Extraction Optimization and Structural Characteristics of Chitosan from Cuttlefish (S. pharaonis sp.) Bone
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Cuttlebones (Sepia pharaonis sp.)
2.3. Extraction of Chitin and Conversion into Chitosan
2.4. Preparation of Chitosan and Response Surface Methodology Experimental Design
2.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.6. Preparation, Identification and Degree of Deacetylation (DD) of Chitosan by Proton Nuclear Magnetic Resonance (1H NMR)
2.7. Scanning Electron Microscopy
2.8. DPPH Free Radical Scavenging Ability Test
2.9. Reducing Power Assay
2.10. Free Radical Scavenging Ability of ABTS
2.11. Absorption Capabilities for Oils
3. Results and Discussion
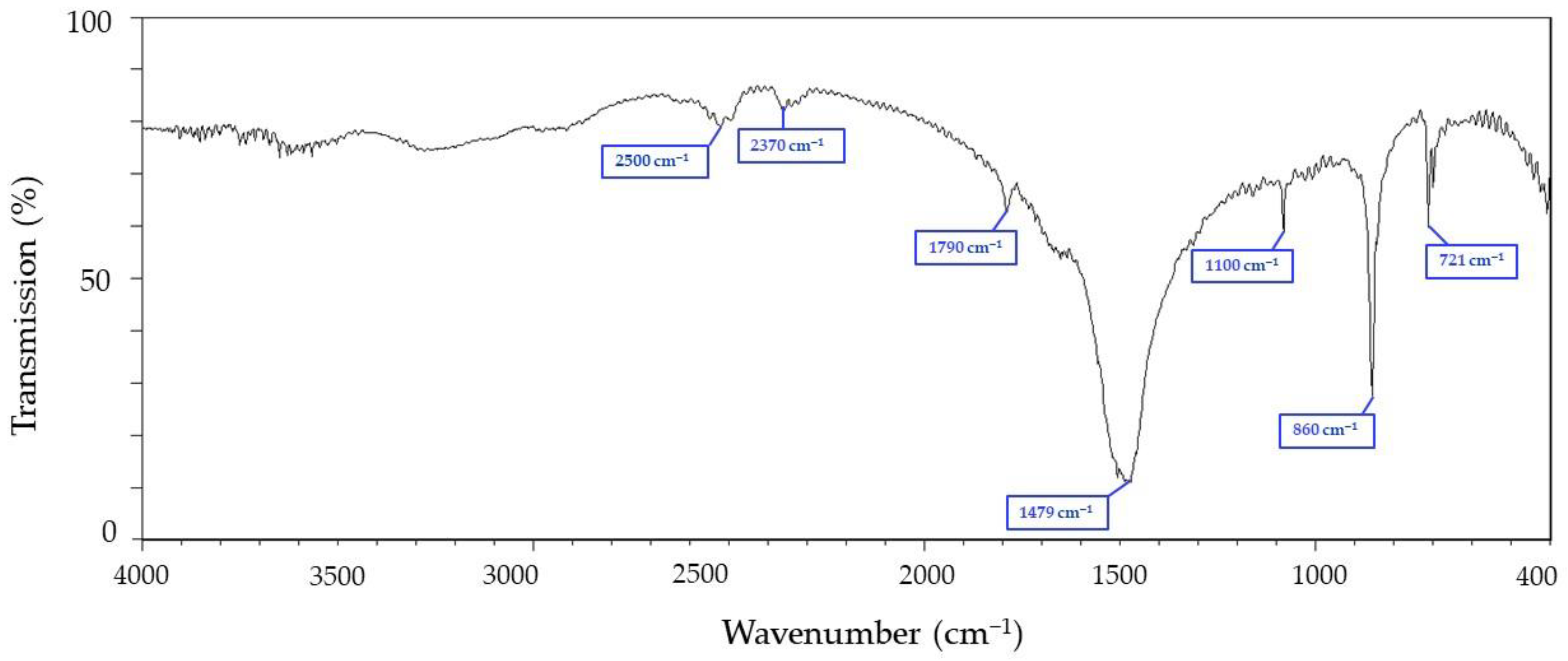
3.1. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis of Chitin from Cuttlefish Bones
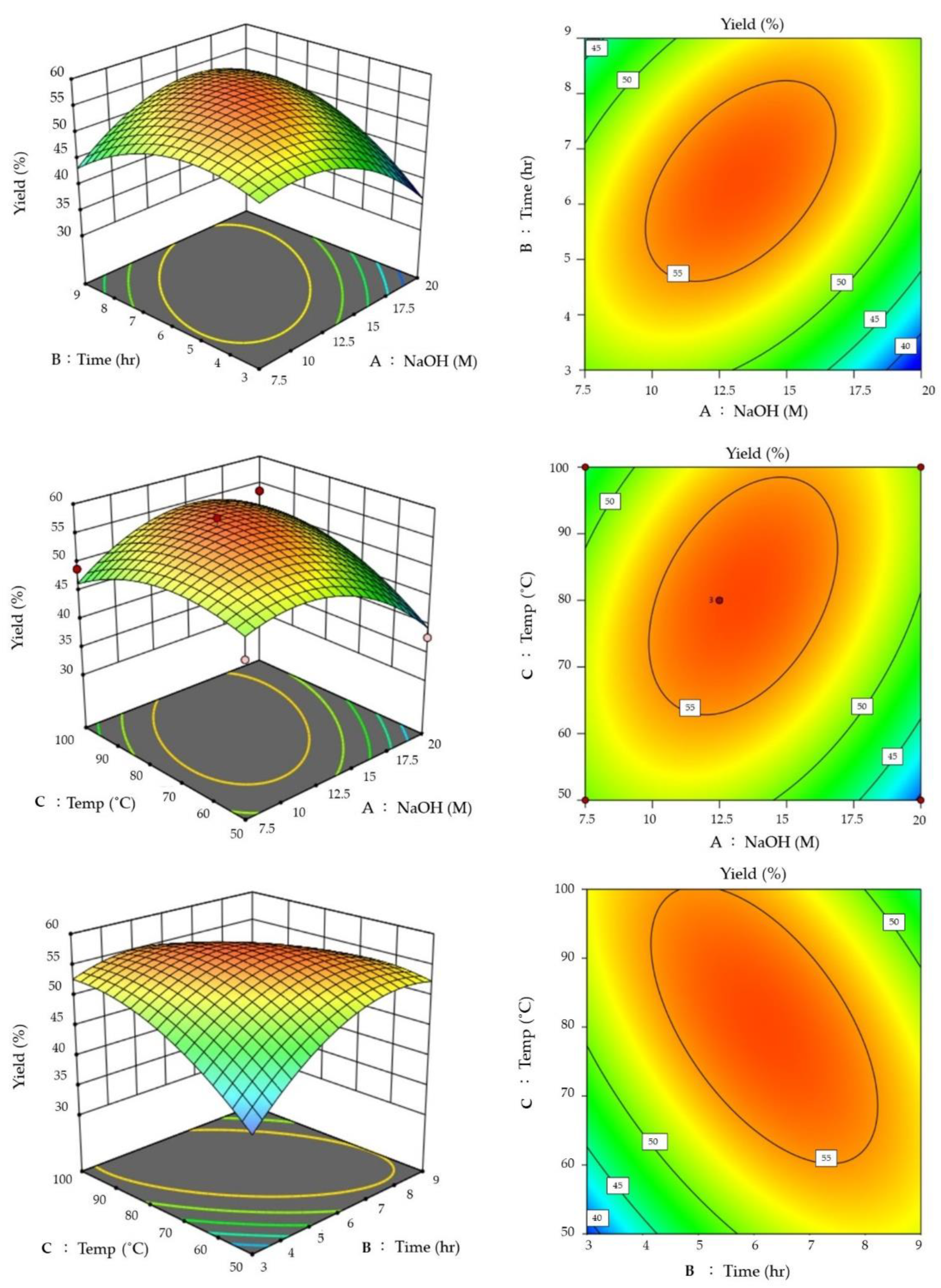
3.2. RSM-Optimized Extraction of Chitosan
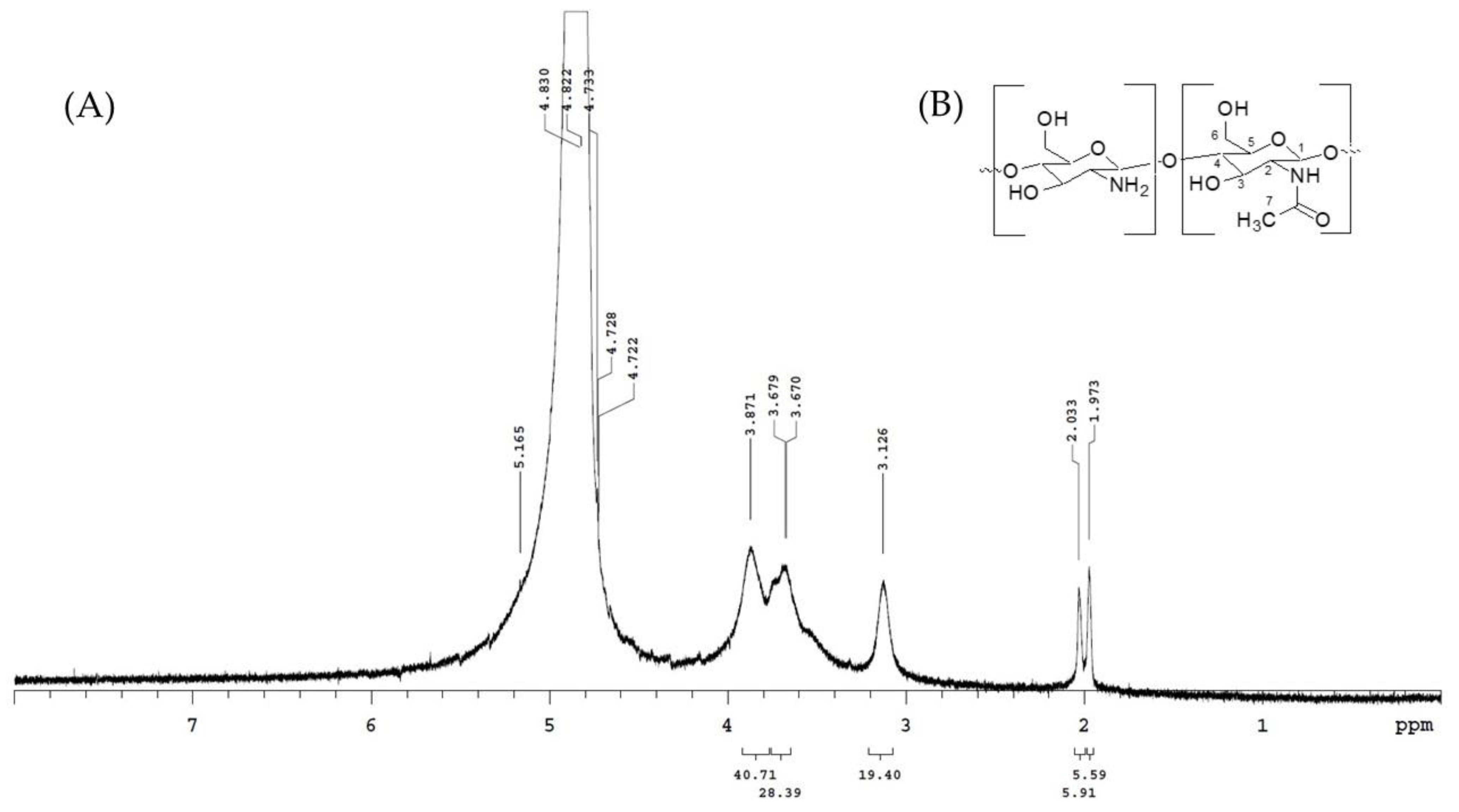
3.3. Chitosan Identification
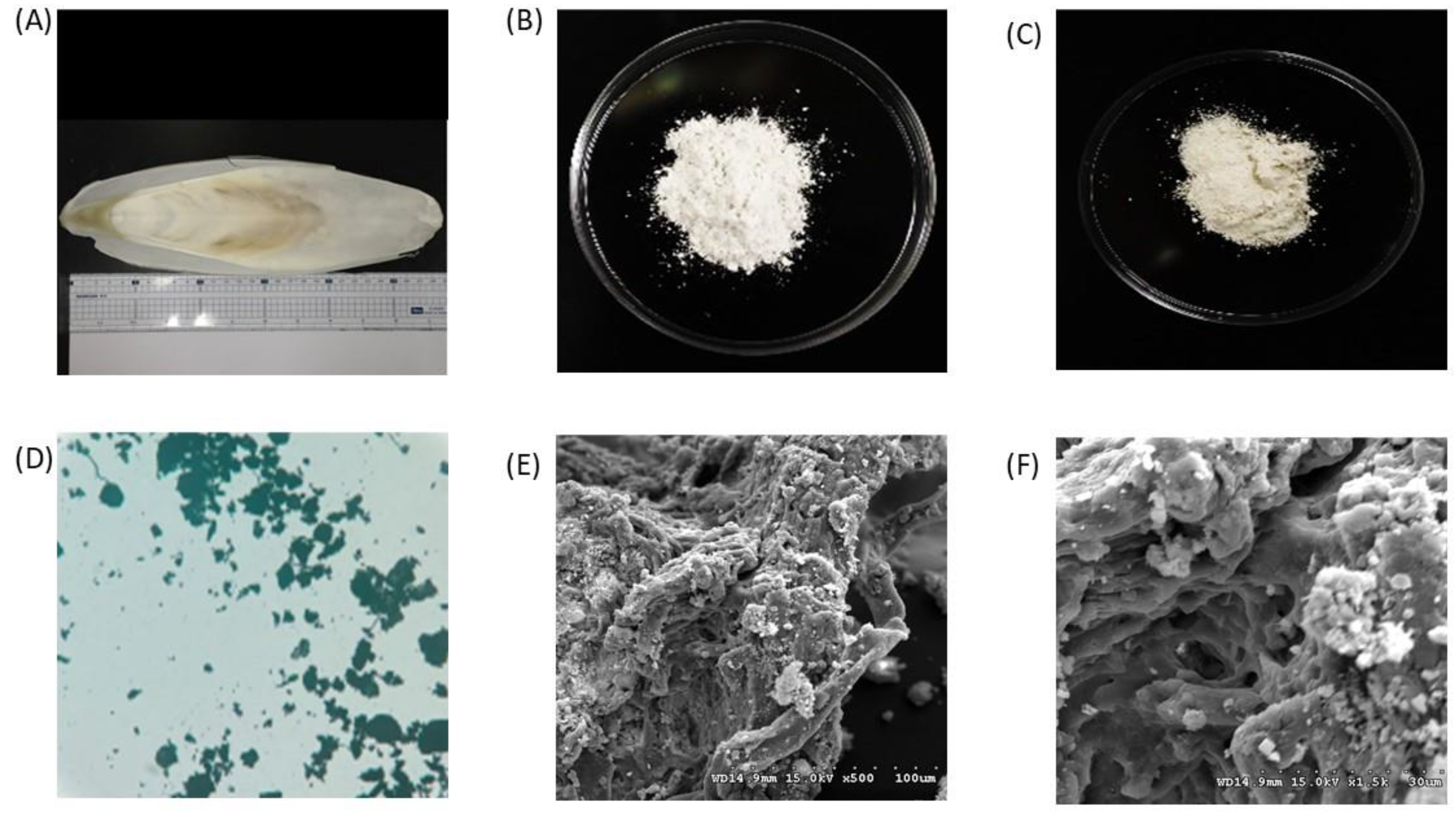
3.4. Scanning Electron Microscope (SEM) Analysis of Cuttlebone Chitosan
3.5. Antioxidative Ability Test and Oil Absorption Test of Chitosan
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dekkers, E.; Raghavan, S.; Kristinsson, H.G.H.G.; Marshall, M.R.M.R. Oxidative stability of mahi mahi red muscle dipped in tilapia protein hydrolysates. Food Chem. 2011, 124, 640–645. [Google Scholar] [CrossRef]
- Lin, L.; Regenstein, J.M.; Lv, S.; Lu, J.; Jiang, S. An overview of gelatin derived from aquatic animals: Properties and modification. Trends Food Sci. Technol. 2017, 68, 102–112. [Google Scholar] [CrossRef]
- Hsu, K.-C. Purification of antioxidative peptides prepared from enzymatic hydrolysates of tuna dark muscle by-product. Food Chem. 2010, 122, 42–48. [Google Scholar] [CrossRef]
- Wang, C.H.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Reclamation of Fishery Processing Waste: A mini review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Kuo, C.Y.; Kong, Z.L.; Lai, C.Y.; Chen, G.W.; Yang, A.J.; Lin, L.H.; Wang, M.F. Anti-Fatigue Effect of a Dietary Supplement from the Fermented By-Products of Taiwan Tilapia Aquatic Waste and Monostroma nitidum Oligosaccharide Complex. Nutrients 2021, 13, 1688. [Google Scholar] [CrossRef]
- Ospina-Alvarez, A.; de Juan, S.; Pita, P.; Ainsworth, G.B.; Matos, F.L.; Pita, C.; Villasante, S. A network analysis of global cephalopod trade. Sci. Rep. 2022, 12, 322. [Google Scholar] [CrossRef]
- Fisheries Agency, Council of Agriculture, Executive Yuan. Fisheries Statistical Yearbook: Quantity of Edible Aquatic Products, Fishery Production. Available online: https://www.fa.gov.tw/view.php?theme=FS_AR&subtheme=&id=20 (accessed on 3 August 2022).
- Aewsiri, T.; Benjakul, S.; Visessanguan, W. Functional properties of gelatin from cuttlefish (Sepia pharaonis) skin as affected by bleaching using hydrogen peroxide. Food Chem. 2009, 115, 243–249. [Google Scholar] [CrossRef]
- Li, F.; Luo, P.; Liu, H. A potential adjuvant agent of chemotherapy: Sepia ink polysaccharides. Mar. Drugs 2018, 16, 106. [Google Scholar] [CrossRef]
- Birchall, J.D.; Thomas, N.L. On the architecture and function of cuttlefish bone. J. Mater. Sci. 1983, 18, 2081–2086. [Google Scholar] [CrossRef]
- Derby, C.D. Cephalopod ink: Production, chemistry, functions and applications. Mar. Drugs 2014, 12, 2700. [Google Scholar] [CrossRef]
- Rolandi, M.; Rolandi, R. Self-assembled chitin nanofibers and applications. Adv. Colloid Interface Sci. 2014, 207, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.B.; He, Y.S.; Li, S.L.; Wang, Y.Z. Chitin whiskers: An overview. Biomacromolecules 2012, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.H.; Williams, P.A.; Tverezovskaya, O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll. 2013, 31, 166–171. [Google Scholar] [CrossRef]
- Hunt, S.; El Sherief, A. A periodic structure in the “pen” chitin of the squid Loligo vulgaris. Tissue Cell 1990, 22, 191–197. [Google Scholar] [CrossRef]
- Jung, H.-S.; Kim, M.H.; Shin, J.Y.; Park, S.R.; Jung, J.-Y.; Park, W.H. Electrospinning and wound healing activity of β-chitin extracted from cuttlefish bone. Carbohydr. Polym. 2018, 193, 205–211. [Google Scholar] [CrossRef]
- Wu, J.; Lin, H.; Meredith, J.C. Poly(ethylene oxide) bionanocomposites reinforced with chitin nanofiber networks. Polymer 2016, 84, 267–274. [Google Scholar] [CrossRef]
- Illanes, A.; Ruiz, A.; Zúñiga, M.E.; Aguirre, C.; O’Reilly, S.; Curotto, E. Immobilization of lactase for the continuous hydrolysis of whey permaete. Bioprocess Eng. 1990, 5, 257–262. [Google Scholar] [CrossRef]
- Shushizadeh, M.R.; Pour, E.M.; Zare, A.; Lashkari, Z. Persian gulf β-chitin extraction from Sepia pharaonis sp. cuttlebone and preparation of its derivatives. Bioact. Carbohydr. Diet. Fibre 2015, 6, 133–142. [Google Scholar] [CrossRef]
- Chassary, P.; Vincent, T.; Sanchez Marcano, J.; MacAskie, L.E.; Guibal, E. Palladium and platinum recovery from bicomponent mixtures using chitosan derivatives. Hydrometallurgy 2005, 76, 131–147. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Sannan, T.; Kurita, K.; Iwakura, Y. Studies on Chitin, 1. Solubility Change by Alkaline Treatment and Film Casting. Available online: https://ur.booksc.me/book/1767281/21b32e (accessed on 23 June 2022).
- Subhapradha, N.; Ramasamy, P.; Shanmugam, V.; Madeswaran, P.; Srinivasan, A.; Shanmugam, A. Physicochemical characterisation of β-chitosan from Sepioteuthis lessoniana gladius. Food Chem. 2013, 141, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, P.; Subhapradha, N.; Shanmugam, V.; Shanmugam, A. Extraction, characterization and antioxidant property of chitosan from cuttlebone Sepia kobiensis (Hoyle 1885). Int. J. Biol. Macromol. 2014, 64, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.N.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and eco-friendly approaches for the extraction of chitin and chitosan: A review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Dhillon, G.S. Recent trends in biological extraction of chitin from marine shell wastes: A review. Crit. Rev. Biotechnol. 2015, 35, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, R.D. Progress in bioextraction processes of chitin from crustacean biowastes. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 545–554. [Google Scholar] [CrossRef]
- Takiguchi, Y. Preparation of chiotosan and partially deacetylated chitin. In Chitin, Chitosanjikken Manual; Otakara, A., Yabuki, M., Eds.; Gihodou Shupan Kabushki Kasisha: Tokyo, Japan, 1991; pp. 9–17. [Google Scholar]
- Sahu, A.; Goswami, P.; Bora, U. Microwave mediated rapid synthesis of chitosan. J. Mater. Sci. Mater. Med. 2009, 20, 171–175. [Google Scholar] [CrossRef]
- Arumugam, G.K.S.; Sharma, D.; Balakrishnan, R.M.; Ettiyappan, J.B.P. Extraction, optimization and characterization of collagen from sole fish skin. Sustain. Chem. Pharm. 2018, 9, 19–26. [Google Scholar] [CrossRef]
- Jridi, M.; Nasri, R.; Marzougui, Z.; Abdelhedi, O.; Hamdi, M.; Nasri, M. Characterization and assessment of antioxidant and antibacterial activities of sulfated polysaccharides extracted from cuttlefish skin and muscle. Int. J. Biol. Macromol. 2019, 123, 1221–1228. [Google Scholar] [CrossRef]
- Novoa-Carballal, R.; Fernandez-Megia, E.; Riguera, R. Dynamics of chitosan by 1H NMR relaxation. Biomacromolecules 2010, 11, 2079–2086. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, Y.-W.; Hou, C.-Y. Antioxidant and antibacterial activity of seven predominant terpenoids. Int. J. Food Prop. 2019, 22, 230–238. [Google Scholar] [CrossRef]
- Li, Z.; Shao, L.; Hu, W.; Zheng, T.; Lu, L.; Cao, Y.; Chen, Y. Excellent reusable chitosan/cellulose aerogel as an oil and organic solvent absorbent. Carbohydr. Polym. 2018, 191, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Sannan, T.; Kurita, K.; Ogura, K.; Iwakura, Y. Studies on chitin: 7. I.r. spectroscopic determination of degree of deacetylation. Polymer 1978, 19, 458–459. [Google Scholar] [CrossRef]
- Beil, S.; Schamberger, A.; Naumann, W.; MacHill, S.; Van Pée, K.H. Determination of the degree of N-acetylation (DA) of chitin and chitosan in the presence of water by first derivative ATR FTIR spectroscopy. Carbohydr. Polym. 2012, 87, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Borsagli, F.G.L.M.; Borsagli, A. Chemically Modified Chitosan Bio-Sorbents for the Competitive Complexation of Heavy Metals Ions: A Potential Model for the Treatment of Wastewaters and Industrial Spills. J. Polym. Environ. 2019, 27, 1542–1556. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitosan composites with inorganics, morphogenetic proteins and stem cells, for bone regeneration. Carbohydr. Polym. 2011, 83, 1433–1445. [Google Scholar] [CrossRef]
- Franca, E.F. Biomolecular Characterization of Biopolymers in Solution Using Computer Simulation. Ph.D. Thesis, Center for Science and Technology of Federal University of Sao Carlos, Sao Carlos, Brazil, 2009. [Google Scholar]
- Delgado, A.V.; González-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electrokinetic phenomena. J. Colloid Interface Sci. 2007, 309, 194–224. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Prodpran, T. Ultrasound-Assisted Extraction of Chitosan from Squid Pen: Molecular Characterization and Fat Binding Capacity. J. Food Sci. 2019, 84, 224–234. [Google Scholar] [CrossRef]
- Ben Seghir, B.; Benhamza, M.H. Preparation, optimization and characterization of chitosan polymer from shrimp shells. J. Food Meas. Charact. 2017, 11, 1137–1147. [Google Scholar] [CrossRef]
- Duarte, M.L.; Ferreira, M.C.; Marvão, M.R.; Rocha, J. An optimised method to determine the degree of acetylation of chitin and chitosan by FTIR spectroscopy. Int. J. Biol. Macromol. 2002, 31, 1–8. [Google Scholar] [CrossRef]
- Varma, R.; Vasudevan, S. Extraction, Characterization, and Antimicrobial Activity of Chitosan from Horse Mussel Modiolus modiolus. ACS Omega 2020, 5, 20224–20230. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Xing, R.; Liu, S.; Yu, H.; Zhang, Q.; Li, Z.; Li, P. Preparation of low-molecular-weight and high-sulfate-content chitosans under microwave radiation and their potential antioxidant activity in vitro. Carbohydr. Res. 2004, 339, 2515–2519. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, H.; Dong, F.; Xue, Q.; Wang, G.; Qin, S.; Guo, Z. The influence of the cation of quaternized chitosans on antioxidant activity. Carbohydr. Polym. 2009, 78, 439–443. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Yen, M.T.; Mau, J.L. Physico-chemical characterization of fungal chitosan from shiitake stipes. LWT Food Sci. Technol. 2007, 40, 472–479. [Google Scholar] [CrossRef]
- Kim, K.W.; Thomas, R.L. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- Chuang, C.M.; Wang, H.E.; Chang, C.H.; Peng, C.C.; Ker, Y.B.; Lai, J.E.; Chen, K.C.; Peng, R.Y. Sacchachitin, a novel chitin-polysaccharide conjugate macromolecule present in Ganoderma lucidum: Purification, composition, and properties. Pharm. Biol. 2013, 51, 84–95. [Google Scholar] [CrossRef][Green Version]
- XiaoPing, C.; Yan, C.; ShuiBing, L.; YouGuo, C.; JianYun, L.; LanPing, L. Free radical scavenging of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes and immunity activities in cervical carcinoma rats. Carbohydr. Polym. 2009, 77, 389–393. [Google Scholar] [CrossRef]
- Avelelas, F.; Horta, A.; Pinto, L.F.V.; Marques, S.C.; Nunes, P.M.; Pedrosa, R.; Leandro, S.M. Antifungal and Antioxidant Properties of Chitosan Polymers Obtained from Nontraditional Polybius henslowii Sources. Mar. Drugs 2019, 17, 239. [Google Scholar] [CrossRef]
- Kuravappullam Vedaiyan, R.; Thyriyalakshmi, P. Utilization of biodegradable chitosan-derived sponges as oil retainers. Environ. Sci. Pollut. Res. 2020, 27, 28123–28131. [Google Scholar] [CrossRef]
- da Silva Alves, D.C.; Healy, B.; Pinto, L.A.d.A.; Cadaval, T.R.S.; Breslin, C.B. Recent Developments in Chitosan-Based Adsorbents for the Removal of Pollutants from Aqueous Environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef]
- Omer, A.M.; Abd El-Monaem, E.M.; Abd El-Latif, M.M.; El-Subruiti, G.M.; Eltaweil, A.S. Facile fabrication of novel magnetic ZIF-67 MOF@aminated chitosan composite beads for the adsorptive removal of Cr(VI) from aqueous solutions. Carbohydr. Polym. 2021, 265, 118084. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, R.E.; Ali, A.A.E.W.; Abo-Zaid, G.; Omer, A.M.; Tamer, T.M.; Ammar, Y.; Mohy Eldin, M.S. Optimization using response surface methodology for the sorptive removal of crude oil spills using a low-cost chitosan-poly(Butyl acrylate) grafted copolymer. Desalin. Water Treat. 2021, 224, 343–353. [Google Scholar] [CrossRef]
- Sokker, H.H.; El-Sawy, N.M.; Hassan, M.A.; El-Anadouli, B.E. Adsorption of crude oil from aqueous solution by hydrogel of chitosan based polyacrylamide prepared by radiation induced graft polymerization. J. Hazard. Mater. 2011, 190, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Farias, P.V.S.; Aragão, D.C.; Farias, M.V.; Correia, L.M.; Carvalho, T.V.; Aguiar, J.E.; Vieira, R.S. Natural and Cross-Linked Chitosan Spheres as Adsorbents for Diesel Oil Removal. Adsorpt. Sci. Technol. 2015, 33, 783–792. [Google Scholar] [CrossRef]
- Sugano, M.; Watanabe, S.; Kishi, A.; Izume, M.; Ohtakara, A. Hypocholesterolemic action of chitosans with different viscosity in rats. Lipids 2006, 23, 187–191. [Google Scholar] [CrossRef]
- Ikeda, I.; Sugano, M.; Yoshida, K.; Sasaki, E.; Iwamoto, Y.; Hatano, K. Effects of Chitosan Hydrolysates on Lipid Absorption and on Serum and Liver Lipid Concentration in Rats. J. Agric. Food Chem. 1993, 41, 431–435. [Google Scholar] [CrossRef]
- Medeiros Borsagli, F.G.L.; Mansur, A.A.P.; Mansur, H.S. Synthesis and Characterization of CMC for Potential Application as Adsorbent in Water Treatment. Mater. Sci. Forum 2016, 869, 750–755. [Google Scholar] [CrossRef]
- Yang, J.; Chen, X.; Zhang, J.; Wang, Y.; Wen, H.; Xie, J. Role of chitosan-based hydrogels in pollutants adsorption and freshwater harvesting: A critical review. Int. J. Biol. Macromol. 2021, 189, 53–64. [Google Scholar] [CrossRef]


| Independent Variables | Symbols | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| NaOH | A | 7.5M | 12.5M | 20M |
| Time | B | 3 h | 6 h | 9 h |
| Temp | C | 50 °C | 80 °C | 100 °C |
| Std | Run | NaOH (M) | Time (h) | Temp (°C) | Yield (%) |
|---|---|---|---|---|---|
| 15 | 1 | 12.5 | 6 | 80 | 57.96 |
| 8 | 2 | 20 | 6 | 100 | 53.55 |
| 2 | 3 | 20 | 3 | 80 | 37.79 |
| 3 | 4 | 7.5 | 9 | 80 | 42.58 |
| 13 | 5 | 12.5 | 6 | 80 | 55.66 |
| 10 | 6 | 12.5 | 9 | 50 | 54.55 |
| 9 | 7 | 12.5 | 3 | 50 | 43.50 |
| 5 | 8 | 7.5 | 6 | 50 | 46.45 |
| 7 | 9 | 7.5 | 6 | 100 | 48.94 |
| 1 | 10 | 7.5 | 3 | 80 | 50.90 |
| 4 | 11 | 20 | 9 | 80 | 50.38 |
| 11 | 12 | 12.5 | 3 | 100 | 50.85 |
| 12 | 13 | 12.5 | 9 | 100 | 41.10 |
| 6 | 14 | 20 | 6 | 50 | 37.79 |
| 14 | 15 | 12.5 | 6 | 80 | 55.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazeena, S.H.; Hou, C.-Y.; Zeng, J.-H.; Li, B.-H.; Lin, T.-C.; Liu, C.-S.; Chang, C.-I.; Hsieh, S.-L.; Shih, M.-K. Extraction Optimization and Structural Characteristics of Chitosan from Cuttlefish (S. pharaonis sp.) Bone. Materials 2022, 15, 7969. https://doi.org/10.3390/ma15227969
Hazeena SH, Hou C-Y, Zeng J-H, Li B-H, Lin T-C, Liu C-S, Chang C-I, Hsieh S-L, Shih M-K. Extraction Optimization and Structural Characteristics of Chitosan from Cuttlefish (S. pharaonis sp.) Bone. Materials. 2022; 15(22):7969. https://doi.org/10.3390/ma15227969
Chicago/Turabian StyleHazeena, Sulfath Hakkim, Chih-Yao Hou, Jing-Huei Zeng, Bo-Heng Li, Tzu-Chih Lin, Cai-Sian Liu, Chi-I Chang, Shu-Ling Hsieh, and Ming-Kuei Shih. 2022. "Extraction Optimization and Structural Characteristics of Chitosan from Cuttlefish (S. pharaonis sp.) Bone" Materials 15, no. 22: 7969. https://doi.org/10.3390/ma15227969
APA StyleHazeena, S. H., Hou, C.-Y., Zeng, J.-H., Li, B.-H., Lin, T.-C., Liu, C.-S., Chang, C.-I., Hsieh, S.-L., & Shih, M.-K. (2022). Extraction Optimization and Structural Characteristics of Chitosan from Cuttlefish (S. pharaonis sp.) Bone. Materials, 15(22), 7969. https://doi.org/10.3390/ma15227969









