chem. 2019, 91, 1318. [Google Scholar] [CrossRef]
- Ibeas, I.L.; Cuevas, A.R.; Andrikopoulou, C.; Person, V.; Baldas, L.; Colin, S.; Calvé, S.L. Sub-ppb level detection of BTEX gaseous mixtures with a compact prototype GC equipped with a preconcentration unit. Micromachines 2019, 10, 187. [Google Scholar]
- Garg, A.; Akbar, M.; Vejerano, E.; Narayanan, S.; Nazhandali, L.; Marr, L.C.; Agah, M. Zebra GC: A mini gas chromatography system for trace-level determination of hazardous air pollutants. Sens. Actuators B Chem. 2015, 212, 145. [Google Scholar] [CrossRef]
| Sorbent Used in Microfluidic Preconcentrator | n-Pentane Concentration in the Model Gas Mixture, Ctrue ± UCtrue, mg/m3 | Experimentally Determined n-Pentane Concentration, CMV ± U, mg/m3 | Deviation from True * Value, mg/m3 |
|---|---|---|---|
| n-Octane ResSil-C | 0.100 ± 0.004 | 0.09 ± 0.02 | 0.01 |
| 1.00 ± 0.04 | 0.92 ± 0.20 | 0.08 | |
| 10.0 ± 0.4 | 9.8 ± 0.6 | 0.2 |
| Sorbent | Initial n-Pentane Concentration C0 ± UC0, mg/m3 | Final n-Pentane Concentration Cdes ± UCdes, mg/m3 | Concentrat ion Factor, Kc |
|---|---|---|---|
| Al2O3 | 0.100 ± 0.004 | 4.20 ± 0.4 | 42.0 |
| 1.00 ± 0.04 | 42.1 ± 0.3 | 42.1 | |
| 10.0 ± 0.4 | 416.6 ± 15.1 | 41.7 | |
| n-octane ResSil-C | 0.100 ± 0.004 | 4.90 ± 0.5 | 49.0 |
| 1.00 ± 0.04 | 45.8 ± 0.3 | 45.8 | |
| 10.0 ± 0.4 | 464.2 ± 18.2 | 46.4 |
| S. No. | Pre-Concentrator | Target Compounds | Analysis Time/Cycle (min) | LOD (ppb) | Detector | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Materials | Adsorbent | Heating system | ||||||
| 1 | ResSil-B | VOC | 35 | 0.1–1.6 (BTEX) | PID | [27] | ||
| 2 | Al | Basolite C300 | Cartridge | BTEX | 19 | 0.002–0.011 (BTEX) | PID | [28] |
| 3 | Si-glass | Tenax | Cr/N | VOC | <12 | ~25 (TEX) | TCD | [29] |
| 4 | Al | n-octane ResSil-C | Peltier elements | n-pentane | <5 | 0.1 (n-pentane) | PID | Our work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platonov, V.; Sharma, P.; Ledyaev, M.; Anikina, M.A.; Djuzhev, N.A.; Chinenkov, M.Y.; Tripathi, N.; Parveen, S.; Ahmad, R.; Pavelyev, V.; et al. Realization of Microfluidic Preconcentrator for N-Pentane Traces Impurities from the Gaseous Media. Materials 2022, 15, 8090. https://doi.org/10.3390/ma15228090
Platonov V, Sharma P, Ledyaev M, Anikina MA, Djuzhev NA, Chinenkov MY, Tripathi N, Parveen S, Ahmad R, Pavelyev V, et al. Realization of Microfluidic Preconcentrator for N-Pentane Traces Impurities from the Gaseous Media. Materials. 2022; 15(22):8090. https://doi.org/10.3390/ma15228090
Chicago/Turabian StylePlatonov, Vladimir, Prachi Sharma, Mikhail Ledyaev, Maria A. Anikina, Nikolay Alekseevich Djuzhev, Maksim Yuryevich Chinenkov, Nishant Tripathi, Sania Parveen, Rafiq Ahmad, Vladimir Pavelyev, and et al. 2022. "Realization of Microfluidic Preconcentrator for N-Pentane Traces Impurities from the Gaseous Media" Materials 15, no. 22: 8090. https://doi.org/10.3390/ma15228090
APA StylePlatonov, V., Sharma, P., Ledyaev, M., Anikina, M. A., Djuzhev, N. A., Chinenkov, M. Y., Tripathi, N., Parveen, S., Ahmad, R., Pavelyev, V., & Melaibari, A. A. (2022). Realization of Microfluidic Preconcentrator for N-Pentane Traces Impurities from the Gaseous Media. Materials, 15(22), 8090. https://doi.org/10.3390/ma15228090










