A New Zn(II) Metal Hybrid Material of 5-Nitrobenzimidazolium Organic Cation (C7H6N3O2)2[ZnCl4]: Elaboration, Structure, Hirshfeld Surface, Spectroscopic, Molecular Docking Analysis, Electric and Dielectric Properties
Abstract
:1. Introduction
2. Experimental Part
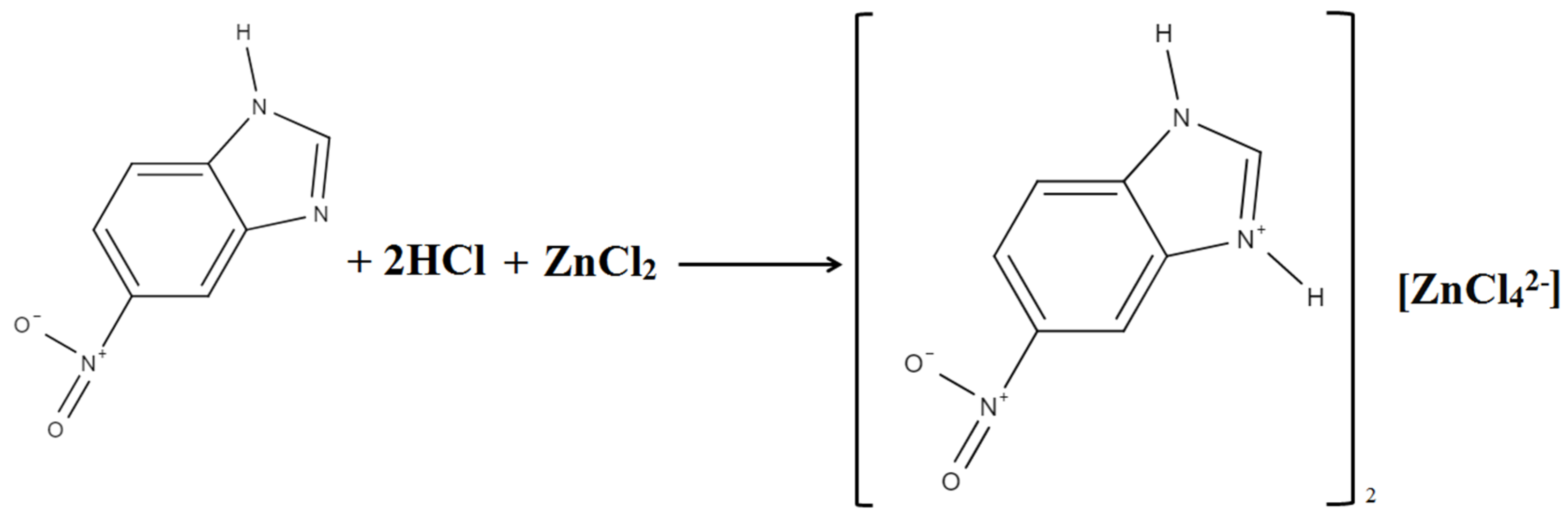
2.1. Chemical Preparation
2.2. Methods Details
3. Discussion Part
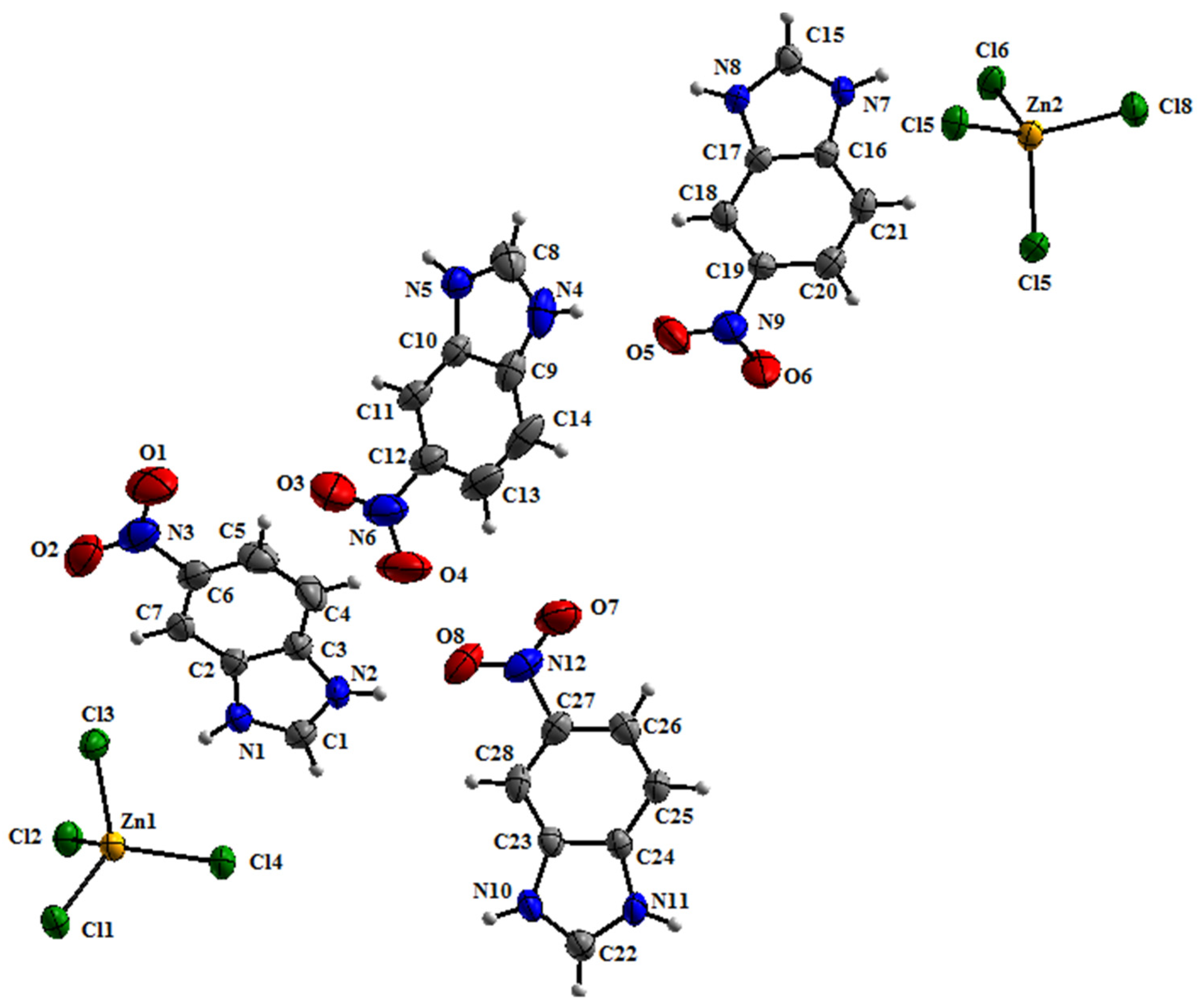
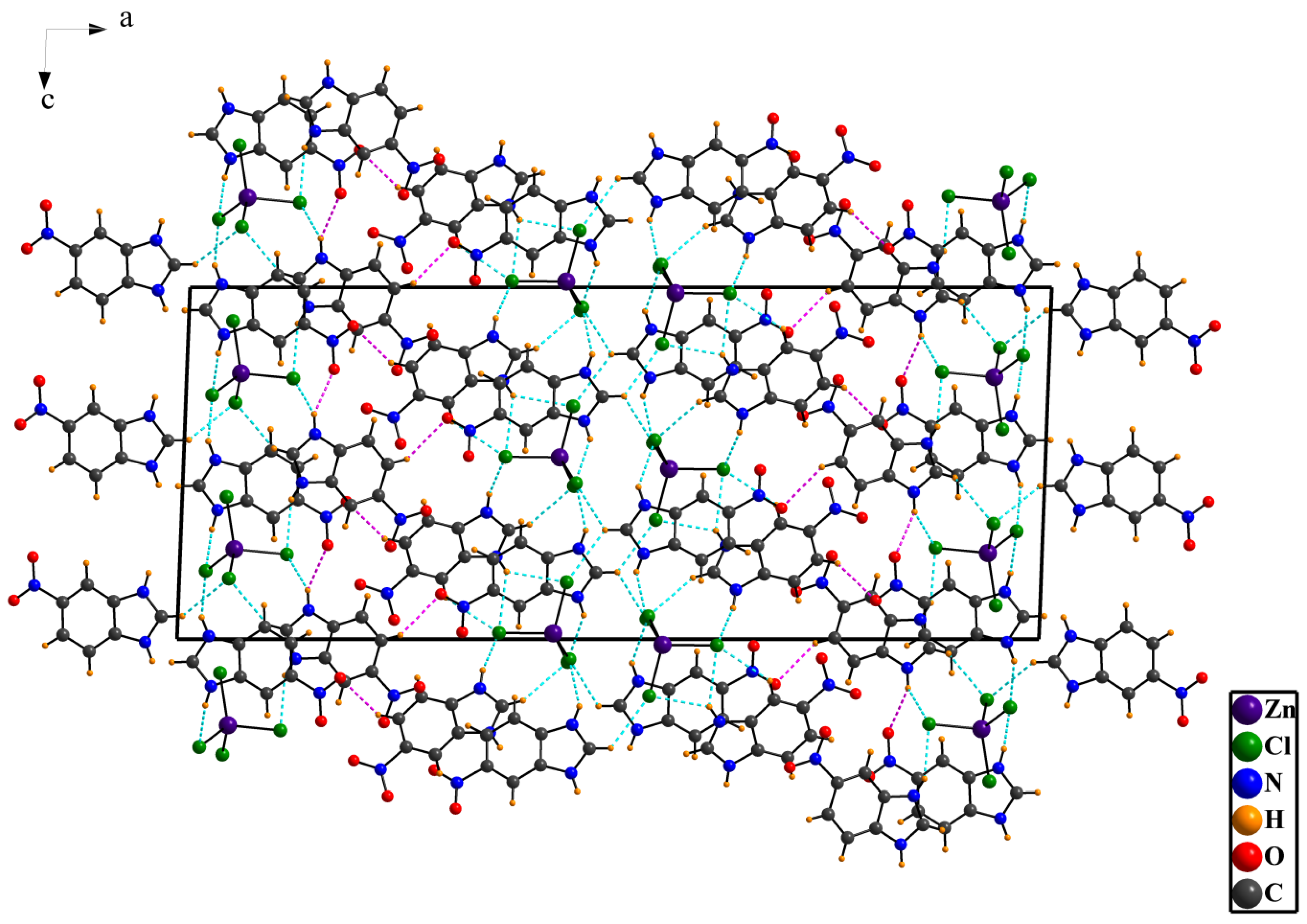
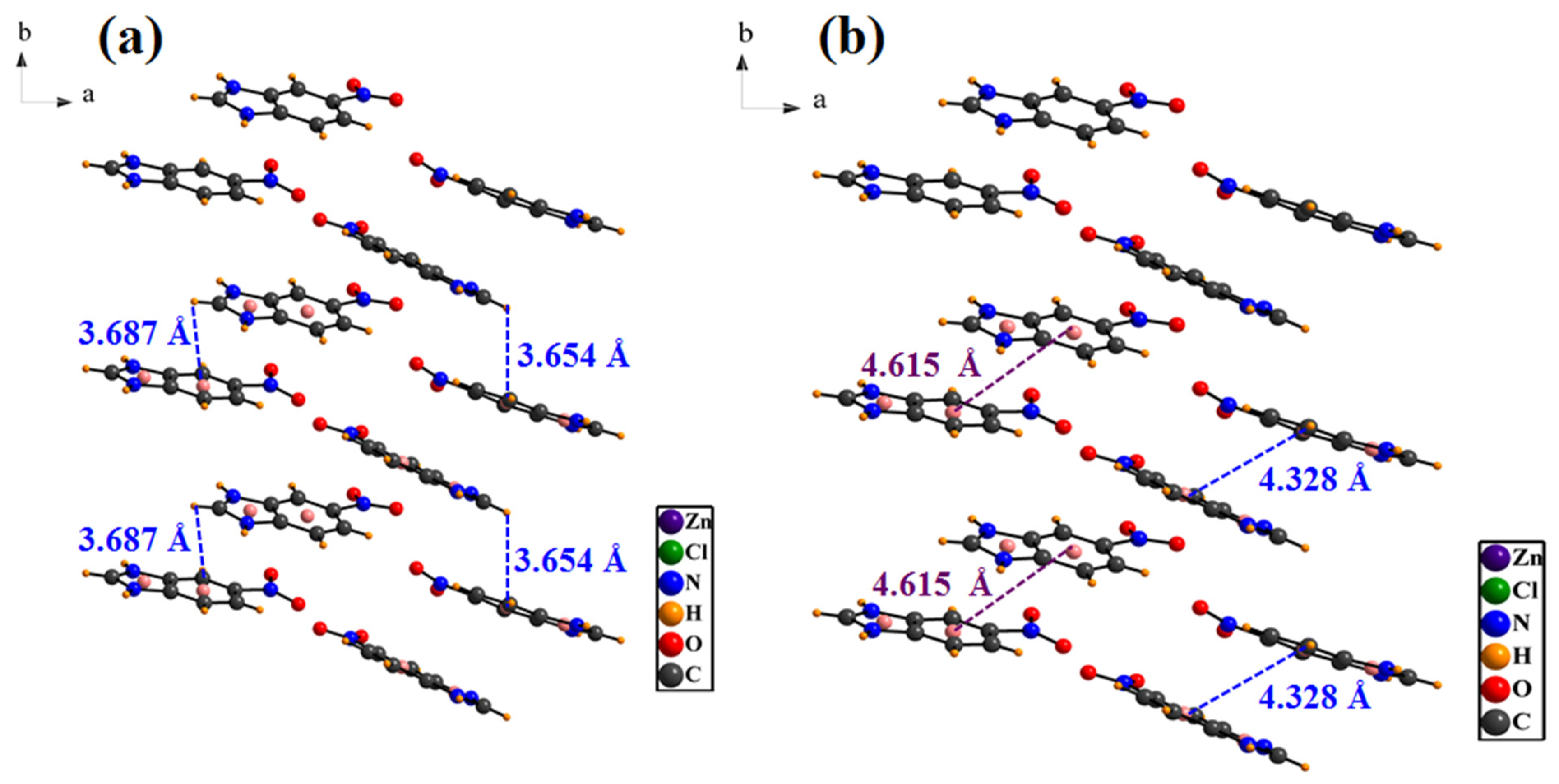
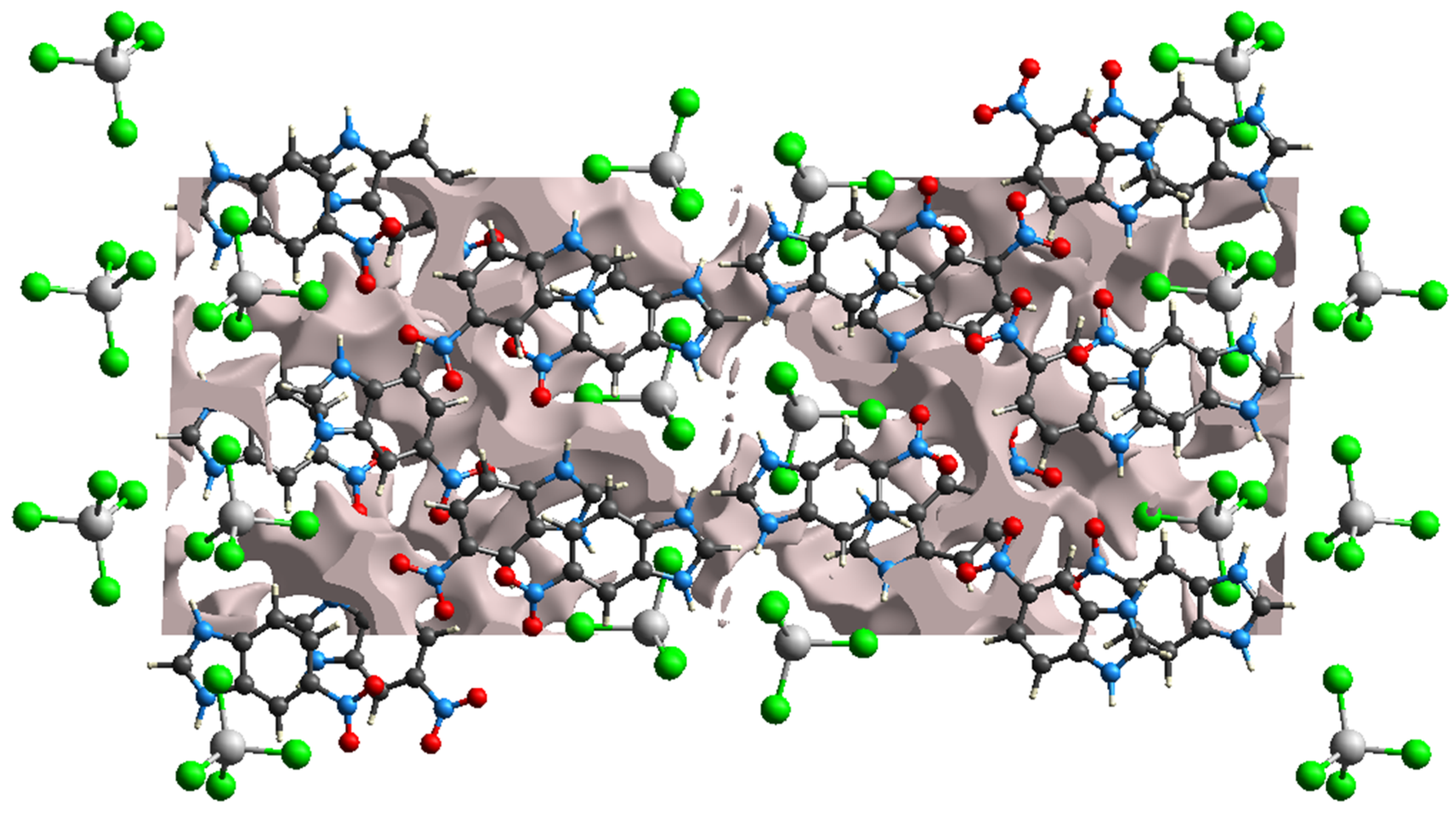
3.1. Crystal Structure Details
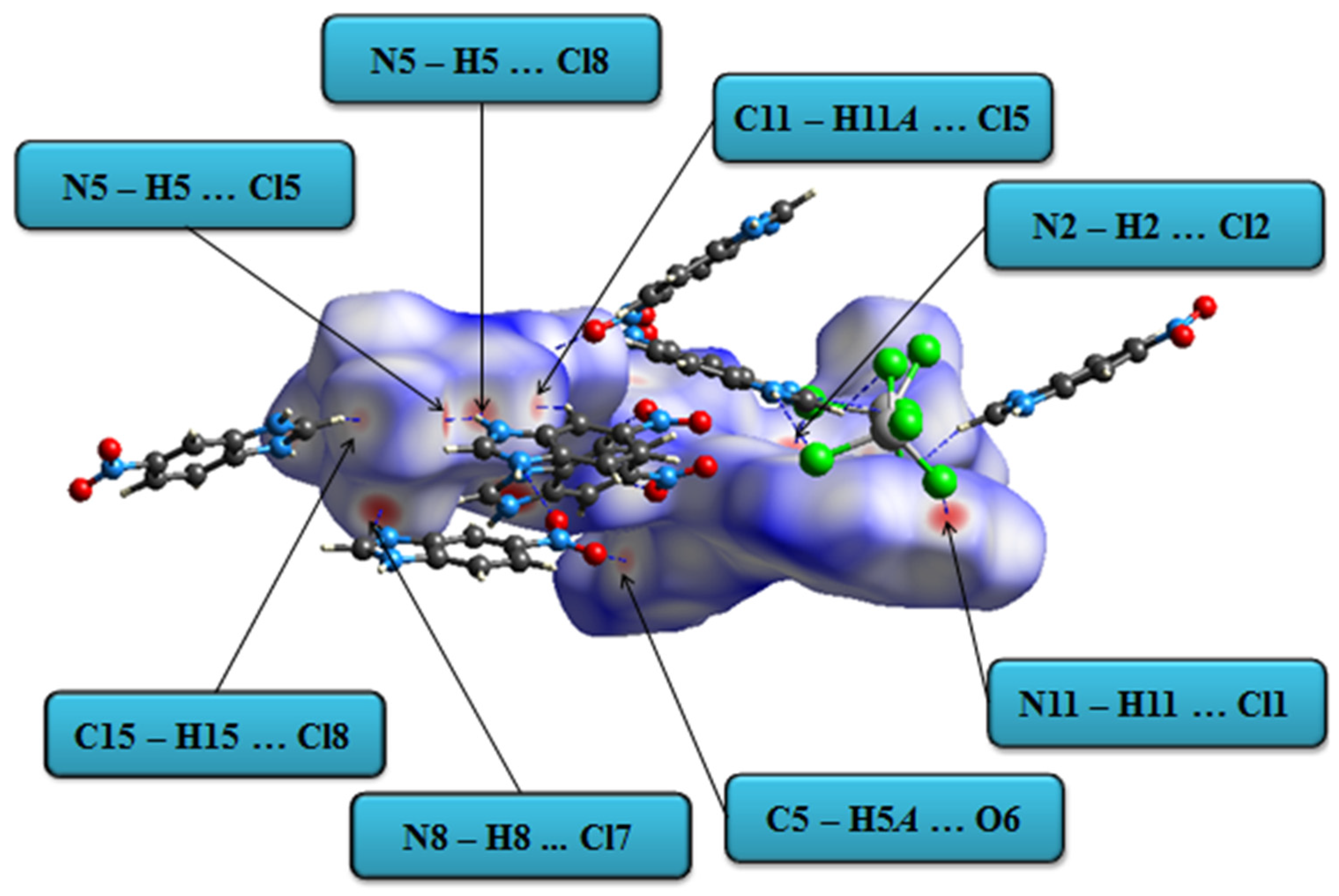
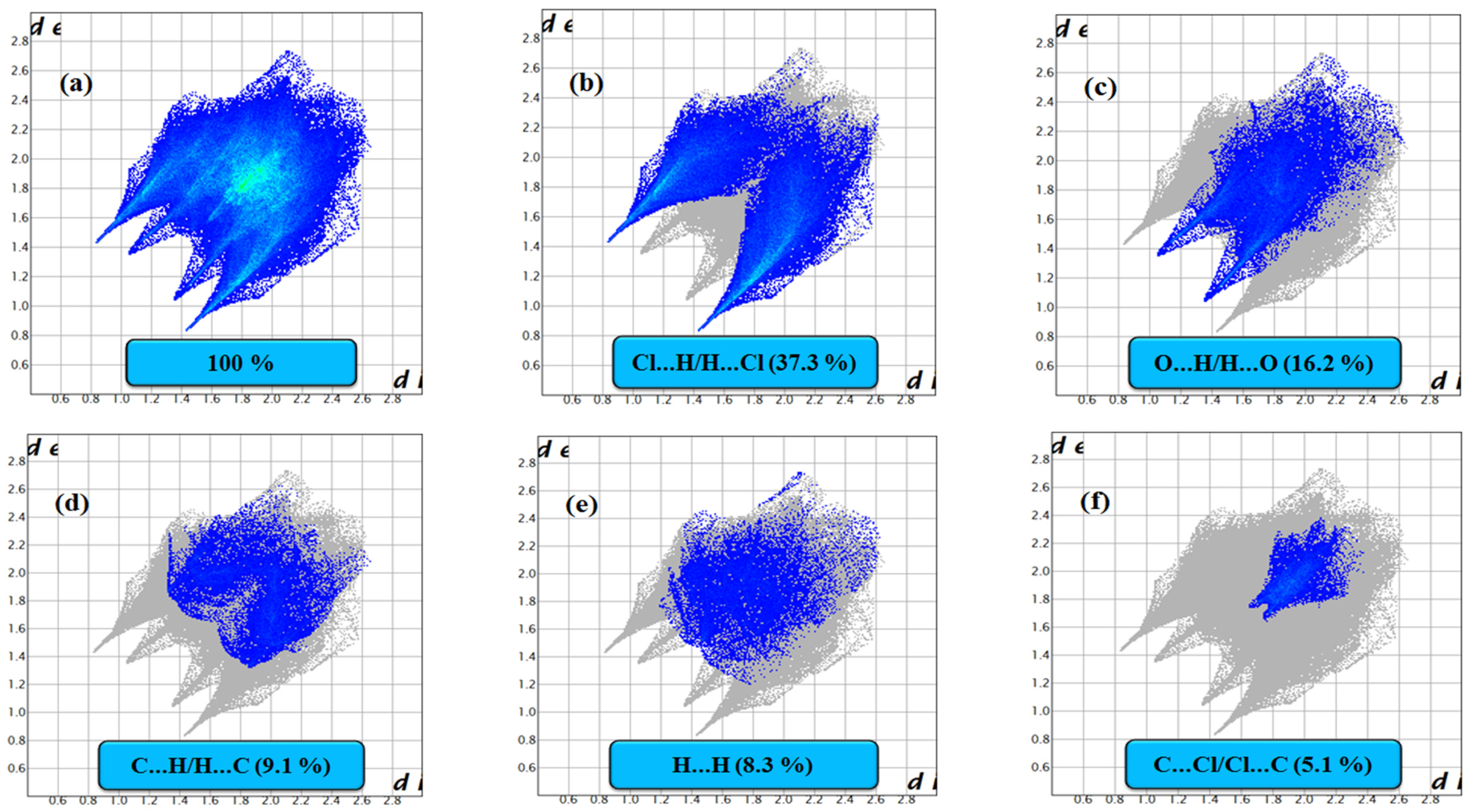
3.2. HS Analysis, 2D Fingerprint Plots and EXY
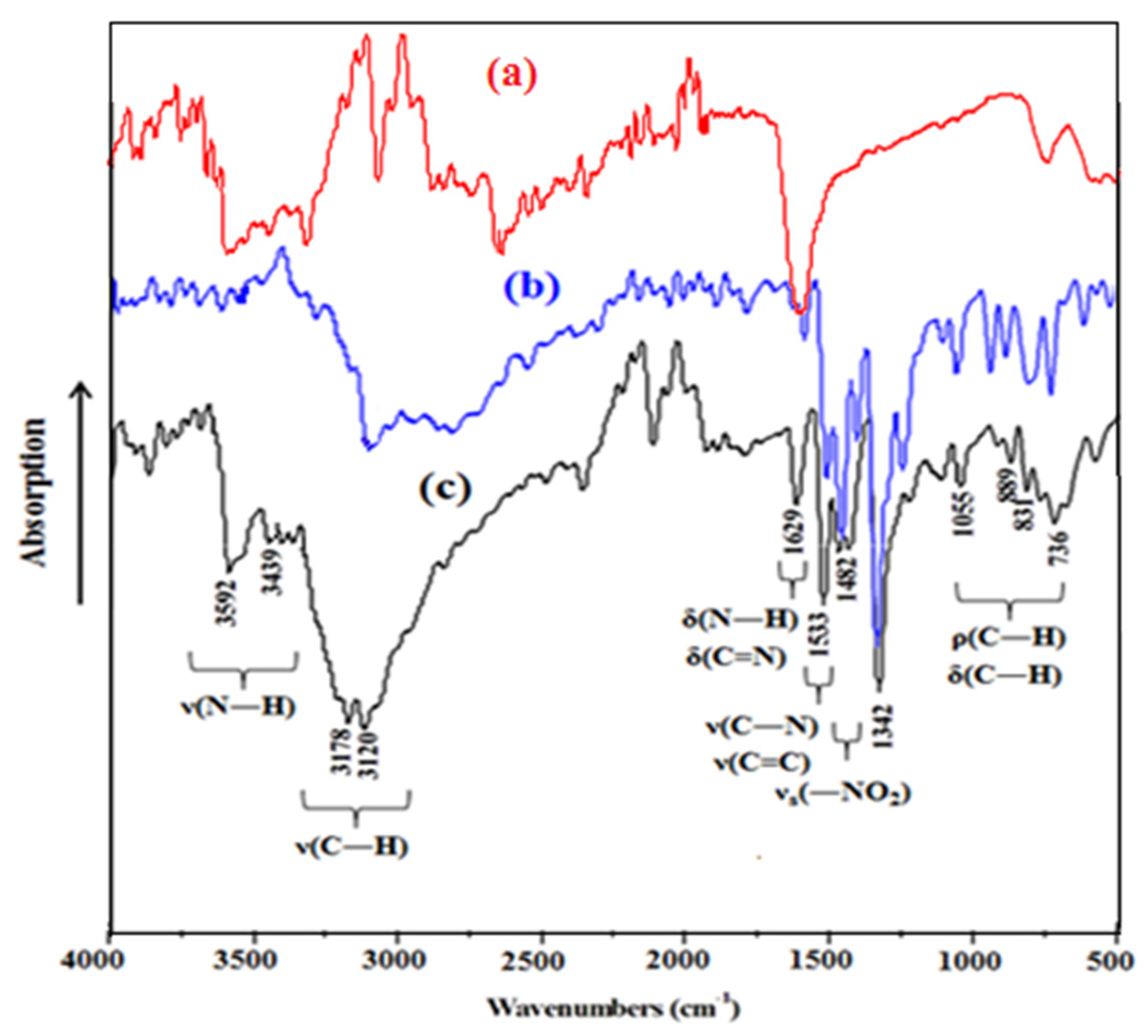
3.3. Infrared Spectroscopy
3.4. Electric and Dielectric Reports
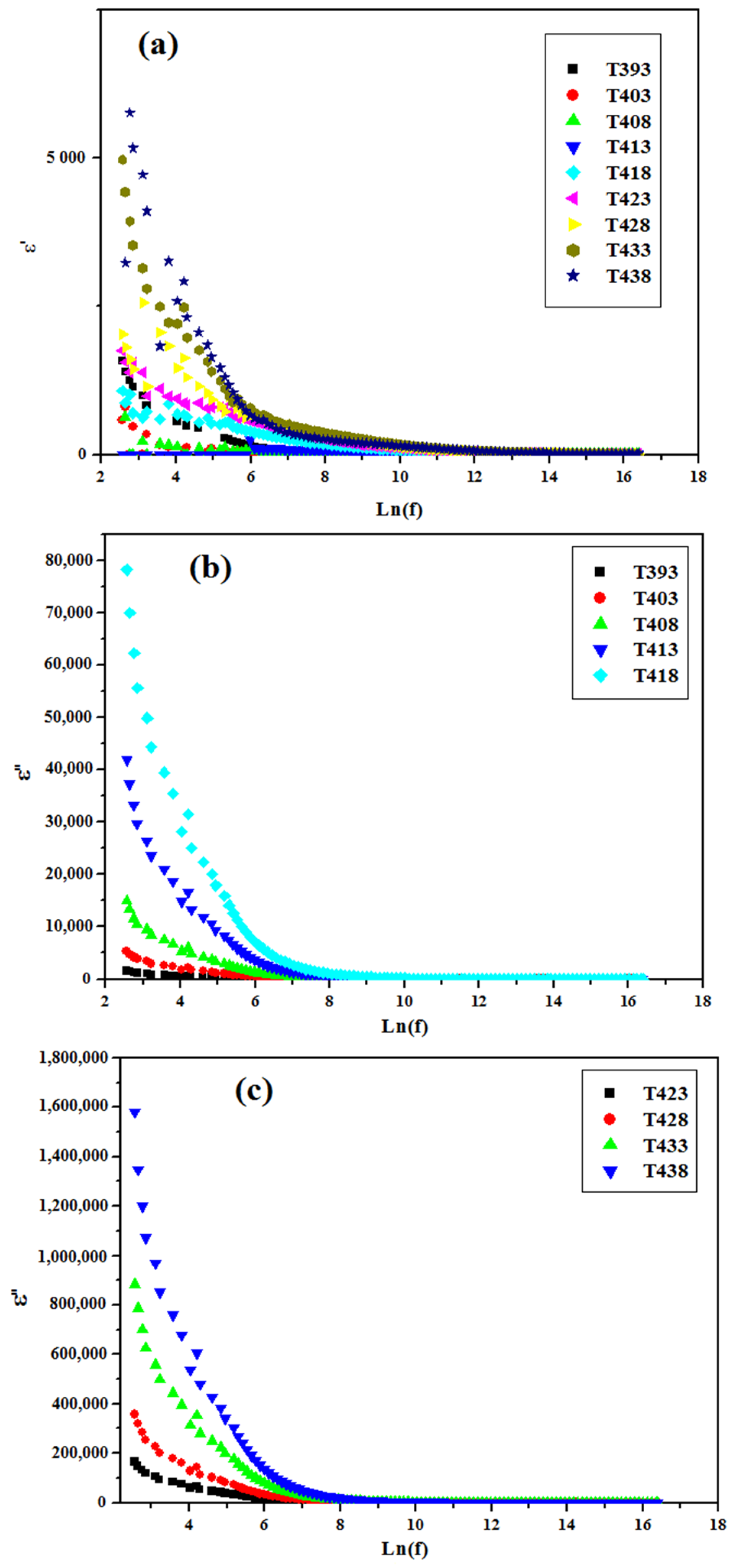
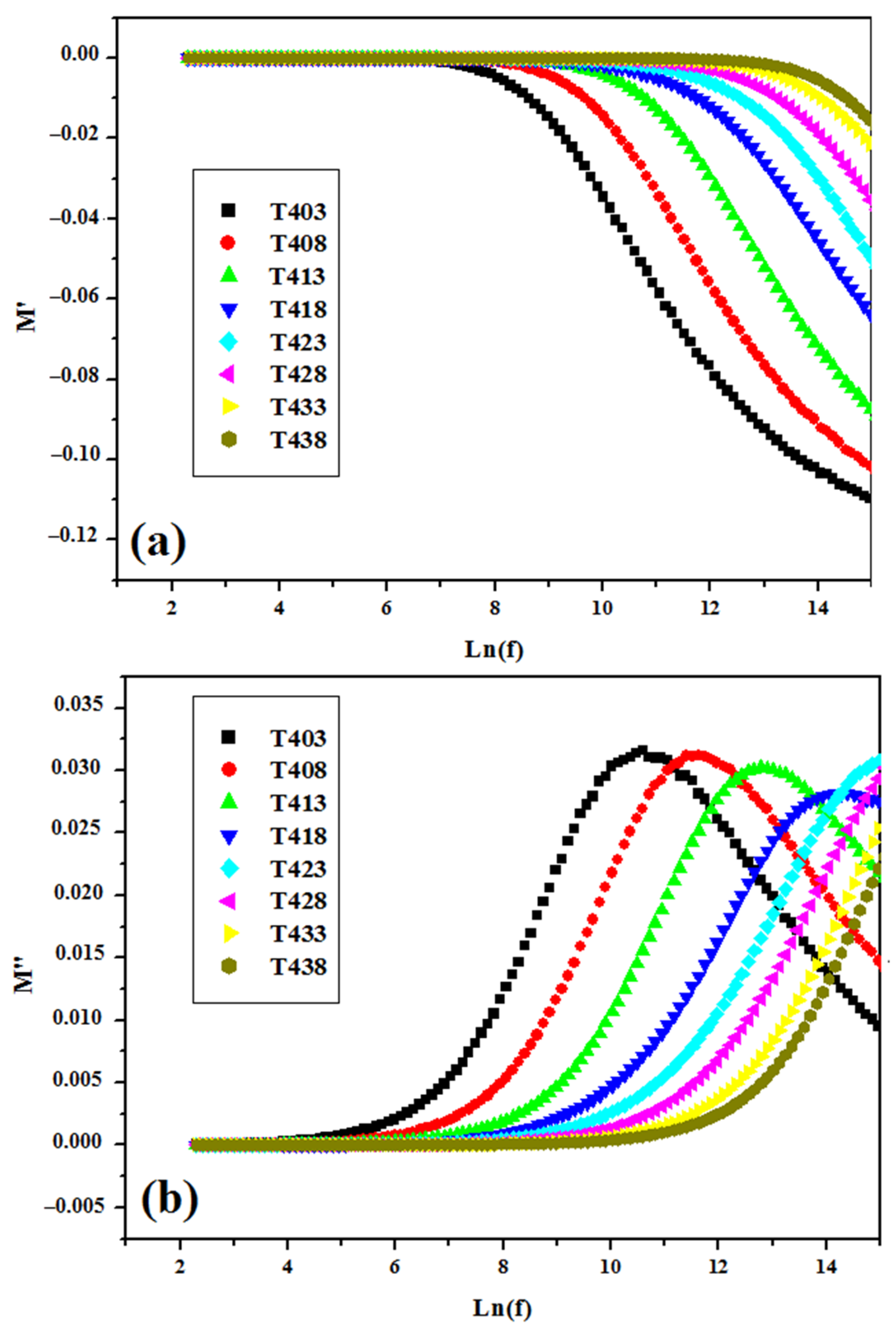
3.4.1. ε’ and ε″ versus Ln(f)
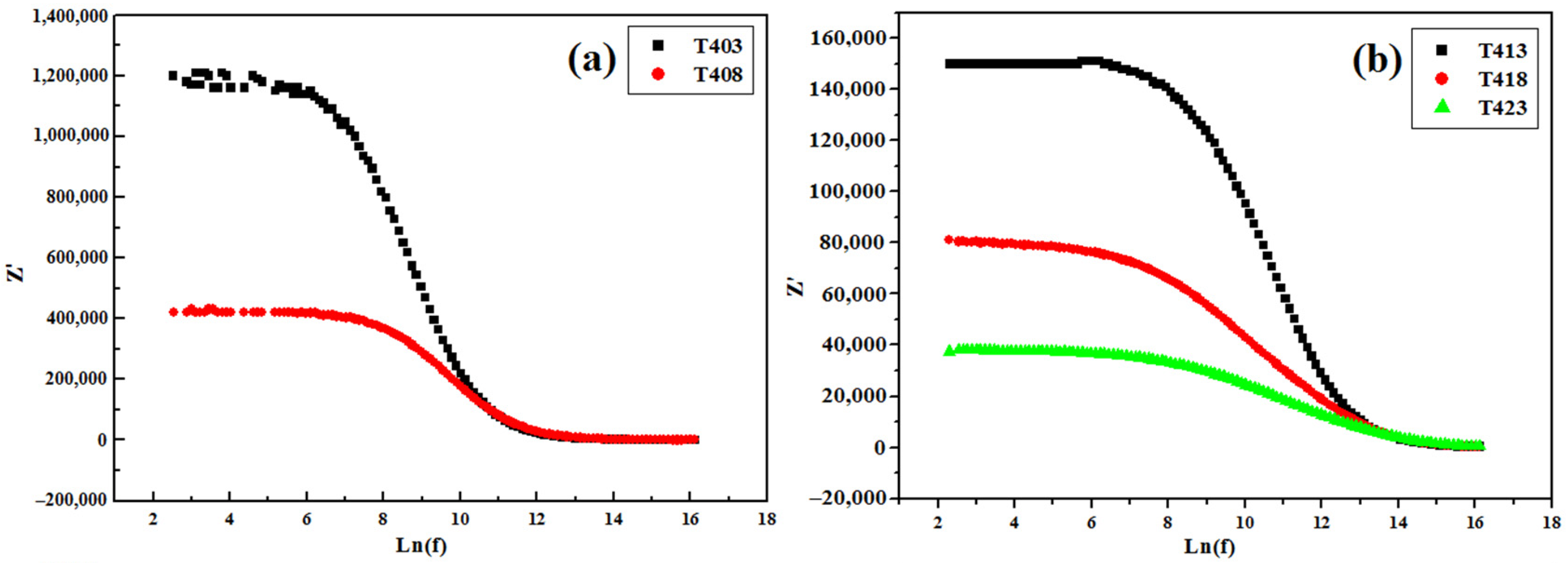
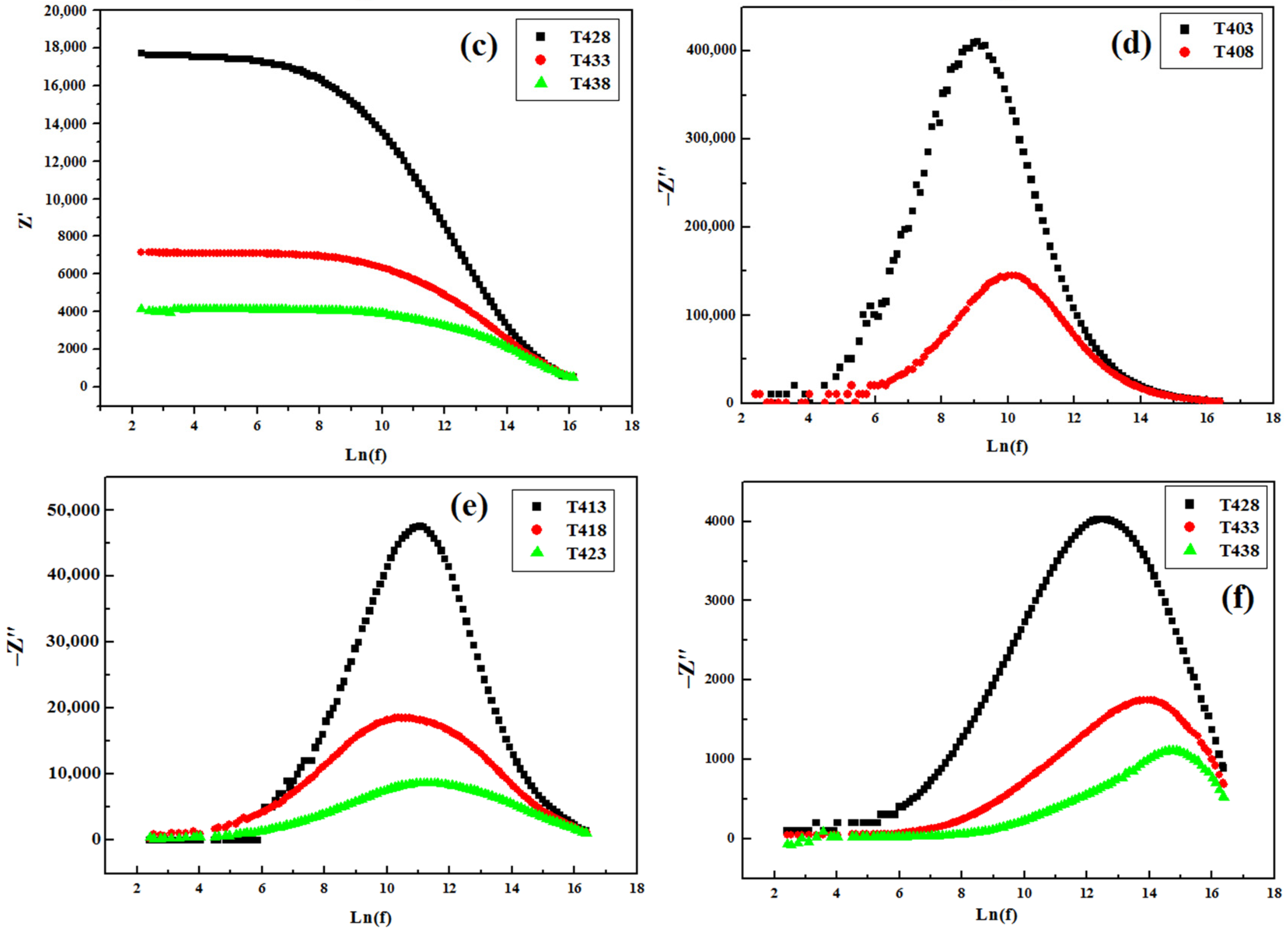
3.4.2. Impedance Spectroscopy
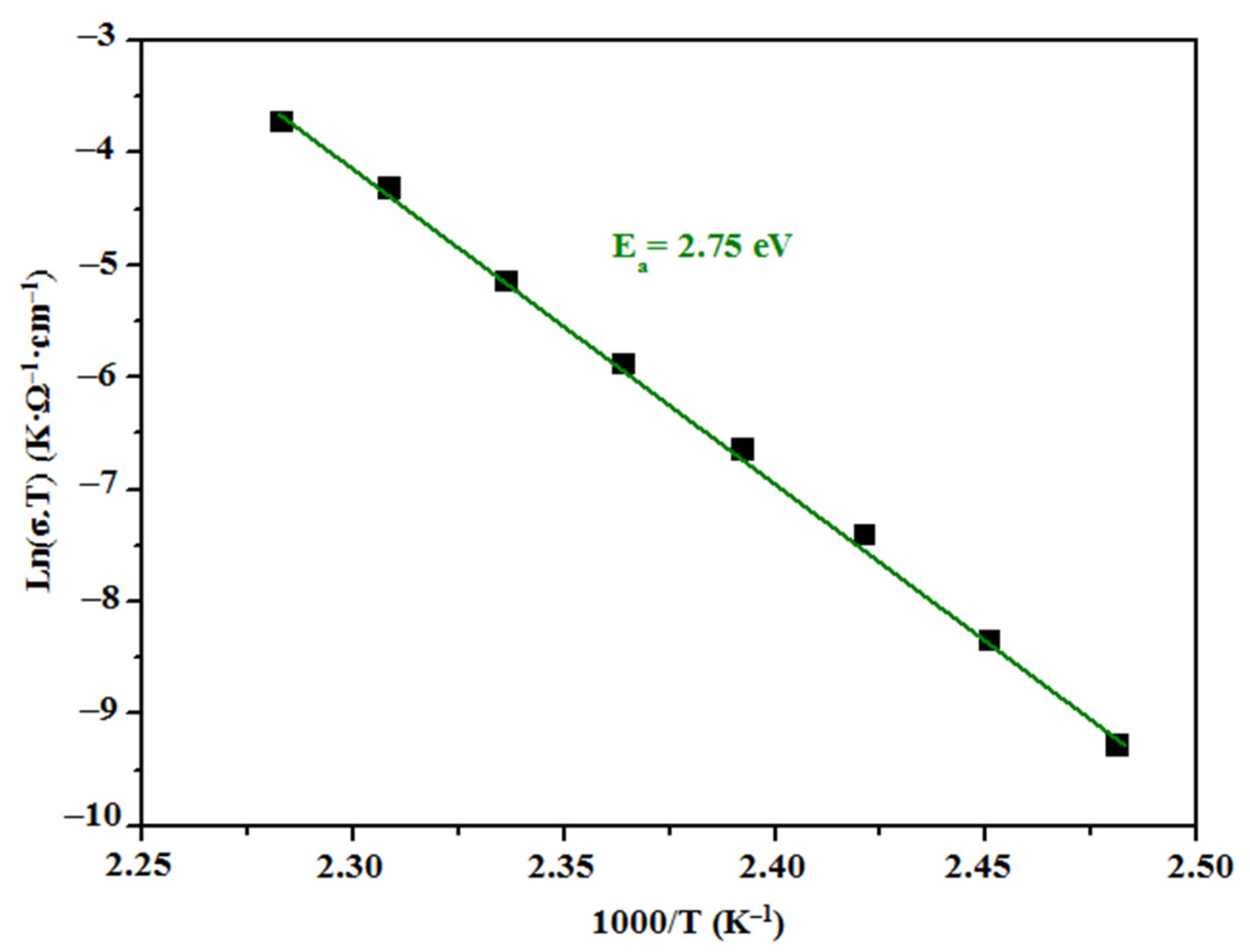
3.4.3. Electric Conductivity
3.4.4. Electrical Modulus
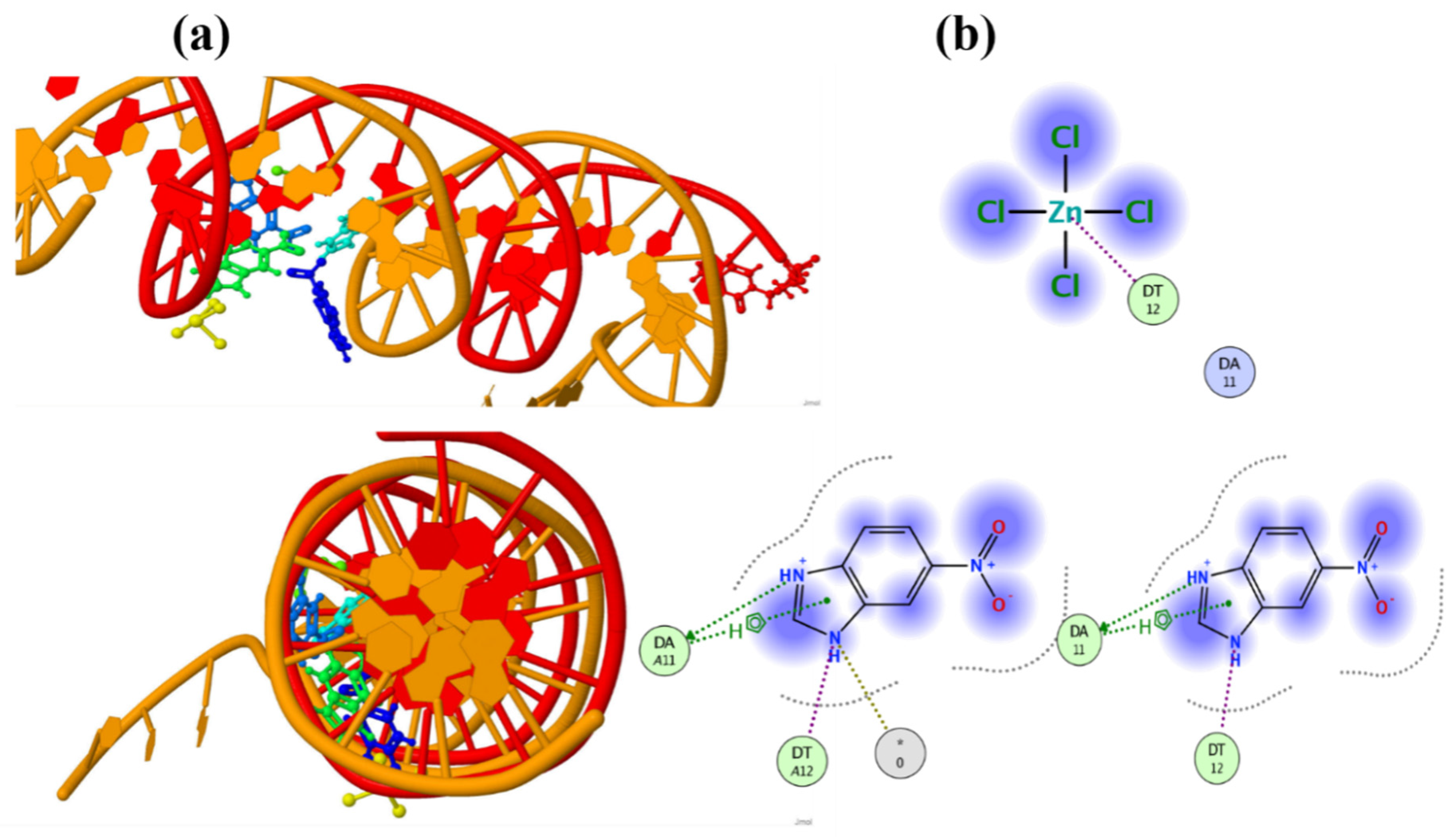
3.5. Molecular Docking Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan-On, W.; Huyen, N.T.B.; Songtawee, N.; Suwanjang, W.; Prachayasittikul, S.; Prachayasittikul, V. Quinoline-based clioquinol and nitroxoline exhibit anticancer activity inducing FoxM1 inhibition in cholangiocarcinoma cells. Drug Des. Dev. Ther. 2015, 9, 2033. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Terwilliger, A.; Maresso, A.W. Iron and zinc exploitation during bacterial pathogenesis. Metallomics 2015, 7, 1541–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrangsu, P.; Rensing, C.; Helmann, J.D. Metal homeostasis and resistance in bacteria. Nat. Rev. Microbiol. 2017, 15, 338–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crans, D.C.; Meade, T.J. Preface for the Forum on Metals in Medicine and Health: New Opportunities and Approaches to Improving Health. Inorg. Chem. 2013, 52, 12181–12183. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.P.; Hann, Z.S.; Cohen, S.M. Metalloprotein–Inhibitor Binding: Human Carbonic Anhydrase II as a Model for Probing Metal–Ligand Interactions in a Metalloprotein Active Site. Inorg. Chem. 2013, 52, 12207–12215. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.-F.; Neumann, H. Zinc-Catalyzed Organic Synthesis: C-C, C-N, C-O Bond Formation Reactions. Adv. Synth. Catal. 2012, 354, 3141–3160. [Google Scholar] [CrossRef]
- Mainar, A.R.; Iruin, E.; Colmenares, L.C.; Kvasha, A.; de Meatza, I.; Bengoechea, M.; Leonet, O.; Boyano, I.; Zhang, Z.; Blazquez, J.A. An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc. J. Energy Storage 2018, 15, 304–328. [Google Scholar] [CrossRef]
- Loseva, O.V.; Lutsenko, I.A.; Rodina, T.A.; Nelyubina, Y.V.; Gerasimenko, A.V.; Bekker, O.B.; Ivanov, A.V.; Eremenko, I.L. An ionic gold(III)–zinc(II) pseudo-polymeric compound of [H3O][Au{S2CN(CH2)5}2]3[ZnCl4]2: Synthesis, supramolecular architecture and anti-tuberculosis activity. Polyhedron 2022, 226, 116097. [Google Scholar] [CrossRef]
- Jasrotia, D.; Verma, S.K.; Sridhar, B.; Alvi, P.A.; Kumar, A. 3D-2D lattice dimensionality, optical Eg and PL energy variations due to organic variant in two [ZnCl4]2− based hybrid materials. Mater. Chem. Phys. 2018, 207, 98–104. [Google Scholar] [CrossRef]
- Zhong, C.; Wu, Q.; Guo, R.; Zhang, H. Synthesis and luminescence properties of polymeric complexes of Cu(II), Zn(II) and Al(III) with functionalized polybenzimidazole containing 8-hydroxyquinoline side group. Opt. Mater. 2008, 30, 870–875. [Google Scholar] [CrossRef]
- Afzaal, M.; Rosenberg, C.L.; Malik, M.A.; White, A.J.; O’Brien, P. Phosphine stabilized copper(i) complexes of dithiocarbamates and xanthates and their decomposition pathways. New J. Chem. 2011, 35, 2773–2780. [Google Scholar] [CrossRef]
- Asghar, M.A.; Zhang, S.; Khan, T.; Sun, Z.; Zeb, A.; Ji, C.; Li, L.; Zhao, S.; Luo, J. Reversible phase transition driven by order–disorder transformations of metal-halide moieties in [(C6H14)NH2]2·CuBr4. J. Mater. Chem. C 2016, 4, 7537–7540. [Google Scholar] [CrossRef]
- Nguyen, L.M.; Dellinger, M.E.; Lee, J.T.; Quinlan, R.A.; Rheingold, A.L.; Pike, R.D. Convenient synthesis of copper (I) thiolates and related compounds. Inorg. Chim. Acta 2005, 358, 1331–1336. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.; Asghar, M.A.; Sun, Z.; Zeb, A.; Ji, C.; Luo, J. A supra-molecular switchable dielectric material with non-linear optical properties. J. Mater. Chem. C 2017, 5, 2865–2870. [Google Scholar] [CrossRef]
- Kore, R.; Kelley, S.P.; Aduri, P.; Rogers, R.D. Mixed metal double salt ionic liquids comprised of [HN222]2[ZnCl4] and AlCl3 provide tunable Lewis acid catalysts related to the ionic environment. Dalton Trans. 2018, 47, 7795–7803. [Google Scholar] [CrossRef]
- Hao, P.; Zhu, H.; Pang, Y.; Shen, J.; Fu, Y. Positional Isomerism Controlled Electronic and Photochromic Properties of Naphthalene Diimide-Based Chlorozincate Hybrids. Cryst. Growth Des. 2019, 20, 345–351. [Google Scholar] [CrossRef]
- Yuan, W.; Zeng, Y.; Tan, Y.-Y.; Zhou, J.-H.; Xu, W.-J.; Zhang, W.-X.; Chen, X.-M. A new ferroelastic hybrid material with a large spontaneous strain: (Me3NOH)2[ZnCl4]. Chem. Commun. 2019, 55, 8983–8986. [Google Scholar] [CrossRef]
- Fan, M.; Zhao, F.; Peng, S.; Dai, Q.; Liu, Y.; Yin, S.; Zhang, Z. Biocompatibility of Zinc Matrix Biodegradable Composites Reinforced by Graphene Nanosheets. Materials 2022, 15, 6481. [Google Scholar] [CrossRef]
- Muslimov, A.E.; Tarasov, A.P.; Kanevsky, V.M. Interference Phenomena and Stimulated Emission in ZnO Films on Sapphire. Materials 2022, 15, 6409. [Google Scholar] [CrossRef]
- Puertas, M.L.; Durán, T.; Bartolomé, J.F.; Esteban-Cubillo, A. Synthesis of a Zinc Hydroxystannate/Sepiolite Hybrid Additive to Avoid Fire Propagation and Reduce Smoke Emission of EPDM Rubber Nanocomposites. Materials 2022, 15, 6297. [Google Scholar] [CrossRef]
- Derbali, L. Electrical and Optoelectronic Properties Enhancement of n-ZnO/p-GaAs Heterojunction Solar Cells via an Optimized Design for Higher Efficiency. Materials 2022, 15, 6268. [Google Scholar] [CrossRef]
- Manna, P.; Seth, S.K.; Mitra, M.; Choudhury, S.R.; Bauzá, A.; Frontera, A.; Mukhopadhyay, S. Experimental and Computational Study of Counterintuitive ClO4−···ClO4− Interactions and the Interplay between π+–π and Anion···π+ Interactions. Cryst. Growth Des. 2014, 14, 5812–5821. [Google Scholar] [CrossRef]
- Manna, P.; Seth, S.K.; Mitra, M.; Das, A.; Singh, N.J.; Choudhury, S.R.; Kar, T.; Mukhopadhyay, S. A successive layer-by-layer assembly of supramolecular frameworks driven by a novel type of face-to-face π+−π+ interactions. CrystEngComm 2013, 15, 7879–7886. [Google Scholar] [CrossRef]
- Reddy, C.M.; Kirchner, M.T.; Gundakaram, R.C.; Padmanabhan, K.A.; Desiraju, G.R. Isostructurality, Polymorphism and Mechanical Properties of Some Hexahalogenated Benzenes: The Nature of Halogen⋅⋅⋅Halogen Interactions. Chem. Eur. J. 2006, 12, 2222–2234. [Google Scholar] [CrossRef]
- Seth, S.K.; Manna, P.; Singh, N.J.; Mitra, M.; Jana, A.D.; Das, A.; Choudhury, S.R.; Kar, T.; Mukhopadhyay, S.; Kim, K.S. Molecular architecture using novel types of non-covalent π-interactions involving aromatic neutrals, aromatic cations and π-anions. CrystEngComm 2013, 15, 1285–1288. [Google Scholar] [CrossRef] [Green Version]
- Salah, S.B.H.; Hermi, S.; Alotaibi, A.A.; Alotaibi, K.M.; Lefebvre, F.; Kaminsky, W.; Ben Nasr, C.; Mrad, M.H. Stabilization of hexachloride net with mixed Sn(IV) metal complex and 2,3-dimethylanilinium organic cation: Elaboration, optical, spectroscopic, computational studies and thermal analysis. Chem. Pap. 2022, 76, 1861–1873. [Google Scholar] [CrossRef]
- Althobaiti, M.G.; Hermi, S.; Alotaibi, A.A.; Alotaibi, K.M.; Hassan, H.A.; Mi, J.-X.; Ben Nasr, C.; Mrad, M.H. A New Cu(II) Metal Complex Template with 4–tert–Butyl-Pyridinium Organic Cation: Synthesis, Structure, Hirshfeld Surface, Characterizations and Antibacterial Activity. Crystals 2022, 12, 254. [Google Scholar] [CrossRef]
- Hermi, S.; Althobaiti, M.G.; Alotaibi, A.A.; Almarri, A.H.; Fujita, W.; Lefebvre, F.; Ben Nasr, C.; Mrad, M.H. Synthesis, Crystal Structure, DFT Theoretical Calculationand Physico-Chemical Characterization of a New Complex Material (C6H8Cl2N2)2[Cd3Cl10]·6H2O. Crystals 2021, 11, 553. [Google Scholar] [CrossRef]
- Hermi, S.; Alotaibi, A.A.; Alswieleh, A.M.; Alotaibi, K.M.; Althobaiti, M.G.; Jelsch, C.; Wenger, E.; Ben Nasr, C.; Mrad, M.H. The Coordination Behavior of Two New Complexes, [(C7H10NO2)CdCl3]n(I) and [(C7H9NO2)CuCl2] (II), Based on 2,6-Dimethanolpyridine; Elaboration of the Structure and Hirshfeld Surface, Optical, Spectroscopic and Thermal Analysis. Materials 2022, 15, 1624. [Google Scholar] [CrossRef]
- Feddaoui, I.; Abdelbaky, M.S.M.; García-Granda, S.; Ben Nasr, C.; Mrad, M.H. Elaboration, crystal structure, vibrational, optical properties, thermal analysis and theoretical study of a new inorganic-organic hybrid salt [C4H12N2]4·Pb2Cl11·Cl·4H2O. J. Mol. Struct. 2020, 1211, 128056. [Google Scholar] [CrossRef]
- Ayari, C.; Althobaiti, M.G.; Alotaibi, A.A.; Almarri, A.; Ferretti, V.; Ben Nasr, C.; Mrad, M.H. Synthesis, Crystal Structure, Hirshfeld Surface, and Physicochemical Characterization of New Salt Bis(2-ethyl-6-methylanilinium)tetrachloromercurate (II) [C9H14N]2HgCl4. J. Chem. 2021, 2021, 2857369. [Google Scholar] [CrossRef]
- Ayari, C.; Alotaibi, A.A.; Alotaibi, K.M.; Ferretti, V.; Kaminsky, W.; Lefebvre, F.; Ben Nasr, C.; Mrad, M.H. A new Hg(II) hybrid compound (C6H9N2)[Hg6Cl13]·H2O elaboration, crystal structure, spectroscopic, thermal, and DFT theoretical calculations. Chem. Pap. 2022, 76, 2327–2340. [Google Scholar] [CrossRef]
- Ayari, C.; Alotaibi, A.A.; Baashen, M.A.; Alotaibi, K.M.; Alharbi, K.H.; Othmani, A.; Fujita, W.; Ben Nasr, C.; Mrad, M.H. Synthesis of New Homopiperazine-1.4-Diium Tetrachloridromercurate (II) Monohydrate (C5H14N2)[HgCl4]·H2O, Crystal Structure, Hirshfeld Surface, Spectroscopy, Thermal Analysis, Antioxidant Activity, Electric and Dielectric Behavior. Crystals 2022, 12, 486. [Google Scholar] [CrossRef]
- Ben Hmamou, D.; Salghi, R.; Zarrouk, A.; Zarrok, H.; Al-Deyab, S.S.; Benali, O.; Hammouti, B. The Inhibited effect of Phenolphthalein towards the corrosion of C38 Steel in Hydrochloric Acid. Int. J. Electrochem. Sci. 2012, 7, 8988–9003. [Google Scholar]
- Luo, X.; Ci, C.; Lia, J.; Lin, K.; Du, S.; Zhang, H.; Li, X.; Cheng, Y.F.; Zang, J.; Liu, Y. 4-aminoazobenzene modified natural glucomannan as a green eco-friendly inhibitor for the mild steel in 0.5 M HCl solution. Corros. Sci. 2019, 151, 132–142. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Tan, B.; Chen, S. Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X-steel in HCl solution. Corros. Sci. 2018, 133, 6–16. [Google Scholar] [CrossRef]
- Akhtar, W.; Faraz Khan, M.; Verma, G.; Shaquiquzzaman, M.; Rizvi, M.A.; Mehdi, S.H.; Akhter, M.; Alam, M.M. Therapeutic evolution of benzimidazole derivatives in the last quinquennial period. Eur. J. Med. Chem. 2017, 126, 705–753. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S.; Zhang, Q.; Long, Z.; Yu, Y.; Fang, H. Fungicides enhanced the abundance of antibiotic resistance genes in greenhouse soil. Environ. Pollut. 2020, 259, 113877. [Google Scholar] [CrossRef]
- Dutta, A.; Saha, S.K.; Banerjee, P.; Sukul, D. Correlating electronic structure with corrosion inhibition potentiality of some bis-benzimidazole derivatives for 10 mild steel in hydrochloric acid: Combined experimental and theoretical studies. Corros. Sci. 2015, 98, 541–550. [Google Scholar] [CrossRef]
- Onyeachua, I.B.; Obot, I.B.; Soroura, A.A.; Abdul-Rashid, M.I. Green corrosion inhibitor for oilfield application I: Electrochemical assessment of 2-(2-pyridyl) benzimidazole for API X60 steel under sweet environment in NACE brine ID196. Corros. Sci. 2019, 51, 132–142. [Google Scholar] [CrossRef]
- Ghanbari, A.; Attar, M.M.; Mahdavian, M. Corrosion inhibition performance of three imidazole derivatives on mild steel in 1 M phosphoric acid. Mater. Chem. Phys. 2010, 124, 1205–1209. [Google Scholar] [CrossRef]
- Abboud, Y.; Abourriche, A.; Saffaj, T.; Berrada, M.; Charrouf, M.; Bennamara, A.; Cherqaoui, A.; Takky, D. The inhibition of mild steel corrosion in acidic medium by 2,20-bis(benzimidazole). Appl. Surf. Sci. 2006, 252, 8178–8184. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, F.; Hu, S.; Cao, Z.; Wu, Z.; Jing, W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part I: Gravimetric, electrochemical, SEM and XPS studies. Corros. Sci. 2013, 74, 271–282. [Google Scholar] [CrossRef]
- Cao, Z.; Tang, Y.; Cang, H.; Xu, J.; Lu, G.; Jing, W. Novel benzimidazole derivatives as corrosion 21 inhibitors of mild steel in the acidic media. Part II: Theoretical studies. Corros. Sci. 2014, 83, 292–298. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, K. Diamond Version 2.0; Impact GbR: Bonn, Germany, 1998. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0–new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Available online: https://www.rcsb.org/structure/6TNY (accessed on 1 May 2022).
- Perveen, F.; Arshad, N.; Qureshi, R.; Nowsherwan, J.; Sultan, A.; Nosheen, B.; Rafique, H. Electrochemical, spectroscopic and theoretical monitoring of anthracyclines’ interactions with DNA and ascorbic acid by adopting two routes: Cancer cell line studies. PLoS ONE 2018, 13, e0205764. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(i) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef]
- Rademeyer, M. Bis(p -toluidinium) tetrachlorozincate(II). Acta Cryst. 2005, E61, m304–m306. [Google Scholar] [CrossRef] [Green Version]
- Fowkes, A.; Harrison, W.T. Bis(piperidinium) tetrachlorozincate. Acta Cryst. 2004, E60, m59–m61. [Google Scholar] [CrossRef]
- Anzellotti, A.; Briceño, A. Hexakis(acetonitrile)ruthenium(II) tetrachlorozincate 2.55-hydrate. Acta Crystallogr. Sect. E 2001, 57, m538–m540. [Google Scholar] [CrossRef]
- Hosseinian, A.; Mahjoub, A.R. 2,2’-Diamino-5,5’-dimethyl-4,4’-bi-1,3-thiazolium tetrachloridozincate(II). Acta Crystallogr. Sect. E 2009, 65, m1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.-Q.; Gao, J.-X.; Hua, X.-N.; Chen, X.-G.; Lu, Y. Unusual two-step sequential reversible phase transitions with coexisting switchable nonlinear optical and dielectric behaviors in [(CH3)3NCH2Cl]2[ZnCl4]. J. Mater. Chem. 2017, 5, 11873–11878. [Google Scholar] [CrossRef]
- Ru, Z.L. (S)-1,2,4-Trimethylpiperazine-1,4-diium tetrachloridozincate (II). Acta Crystallogr. Sect. E 2010, 66, m1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bofill, L.; Prohens, R.; Barbas, R.; Frontera, A. DFT Analysis of Uncommon π···H-Bond Array Interaction in a New Pterostilbene/Theophylline Cocrystal. Cryst. Growth Des. 2020, 20, 6691–6698. [Google Scholar] [CrossRef]
- Rakhmonova, D.; Kadirova, Z.; Torambetov, B.; Kadirova, S.; Ashurov, J.; Shishkina, S. The molecular and crystal structures of 2-(3-hydroxypropyl)benzimidazole and its nitrate salt. Acta Crystallogr. Sect. E 2022, 78, 211–215. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17; The University of Western Australia: Crawley, Australia, 2017. [Google Scholar]
- Chebbi, H.; Mezrigui, S.; Ben Jomaa, M.; Zid, M.F. Crystal structure, Hirshfeld surface analysis and energy framework calculation of the first oxoanion salt containing 1,3-cyclohexanebis(methylammonium): [3-(azaniumylmethyl)cyclohexyl]methanaminium dinitrate. Acta Crystallogr. Sect. E 2018, 74, 949–954. [Google Scholar] [CrossRef]
- Sudha, S.; Karabacak, M.; Kurt, M.; Cinar, M.; Sundaraganesan, N. Molecular structure, vibrational spectroscopic, first-order hyperpolarizability and HOMO, LUMO studies of 2-aminobenzimidazole. Spectrochim. Acta 2011, 84A, 184–195. [Google Scholar] [CrossRef]
- Nami, S.A.; Husain, A.; Siddiqi, K.; Westcott, B.L.; Kopp-Vaughn, K. Synthesis, spectroscopic, magnetic and thermal properties of bimetallic salts, [Ni(L)][MCl4] {where M=Co(II), Zn(II), Hg(II) and L=3,7-bis(2-aminoethyl)-1,3,5,7-tetraazabicyclo(3.3.1)nonane}. X-ray structure of [Ni(L)][CoCl4]. Spectrochim. Acta 2010, 75, 444–447. [Google Scholar] [CrossRef]
- Abo-Aly, M.; Salem, A.; Sayed, M.; Aziz, A.A. Spectroscopic and structural studies of the Schiff base 3-methoxy-N-salicylidene-o-amino phenol complexes with some transition metal ions and their antibacterial, antifungal activities. Spectrochim. Acta 2014, 136, 993–1000. [Google Scholar] [CrossRef]
- Abu Al-Nasr, A.K.; Ramadan, R.M. Spectroscopic studies and biological activity of some transition metal complexes of unusual Schiff base. Spectrochim. Acta 2013, 105A, 14–19. [Google Scholar] [CrossRef]
- Hassen, S.; Chebbi, H.; Zid, M.F.; Arfaoui, Y. Crystal structure, spectroscopic study, photoluminescent properties and DFT calculations of the 2-guanidinobenzimidazolium dichloride and dibromide monohydrate salts. J. Mol. Struct. 2018, 1167, 1–10. [Google Scholar] [CrossRef]
- Ennaceur, N.; Henchiri, R.; Jalel, B.; Cordier, M.; Ledoux-Rak, I.; Elaloui, E. Synthesis, crystal structure, and spectroscopic characterization supported by DFT calculations of organoarsenic compound. J. Mol. Struct. 2017, 1144, 25–32. [Google Scholar] [CrossRef]
- Mahadevan, D.; Periandy, S.; Karabacak, M.; Ramalingam, S.; Puviarasan, N. Spectroscopic (FT-IR, FT-Raman and UV–vis) investigation and frontier molecular orbitals analysis on 3-methyl-2-nitrophenol using hybrid computational calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc 2012, 86, 139–151. [Google Scholar] [CrossRef]
- Dutta, A.; Sinha, T.P. Structural and dielectric properties of A(Fe1/2Ta1/2)O3 [A=Ba, Sr, Ca]. Mater. Res. Bull. 2011, 46, 518–524. [Google Scholar] [CrossRef]
- Rao, K.S.; Prasad, D.M.; Krishna, P.M.; Tilak, B.; Varadarajulu, K.C. Impedance and modulus spectroscopy studies on Ba0.1Sr0.81La0.06Bi2Nb2O9 ceramic. Mater. Sci. Eng. B 2006, 133, 141–150. [Google Scholar] [CrossRef]
- Chandra, K.P.; Prasad, K.; Gupta, R.N. Impedance spectroscopy study of an organic semiconductor: Alizarin. Phys. B Condens. Matter 2007, 388, 118–123. [Google Scholar] [CrossRef]
- Rao, K.S.; Krishna, P.M.; Prasad, D.M.; Lee, J.-H.; Kim, J.-S. Electrical, electromechanical and structural studies of lead potassium samarium niobate ceramics. J. Alloys Compd. 2008, 464, 497–507. [Google Scholar] [CrossRef]
- Anantha, P.S.; Hariharan, K. ac Conductivity analysis and dielectric relaxation behaviour of NaNO3–Al2O3 composites. J. Mater. Sci. Eng. B 2005, 121, 12–19. [Google Scholar] [CrossRef]
- Macedo, P.B. The role of ionic diffusion in polarisation in vitreous ionic conductors. Phys. Chem. Glasses 1972, 13, 171–179. [Google Scholar]
- Akki, M.; Reddy, D.S.; Katagi, K.S.; Kumar, A.; Devarajegowda, H.C.; Babagond, V.; Mane, S.; Joshi, S.D. Synthesis of coumarin-thioether conjugates as potential anti-tubercular agents: Their molecular docking and X-ray crystal studies. J. Mol. Struct. 2022, 1266, 133452. [Google Scholar] [CrossRef]
- Naik, S.D.; Hosamani, K.M. Design and Synthesis of Novel C4-Linked Substituted 2H-Chromen-2-one-hypoxanthine Hybrids as Potential Antimicrobial Agents: An Approach to Molecular Docking Studies. J. Heterocycl. Chem. 2019, 56, 579–588. [Google Scholar] [CrossRef]
- Perveen, F.; Qureshi, R.; Ansari, F.L.; Kalsoom, S.; Ahmed, S. Investigations of drug–DNA interactions using molecular docking, cyclic voltammetry and UV–Vis spectroscopy. J. Mol. Struct. 2011, 1004, 67–73. [Google Scholar] [CrossRef]
- Honório, K.M.; Da Silva, A.B.F. An AM1 study on the electron-donating and electron-accepting character of biomolecules. Int. J. Quant. Chem. 2003, 95, 126–132. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comput. Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef]
- Ahmad, S.; Raza, S.; Uddin, R.; Azam, S.S. Binding mode analysis, dynamic simulation and binding free energy calculations of the MurF ligase from Acinetobacter baumannii. J. Mol. Graph. Model. 2017, 77, 72–85. [Google Scholar] [CrossRef]


| Atoms | Zn | Cl | N | O | C | H |
|---|---|---|---|---|---|---|
| % Surface | 0.8 | 25.3 | 4.85 | 14.7 | 13.35 | 41.1 |
| Zn | - | 0.988 | - | 0.760 | ||
| Cl | 0.249 | 1.344 | ||||
| N | 0.771 | - | 0.627 | |||
| O | 0.174 | 1.52 | 1.070 | 1.340 | ||
| C | 0.754 | 1.402 | 0.829 | |||
| H | 1.793 | 0.491 |
| Complex Code | Molecular Docking | |
|---|---|---|
| ″Kb″/M−1 | (−ΔG) KJ·mol−1 | |
| (C7H6N3O2)2[ZnCl4] | 7.99 × 107 | 45.01 |
| Complex | EHOMO | ELUMO | Eele | EIP | ETotal |
|---|---|---|---|---|---|
| (C7H6N3O2)2[ZnCl4]-DNA | −16.88 | −9.36 | −2,294,329.0 | 16.88 | −275,981.313 |
| Complex | MR | Hf | SlogP | Dipole |
|---|---|---|---|---|
| (C7H6N3O2)2[ZnCl4]-DNA | 24.61 | 887.22 | 7.61 | 33.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayari, C.; Alotaibi, A.A.; Baashen, M.A.; Perveen, F.; Almarri, A.H.; Alotaibi, K.M.; Abdelbaky, M.S.M.; Garcia-Granda, S.; Othmani, A.; Nasr, C.B.; et al. A New Zn(II) Metal Hybrid Material of 5-Nitrobenzimidazolium Organic Cation (C7H6N3O2)2[ZnCl4]: Elaboration, Structure, Hirshfeld Surface, Spectroscopic, Molecular Docking Analysis, Electric and Dielectric Properties. Materials 2022, 15, 7973. https://doi.org/10.3390/ma15227973
Ayari C, Alotaibi AA, Baashen MA, Perveen F, Almarri AH, Alotaibi KM, Abdelbaky MSM, Garcia-Granda S, Othmani A, Nasr CB, et al. A New Zn(II) Metal Hybrid Material of 5-Nitrobenzimidazolium Organic Cation (C7H6N3O2)2[ZnCl4]: Elaboration, Structure, Hirshfeld Surface, Spectroscopic, Molecular Docking Analysis, Electric and Dielectric Properties. Materials. 2022; 15(22):7973. https://doi.org/10.3390/ma15227973
Chicago/Turabian StyleAyari, Chaima, Abdullah A. Alotaibi, Mohammed A. Baashen, Fouzia Perveen, Abdulhadi H. Almarri, Khalid M. Alotaibi, Mohammed S. M. Abdelbaky, Santiago Garcia-Granda, Abdelhak Othmani, Cherif Ben Nasr, and et al. 2022. "A New Zn(II) Metal Hybrid Material of 5-Nitrobenzimidazolium Organic Cation (C7H6N3O2)2[ZnCl4]: Elaboration, Structure, Hirshfeld Surface, Spectroscopic, Molecular Docking Analysis, Electric and Dielectric Properties" Materials 15, no. 22: 7973. https://doi.org/10.3390/ma15227973








