The Effect of Changes in the Separation Process for the Performance of Recycled Cement Powder: A Comparison with a Previous Study for Radioactive Waste Immobilization
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Concrete Waste
2.2. Recycled Cement Powder
2.3. Characterization of Recycled Cement Powder
2.4. Preparation of Wasteform Specimen Using Liquid Waste Samples
2.5. Hydration Study
2.6. Compressive Strength
2.7. Thermal Cycling
2.8. Leachability
3. Results
3.1. Characteristics of Recycled Cement Powder
3.1.1. Chemical Composition
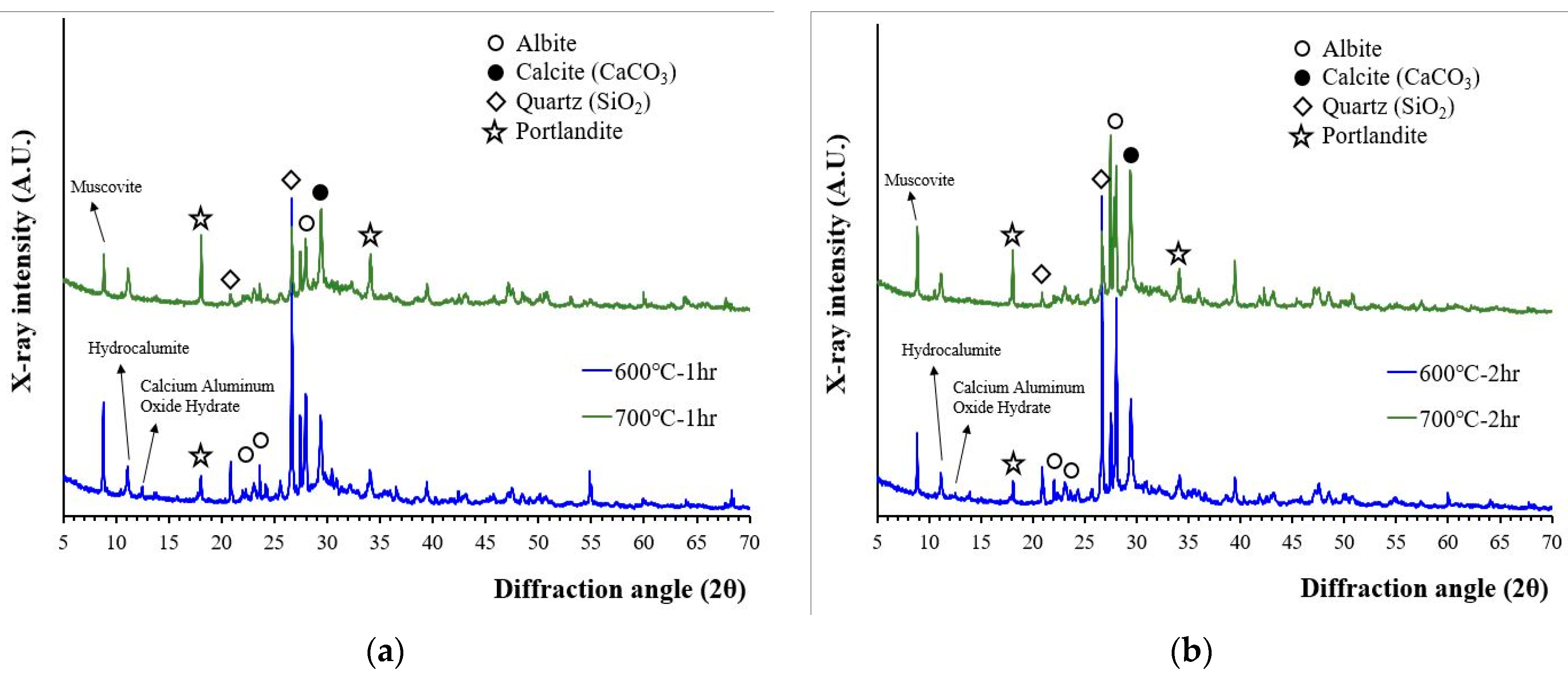
3.1.2. Mineralogy
3.1.3. Surface Area
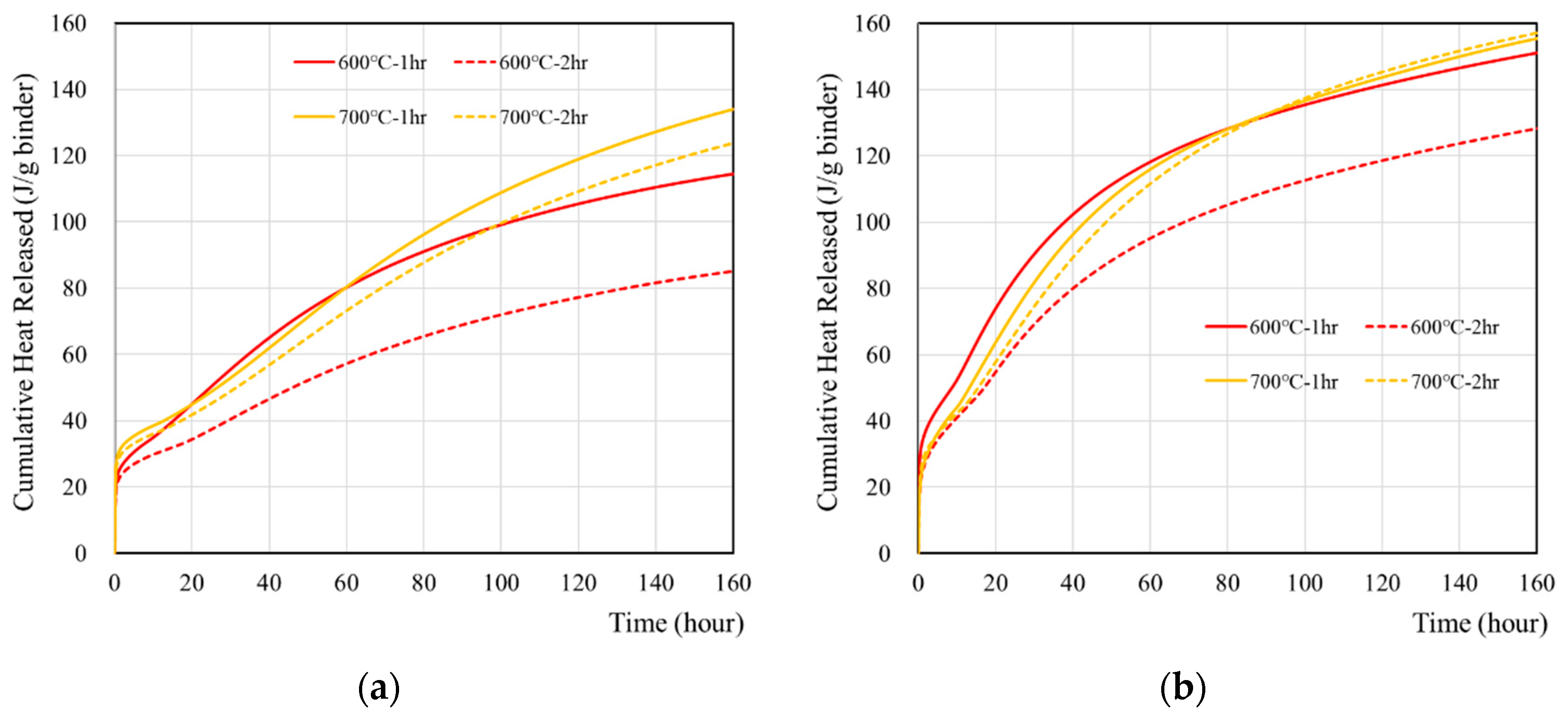
3.2. Reaction of Recycled Cement Paste
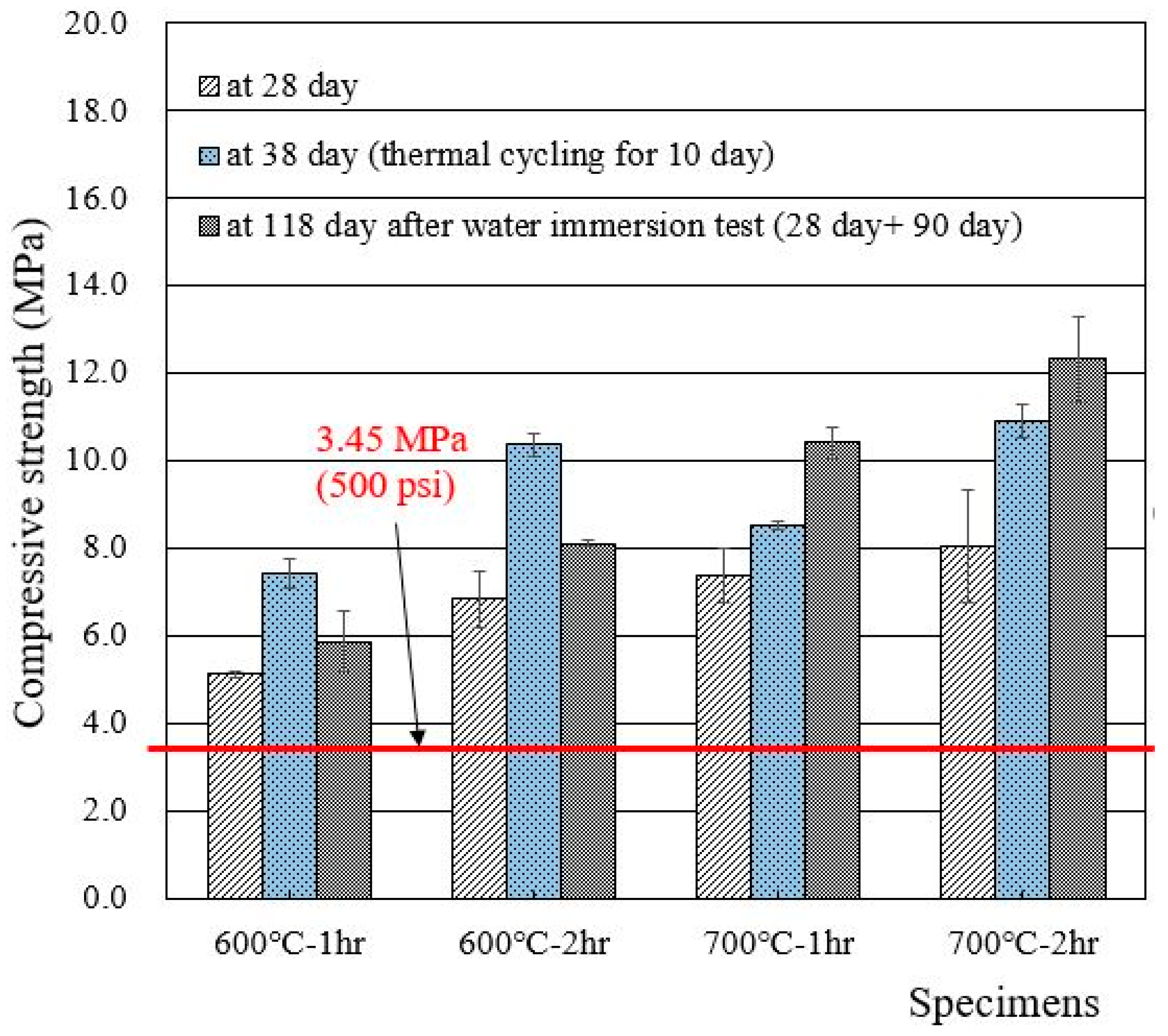
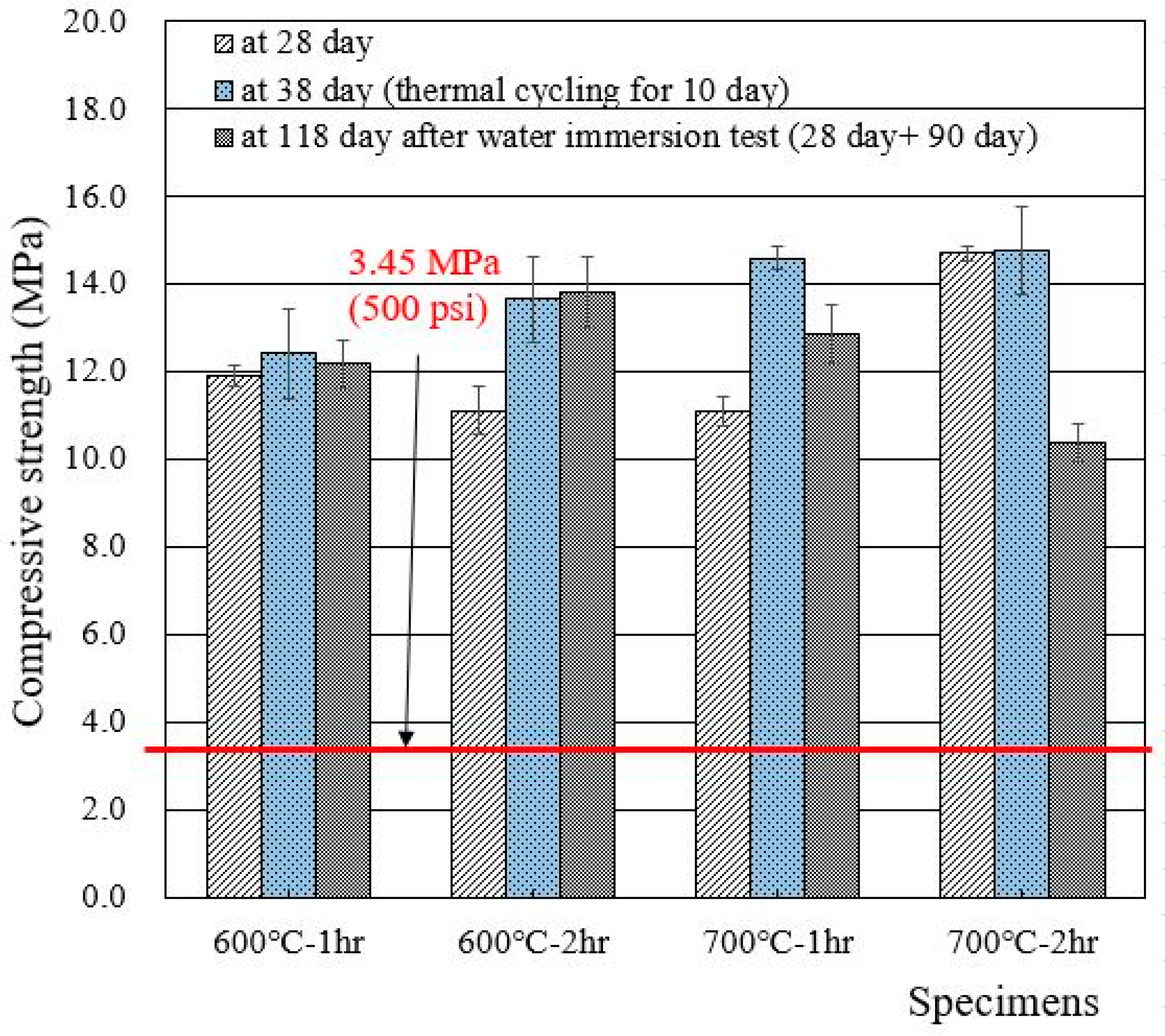
3.3. Compressive Strengths
3.4. Leachability
4. Discussion
5. Conclusions
- The recycled cement powder used in this work (experienced heat treatment after separation process) met the wasteform acceptance criteria. The effects caused by heat treatments were also observed, and heat treatment at 600 °C was found to be a better option than heat treatment at 700 °C.
- Recycled cement powder used in this work required less water demand for mixing compared to the recycled cement powder used in the previous work (experienced heat treatment prior to the separation process). This was mainly related to the reduction in the surface area due to a higher inclusion of crystalline aggregate powder.
- A reduction in surface area enabled a lower solution to binder ratio (S/B 0.5) of wasteform, and, as a result, a relatively higher compressive strength was obtained compared to the case observed from previous work which used a higher solution to binder ratio (0.7).
- A reduction in surface area seemed to be a reason for lower final leachability indices of Cs from the wasteform.
- Application of heat treatment after complete separation of recycled cement powder can be an applicable alternative for the immobilization of liquid waste. However, when using such an approach, the amount of aggregate particle in recycled cement powder should always be accurately monitored because it will eventually reduce the compressive strength of the wasteform.
- Separation of recycled cement powder from concrete and its utilization as a solidifying agent for immobilization of other radioactive waste is a way to achieve “zero” waste production (in an ideal situation) during the decommissioning of concrete in a nuclear power plant.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sasaki, T.; Sone, T.; Koyama, H.; Yamaguchi, H. Steam-assisted pyrolysis system for decontamination and volume reduction of radioactive organic waste. J. Nucl. Sci. Technol. 2009, 46, 232–238. [Google Scholar] [CrossRef]
- Sawada, K.; Uruga, K.; Koyama, T.; Shimada, T.; Mori, Y.; Enokida, Y.; Yamamoto, I. Stoichiometric relation for extraction of uranium from UO2 powder using TBP complex with HNO3 and H2O in supercritical CO2. J. Nucl. Sci. Technol. 2005, 42, 301–304. [Google Scholar] [CrossRef]
- Cheon, J.H.; Lee, S.C.; Kim, C.L.; Park, H.G. Feasibility Study on Recycling of Concrete Waste form NPP Decommissioning Through Literature Review. J. Korean Recycl. Constr. Resour. Inst. 2018, 6, 115–122. [Google Scholar]
- O’Sullivan, P.; Nokhamzon, J.G.; Cantrel, E. Decontamination and dismantling of radioactive concrete structures. NEA News 2010, 25, 27–29. [Google Scholar]
- Deju, R.; Dragusin, M.; Robu, I.; Mazilu, C.; Tuca, C. Review on radioactive concrete recycling methods. Rom. Rep. Phys. 2013, 65, 1485–1504. [Google Scholar]
- Department of Nuclear Environment. Code of Determination of Maintenance cost for Radioactive Waste and Spent Nuclear Fuel; Department of Nuclear Environment in Korean Ministry of Trade, Industry, and Energy: Sejong, Korean, 2022; Volume 1. [Google Scholar]
- Yarar, Y. Activation characteristics of concrete shields containing colemanite. J. Nucl. Mater. 1996, 233–237, 1511–1515. [Google Scholar] [CrossRef]
- Alhajali, S.; Kharita, M.H.; Naoom, B.; Yousef, S.; AlNassar, M. Estimation of the activation of local reactor shielding concretes. Prog. Nucl. Energy 2009, 51, 374–377. [Google Scholar] [CrossRef]
- Lee, K.Y.; Oh, M.K.; Kim, J.M.; Lee, E.H.; Kim, I.S.; Kim, K.W.; Chung, D.Y.; Seo, B.K. Trends in Technology Development for the Treatment of Radioactive Concrete Waste. J. Nucl. Fuel Cycle Waste Technol. 2018, 16, 93–105. [Google Scholar] [CrossRef]
- Pantelias, M.; Volmert, B. Activation neutronics for a swiss pressurized water reactor. J. Nucl. Technol. Tech. Pap. 2017, 192, 278–285. [Google Scholar] [CrossRef]
- Koga, Y.; Inoue, T.; Tateyashiki, H.; Sukekiyo, M.; Okamotom, M.; Asano, T. A process for separating aggregate from concrete waste surging the dismantlement of nuclear power plant. Radioact. Waste Res. 1997, 3, 17–25. [Google Scholar]
- Min, B.Y.; Park, J.W.; Choi, W.K.; Lee, K.W. Separation of Radionuclide from Dismantled Concrete Wates. J. Nucl. Fuel Cycle Waste Technol. 2009, 7, 79–86. [Google Scholar]
- COX, E.J.; GARDE, R. Decontamination of concrete surfaces at the Los Alamos Scientific Laboratory. In Proceedings of the DOE Concrete Decontamination Workshop, Battelle Conference Center, Seattle, WA, USA, 28–29 May 1980; The Los Alamos Scientific Laboratory (No. LA-UR-80-1460; CONF-800542-3), 1980. pp. 109–123. [Google Scholar]
- Kim, H.J.; Lee, J.Y.; Kim, K.M.; Kim, Y.S. A Study on the Review of Concrete Waste Generated by Decommissioning of Nuclear Power Plant. In Proceedings of the Transaction of the Korean Nuclear Society Virtual Spring Meeting, Virtual, 9–10 July 2020. [Google Scholar]
- Ham, S.S.; Hong, S.K.; Nam, S.S.; Kim, W.S.; Um, W.Y. Decontamination of concrete waste from nuclear power plan decommissioning in South Korea. Ann. Nucl. Energy 2020, 149, 107795. [Google Scholar]
- Kim, J.H.; Seo, E.A.; Kim, D.G.; Chung, C.W. Utilization of recycled cement powder as a solidifying agent for radioactive waste immobilization. Constr. Build. Mater. 2021, 289, 123126. [Google Scholar] [CrossRef]
- Wasim, M.; Abadel, A.; Bakar, B.H.A.; Alshaikh, I.M. Future Directions for the Application of Zero Carbon Concrete in Civil Engineering-A Review. Case Stud. Constr. Mater. 2022, 17, e01318. [Google Scholar] [CrossRef]
- Shui, Z.; Xuan, D.; Wan, H.; Cao, B. Rehydration reactivity of recycled mortar from concrete waste experienced to thermal treatment. Constr. Build. Mater. 2008, 22, 1723–1729. [Google Scholar] [CrossRef]
- Wang, J.; Mu, M.; Liu, Y. Recycled cement. Constr. Build. Mater. 2018, 190, 1124–1132. [Google Scholar] [CrossRef]
- Gastaldi, D.; Canonico, F.; Capelli, L.; Buzzi, L.; Boccaleri, E.; Irico, S. An investigation on the recycling of hydrated cement from concrete demolition waste. Cem. Concr. Compos. 2015, 61, 29–35. [Google Scholar] [CrossRef]
- Yoon, E.S.; Paek, Y.L.; Lim, J.H.; Chung, Y.S.; Choi, K.R. Long-Term Performance of Safety Related Concrete Structures in Nuclear Power Plants. Proc. Korea Inst. Conf. 2006, 18, 237–240. [Google Scholar]
- Cho, M.S.; Song, Y.C.; Kim, S.W.; Ko, K.T. Evaluation of the suitability for concrete using fly ash in N.P.P structures. Proc. Korean Nucl. Soc. Autumn Meet. 2002, 34, 34063809. [Google Scholar]
- Alonso, C.; Fernandez, L. Dehydration and rehydration processes of cement paste exposed to high temperature environments. J. Mater. Sci. 2004, 39, 3015–3024. [Google Scholar] [CrossRef]
- Real, S.; Carriço, A.; Bogas, J.A.; Guedes, M. Influence of the treatment temperature on the microstructure and hydration behavior of thermoactivated recycled cement. Materials 2020, 13, 3937. [Google Scholar] [CrossRef] [PubMed]
- Xuan, D.X.; Shui, Z.H. Rehydration activity of hydrated cement paste exposed to high temperature. Fire Mater. 2011, 35, 481–490. [Google Scholar] [CrossRef]
- Shui, Z.H.; Xuan, D.X.; Chen, W.; Yu, R.; Zhang, R. Cementitious characteristics of hydrated cement paste subjected to various dehydration temperature. Constr. Build. Mater. 2009, 23, 531–537. [Google Scholar] [CrossRef]
- ASTM C 39; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2001.
- ASTM B 553; Test Method for Thermal Cycling of Electroplated Plastics. ASTM International: West Conshohocken, PA, USA, 1979.
- ANSI/ANS-16.1; Measurement of the Leachability of Solidified Low-level Radioactive Wastes by a Short-term Test Procedure. American National Standard: New York, NY, USA, 2003.
- Moon, Y.B.; Choi, H.K.; Kim, J.Y.; Lee, J.H.; Chung, C.W.; Kim, J.H. Recycling Waste Paste from Concrete for Solidifying Agent. J. Korea Inst. Build. Constr. 2017, 17, 269–277. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Paroli, R.M.; Beaudoin, J.J.; Delgado, A.H. Handbook of Thermal Analysis of Construction Materials; Noyes Publications: New York, NY, USA, 2003. [Google Scholar]
- Tanaka, K.K.; Yamamoto, T.; Kimura, H. Low-temperature crystallization of amorphous silicate in astrophysical environments. Astrophys. J. 2010, 717, 586. [Google Scholar] [CrossRef] [Green Version]
- Hallenbeck, S.L.; Nuth, J.A., III; Daukantas, P.L. Mid-infrared spectral evolution of amorphous magnesium silicate smokes annealed in vacuum: Comparison to cometary spectra. Icarus 1998, 131, 198–209. [Google Scholar] [CrossRef]
- Fabian, D.; Jager, C.; Henning Th, D.J.; Mutschke, H. Steps toward interstellar silicate mineralogy. Astron. Astrophys. 2000, 364, 282–292. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.; Chihara, H.; Tsuchiyama, A.; Koike, C.; Takakura, T.; Noguchi, T.; Nakamura, T. Crystallization experiments on amorphous silicates with chondritic composition: Quantitative formulation of the crystallization. Astrophys. J. 2007, 668, 285. [Google Scholar] [CrossRef]
- Xue, S.H.; Xie, H.; Ping, H.; Li, Q.C.; Su, B.L.; Fu, Z.Y. Induced transformation of amorphous silica to cristobalite on bacterial surfaces. RSC Adv. 2015, 5, 71844–71848. [Google Scholar] [CrossRef]
- Pinckney, L.R.; Beall, G.H. Microstructural evolution in some silicate glass ceramics: A review. J. Am. Ceram. Soc. 2008, 91, 773–779. [Google Scholar] [CrossRef]
- Leenakul, W.; Tunkasiri, T.; Tongsiri, N.; Pengpat, K.; Ruangsuriya, J. Effect of sintering temperature variations on fabrication of 45S5 bioactive glass-ceramics using rice husk as a source for silica. Mater. Sci. Eng. C 2016, 61, 695–704. [Google Scholar] [CrossRef]
- Pang, X. The effect of water-to-cement ratio on the hydration kinetics of Portland cement at different temperatures. In Proceedings of the 14th International Congress on Cement Chemistry, Beijing, China, 13–16 October 2015. [Google Scholar]
- Sedaghat, A.; Bien-Aime, A.; Zayed, A.; Sandberg, P. Measurements and Predictions of Heat of Hydration of Portland Cement Using Isothermal Conduction Calorimetry. 2011. Available online: http://dl.lib.uom.lk/handle/123/9538 (accessed on 5 December 2013).
- De Matos, P.R.; Sakata, R.D.; Onghero, L.; Uliano, V.G.; de Brito, J.; Campos, C.E.; Gleize, P.J. Utilization of ceramic tile demolition waste as supplementary cementitious material: An early-age investigation. J. Build. Eng. 2021, 38, 102187. [Google Scholar] [CrossRef]
- Chung, C.W.; Um, W.; Valenta, M.M.; Sundaram, S.K.; Chun, J.; Parker, K.E.; Westsik, J.H., Jr. Characteristics of Cast Stone cementitious waste form for immobilization of secondary wastes from vitrification process. J. Nucl. Mater. 2012, 420, 164–174. [Google Scholar] [CrossRef]
- Dorn, T.; Blask, O.; Stephan, D. Acceleration of cement hydration–A review of the working mechanisms, effects on setting time, and compressive strength development of accelerating admixtures. Constr. Build. Mater. 2022, 323, 126554. [Google Scholar] [CrossRef]
- Shanahan, N.; Sedaghat, A.; Zayed, A. Effect of cement mineralogy on the effectiveness of chloride-based accelerator. Cem. Concr. Compos. 2016, 73, 226–234. [Google Scholar] [CrossRef]


| Type | Strength (psi) | W/B | S/a (%) | Unit Contents (kg/m3) | Admixture (mL) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Water | Cement | Fine Aggregate | Coarse Aggregate | WRA | AEA | ||||
| Concrete | 3000 | 0.621 | 45 | 164.93 | 265.79 | 806.26 | 983.06 | 482.33 | 18.39 |
| Recycled Cement Powder (Heat Treatment Time) | S/B | Mixing Water (Simulant Solution) | |
|---|---|---|---|
| Temperature | Time | ||
| 600 °C | 1 h, 2 h | 0.5 | DI-water CsCl 1 M |
| 700 °C | 1 h, 2 h | ||
| Ca | Si | Al | Fe | K | Mg | S | Na | Ti | P | Mn | Zn | Sr | O | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 600 °C 1 h | 40.47 | 14.01 | 2.63 | 1.03 | 1.38 | 1.70 | 0.61 | 0.69 | 0.21 | 0.05 | 0.04 | 0.04 | 0.03 | 37.07 |
| 600 °C 2 h | 38.58 | 15.26 | 2.70 | 0.97 | 1.44 | 1.68 | 0.58 | 0.68 | 0.21 | 0.04 | 0.04 | 0.04 | 0.03 | 37.72 |
| 700 °C 1 h | 44.44 | 11.69 | 2.43 | 0.98 | 1.15 | 1.82 | 0.72 | 0.51 | 0.19 | 0.06 | 0.04 | 0.04 | 0.03 | 35.88 |
| 700 °C 2 h | 43.99 | 12.01 | 2.42 | 0.98 | 1.17 | 1.81 | 0.73 | 0.43 | 0.21 | 0.05 | 0.04 | 0.04 | 0.02 | 36.07 |
| Specimens | Surface Area | Pore Size | |||
|---|---|---|---|---|---|
| SABET (m2/g) | SAMicro (a) (m2/g) | SAExt (m2/g) | PSDia (b) (Å) | ||
| Portland Cement | 1.2189 | 0.2506 | 0.9683 | 196.2565 | |
| Recycled cement powder | 600 °C-1 h | 7.4878 | 1.5073 | 5.9805 | 28.9918 |
| 600 °C-2 h | 7.0737 | 1.5904 | 5.4832 | 35.5834 | |
| 700 °C-1 h | 3.7347 | 1.0372 | 2.6975 | 31.7818 | |
| 700 °C-2 h | 4.0215 | 0.9757 | 3.0458 | 36.1131 | |
| No. | Specimen | Cumulative Heat Flow (J/g Cement) | |
|---|---|---|---|
| Di-Water | CsCl 1 M | ||
| 1 | OPC | 359.62 | 323.34 |
| 2 | 600 °C-1 h | 115.83 | 145.24 |
| 600 °C-2 h | 86.30 | 125.41 | |
| 3 | 700 °C-1 h | 136.077 | 157.18 |
| 700 °C-2 h | 125.83 | 155.26 | |
| Leaching Time (Days) | LI | |||
|---|---|---|---|---|
| 600 °C-1 h | 600 °C-2 h | 700 °C-1 h | 700 °C-2 h | |
| 0.083 | 8.63 | 8.80 | 8.90 | 8.87 |
| 0.292 | 8.73 | 8.88 | 9.08 | 9.07 |
| 1 | 8.80 | 8.90 | 9.17 | 9.17 |
| 2 | 9.06 | 9.09 | 9.28 | 9.25 |
| 3 | 9.01 | 9.28 | 9.42 | 9.41 |
| 4 | 9.47 | 9.44 | 9.46 | 9.49 |
| 5 | 9.69 | 9.60 | 9.52 | 9.56 |
| 19 | 10.75 | 10.44 | 10.01 | 10.04 |
| 47 | 10.54 | 10.82 | 10.43 | 10.84 |
| 90 | 13.39 | 13.20 | 10.71 | 11.55 |
| In Previous Work | In This Work | ||
|---|---|---|---|
| Heat Treatment | Prior to the Separation Process | After Separation Process | |
| Separation process | 1. Jaw crushing to separate coarse and fine aggregates 2. Heat treatment of aggregate (600 °C 2 h) 3. Milling without ball (240 rpm, 22 h) 4. Recovery of cement powder less than 150 μm | 1. Jaw crushing and roll crushing 2. Powder separated using 150 μm sieve after vibration 3. Heat treatment (600 °C 2 h) | |
| The specific surface area | 12.37 m2/g | 7.07 m2/g | |
| Solution to binder ratio (S/B) | 0.7 | 0.5 | |
| Solution type | CsCl 3 M solution | CsCl 1 M solution | |
| Superplasticizer | none | 0.67 % by wt. of binder | none |
| Compressive strength at 28 days | 5.20 MPa | 6.36 MPa | 11.9 MPa |
| Final LI values | 15.30 | 15.29 | 13.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Seo, E.-A.; Kim, D.-G.; Chung, C.-W. The Effect of Changes in the Separation Process for the Performance of Recycled Cement Powder: A Comparison with a Previous Study for Radioactive Waste Immobilization. Materials 2022, 15, 7972. https://doi.org/10.3390/ma15227972
Kim J-H, Seo E-A, Kim D-G, Chung C-W. The Effect of Changes in the Separation Process for the Performance of Recycled Cement Powder: A Comparison with a Previous Study for Radioactive Waste Immobilization. Materials. 2022; 15(22):7972. https://doi.org/10.3390/ma15227972
Chicago/Turabian StyleKim, Ji-Hyun, Eun-A Seo, Do-Gyeum Kim, and Chul-Woo Chung. 2022. "The Effect of Changes in the Separation Process for the Performance of Recycled Cement Powder: A Comparison with a Previous Study for Radioactive Waste Immobilization" Materials 15, no. 22: 7972. https://doi.org/10.3390/ma15227972






