Theoretical and Experimental Studies of Al-Impurity Effect on the Hydrogenation Behavior of Mg
Abstract
1. Introduction
2. Materials and Experimental Methods
3. Ab Initio Methods
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramachandran, R.; Menon, R.K. An overview of industrial uses of hydrogen. Int. J. Hydrog. Energy 1998, 23, 593–598. [Google Scholar] [CrossRef]
- Zhang, Q.; Liao, X.; Liu, S.; Wang, H.; Zhang, Y.; Zhao, Y. Tuning Particle Sizes and Active Sites of Ni/CeO2 Catalysts and Their Influence on Maleic Anhydride Hydrogenation. Nanomaterials 2022, 12, 2156. [Google Scholar] [CrossRef] [PubMed]
- Komova, O.V.; Simagina, V.I.; Pochtar, A.A.; Bulavchenko, O.A.; Ishchenko, A.V.; Odegova, G.V.; Gorlova, A.M.; Ozerova, A.M.; Lipatnikova, I.L.; Tayban, E.S.; et al. Catalytic behavior of iron-containing cubic spinel in the hydrolysis and hydrothermolysis of ammonia boran. Materials 2021, 14, 5422. [Google Scholar] [CrossRef] [PubMed]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sust. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Ismail, M.; Sinin, A.M.; Sheng, C.K.; Nik, W.W. Desorption behaviours of lithium alanate with metal oxide nanopowder additives. Int. J. Electrochem. Sci. 2014, 9, 4959–4973. [Google Scholar]
- Wang, Y.; Wang, Y. Recent advances in additive-enhanced magnesium hydride for hydrogen storage. Prog. Nat. Sci. Mater. Int. 2017, 27, 41–49. [Google Scholar] [CrossRef]
- Perejón, A.; Sánchez-Jiménez, P.E.; Criado, J.M.; Pérez-Maqueda, L.A. Magnesium hydride for energy storage applications: The kinetics of dehydrogenation under different working conditions. J. Alloys Compd. 2016, 681, 571–579. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The hydrogen issue. ChemSusChem 2011, 4, 21–36. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen storage for mobility: A review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Ren, Z.; Zhang, X.; Hu, J.; Huang, Z.; Lu, Y.; Gao, M.; Pan, H. Realizing 6.7 wt% reversible storage of hydrogen at ambient temperature with non-confined ultrafine magnesium hydrides. Energy Environ. Sci. 2021, 14, 2302–2313. [Google Scholar] [CrossRef]
- Crivello, J.C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Walker, G.S.; et al. Mg-based compounds for hydrogen and energy storage. Appl. Phys. A 2016, 122, 85. [Google Scholar] [CrossRef]
- Lyu, J.; Lider, A.M.; Kudiiarov, V.N. Using ball milling for modification of the hydrogenation/dehydrogenation process in magnesium-based hydrogen storage materials: An overview. Metals 2019, 9, 768. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, X.; Nyahuma, F.M.; Yan, N.; Xiao, J.; Su, S.; Zhang, L. Enhancing hydrogen storage properties of MgH2 by transition metals and carbon materials: A brief review. Front. Chem. 2020, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Kudiyarov, V.N.; Elman, R.R.; Kurdyumov, N.E. The Effect of High-Energy Ball Milling Conditions on Microstructure and Hydrogen Desorption Properties of Magnesium Hydride and Single-Walled Carbon Nanotubes. Metals 2021, 11, 1409. [Google Scholar] [CrossRef]
- Verón, M.G.; Troiani, H.; Gennari, F.C. Synergetic effect of Co and carbon nanotubes on MgH2 sorption properties. Carbon 2011, 49, 2413–2423. [Google Scholar] [CrossRef]
- Kudiiarov, V.; Lyu, J.; Semenov, O.; Lider, A.; Chaemchuen, S.; Verpoort, F. Prospects of hybrid materials composed of MOFs and hydride-forming metal nanoparticles for light-duty vehicle hydrogen storage. Appl. Mater. Today 2021, 25, 101208. [Google Scholar] [CrossRef]
- Ismail, M.; Yahya, M.S.; Sazelee, N.A.; Ali, N.A.; Yap, F.H.; Mustafa, N.S. The effect of K2SiF6 on the MgH2 hydrogen storage properties. J. Magnes. Alloy. 2020, 8, 832–840. [Google Scholar] [CrossRef]
- Imamura, H.; Hashimoto, Y.; Aoki, T.; Ushijima, T.; Sakata, Y. Preparation and properties of ball-milled MgH2/Al nanocomposites for hydrogen storage. Mater. Trans. 2014, 55, 572–576. [Google Scholar] [CrossRef]
- Nalbant, M.A.; Korkut, Ö. Hydrogen Absorption and Desorption Performance of magnesium-aluminium alloys produced by powder metallurgy. J. Chem. Soc. Pak. 2014, 36, 439. [Google Scholar]
- Kan, H.M.; Zhang, N.; Wang, X.Y. Al-Mg Alloy powders for hydrogen storage. Adv. Mater. Res. 2012, 550, 497–501. [Google Scholar] [CrossRef]
- Lyu, J.; Elman, R.R.; Svyatkin, L.A.; Kudiiarov, V.N. Theoretical and Experimental Research of Hydrogen Solid Solution in Mg and Mg-Al System. Materials 2022, 15, 1667. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Q.-F.; Liu, S.-S.; Sun, L.-X. The destabilization mechanism and de/re-hydrogenation kinetics of MgH2–LiAlH4 hydrogen storage system. J. Power Sources 2008, 185, 1514–1518. [Google Scholar] [CrossRef]
- Milanović, I.; Milošević, S.; Matović, L.; Vujasin, R.; Novaković, N.; Checchetto, R.; Novaković, J.G. Hydrogen desorption properties of MgH2/LiAlH4 composites. Int. J. Hydrog. Energy 2013, 38, 12152–12158. [Google Scholar] [CrossRef]
- Hsu, W.-C.; Yang, C.-H.; Tan, C.-Y.; Tsai, W.-T. In situ synchrotron X-ray diffraction study on the dehydrogenation behavior of LiAlH4–MgH2 composites. J. Alloys Compd. 2014, 599, 164–169. [Google Scholar] [CrossRef]
- You, Y.W.; Kong, X.S.; Wu, X.B.; Xu, Y.C.; Fang, Q.F.; Chen, J.L.; Luo, G.N.; Liu, C.S.; Pan, B.C.; Wang, Z. Dissolving, trapping and detrapping mechanisms of hydrogen in bcc and fcc transition metals. AIP Adv. 2013, 3, 012118. [Google Scholar] [CrossRef]
- Zhang, Y.; Tsushio, Y.; Enoki, H.; Akiba, E. The study on binary Mg-Co hydrogen storage alloys with BCC phase. J. Alloys Compd. 2005, 393, 147–153. [Google Scholar] [CrossRef]
- Zhang, Y.; Tsushio, Y.; Enoki, H.; Akiba, E. The hydrogen absorption-desorption performances of Mg-Co-X ternary alloys with BCC structure. J. Alloys Compd. 2005, 393, 185–193. [Google Scholar] [CrossRef]
- Shao, H.; Asano, K.; Enoki, H.; Akiba, E. Fabrication and hydrogen storage property study of nanostructured Mg-Ni-B ternary alloys. J. Alloys Compd. 2009, 479, 409–413. [Google Scholar] [CrossRef]
- Shao, H.; Asano, K.; Enoki, H.; Akiba, E. Preparation and hydrogen storage properties of nanostructured Mg-Ni BCC alloys. J. Alloys Compd. 2009, 477, 301–306. [Google Scholar] [CrossRef]
- Shao, H.; Asano, K.; Enoki, H.; Akiba, E. Correlation study between hydrogen absorption property and lattice structure of Mg-based BCC alloys. Int. J. Hydrog. Energy 2009, 34, 2312–2318. [Google Scholar] [CrossRef]
- Klyukin, K.; Shelyapina, M.G.; Fruchart, D. DFT calculations of hydrogen diffusion and phase transformations in magnesium. J. Alloys Compd. 2015, 644, 371–377. [Google Scholar] [CrossRef]
- Shao, H.; Matsuda, J.; Li, H.W.; Akiba, E.; Jain, A.; Ichikawa, T.; Kojima, Y. Phase and morphology evolution study of ball milled Mg-Co hydrogen storage alloys. Int. J. Hydrog. Energy 2013, 38, 7070–7076. [Google Scholar] [CrossRef]
- Li, B.; Yu, X.; Zhao, H.; Shao, H. Geometrical effect in Mg-based metastable nano alloys with BCC structure for hydrogen storage. Int. J. Hydrog. Energy 2019, 44, 29291–29296. [Google Scholar] [CrossRef]
- Hamann, D.R. Optimized norm-conserving Vanderbilt pseudopotentials. Phys. Rev. B 2013, 88, 085117. [Google Scholar] [CrossRef]
- Gonze, X.; Amadon, B.; Antonius, G.; Arnardi, F.; Baguet, L.; Beuken, J.-M.; Bieder, J.; Bottin, F.; Bouchet, J.; Bousquet, E.; et al. The Abinitproject: Impact, environment and recent developments. Comput. Phys. Commun. 2020, 248, 107042. [Google Scholar] [CrossRef]
- ARomero, H.; Allan, D.C.; Amadon, B.; Antonius, G.; Applencourt, T.; Baguet, L.; Bieder, J.; Bottin, F.; Bouchet, J.; Bousquet, E.; et al. ABINIT: Overview and focus on selected capabilities. J. Chem. Phys. 2020, 152, 124102. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Klyukin, K.; Shelyapina, M.G.; Fruchart, D. Hydrogen induced phase transition in magnesium: An Ab initio study. J. Alloys Compd. 2013, 580, S10–S12. [Google Scholar] [CrossRef]
- Crivello, J.C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Milanese, C.; et al. Review of magnesium hydride-based materials: Development and optimization. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef]
- Kondo, R.; Hiroyuki, T.T. Magnesium-Based Materials for Hydrogen Storage: Microstructural Properties. In Magnesium: The Wonder Element for Engineering/Biomedical Applications; Gupta, M., Ed.; IntechOpen: London, UK, 2019; pp. 1–13. [Google Scholar] [CrossRef]
- Antisari, M.V.; Montone, A.; Aurora, A.; Mancini, M.R.; Gattia, D.M.; Pilloni, L. Scanning electron microscopy of partially de-hydrogenated MgH2 powders. Intermetallics 2009, 17, 596–602. [Google Scholar] [CrossRef]
- Popilevsky, L.; Skripnyuk, V.M.; Amouyal, Y.; Rabkin, E. Tuning the thermal conductivity of hydrogenated porous magnesium hydride composites with the aid of carbonaceous additives. Int. J. Hydrog. Energy 2017, 42, 22395–22405. [Google Scholar] [CrossRef]
- Tien, H.-Y.; Tanniru, M.; Wu, C.-Y.; Ebrahimi, F. Effect of hydride nucleation rate on the hydrogen capacity of Mg. Int. J. Hydrog. Energy 2009, 34, 6343–6349. [Google Scholar] [CrossRef]
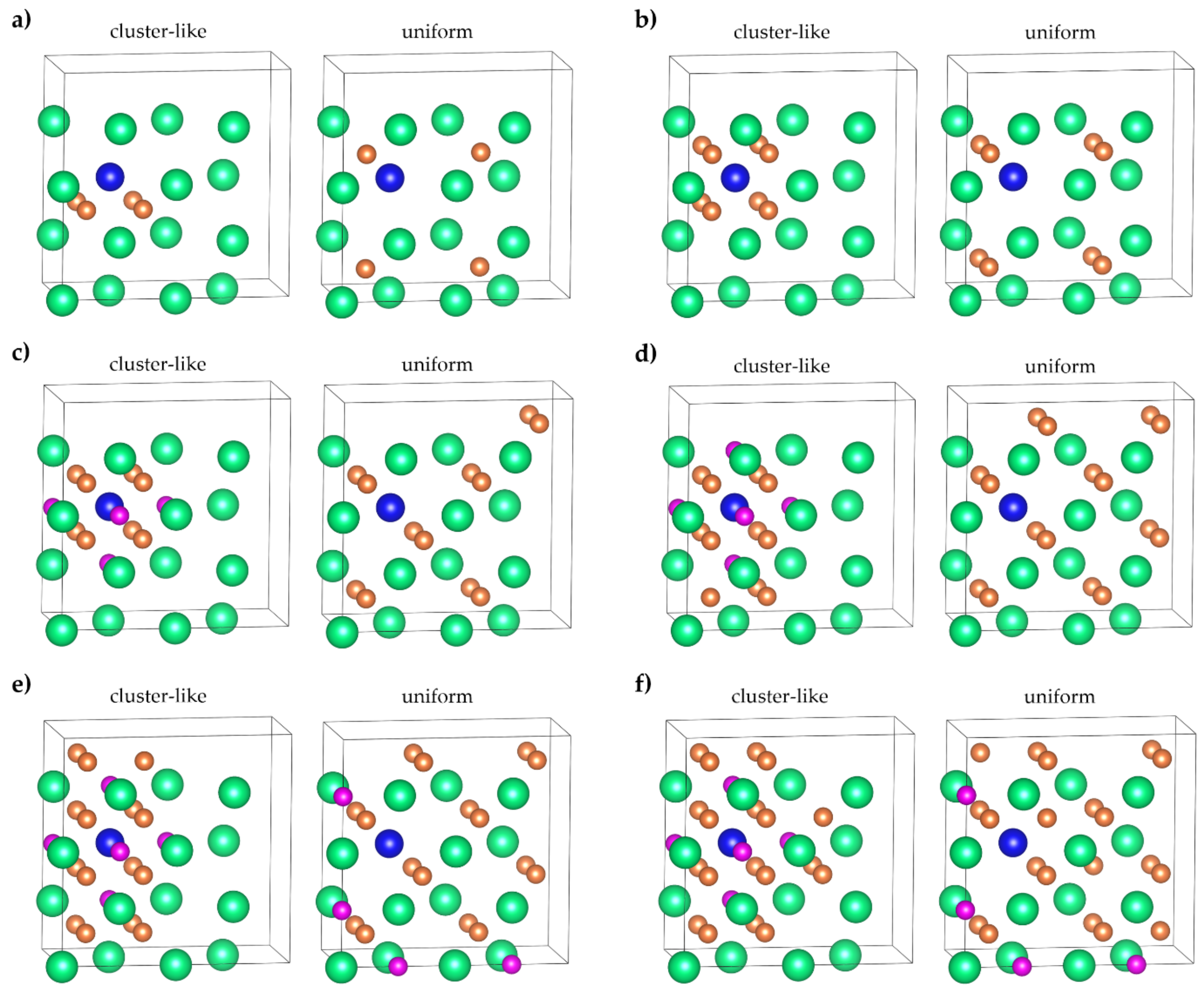

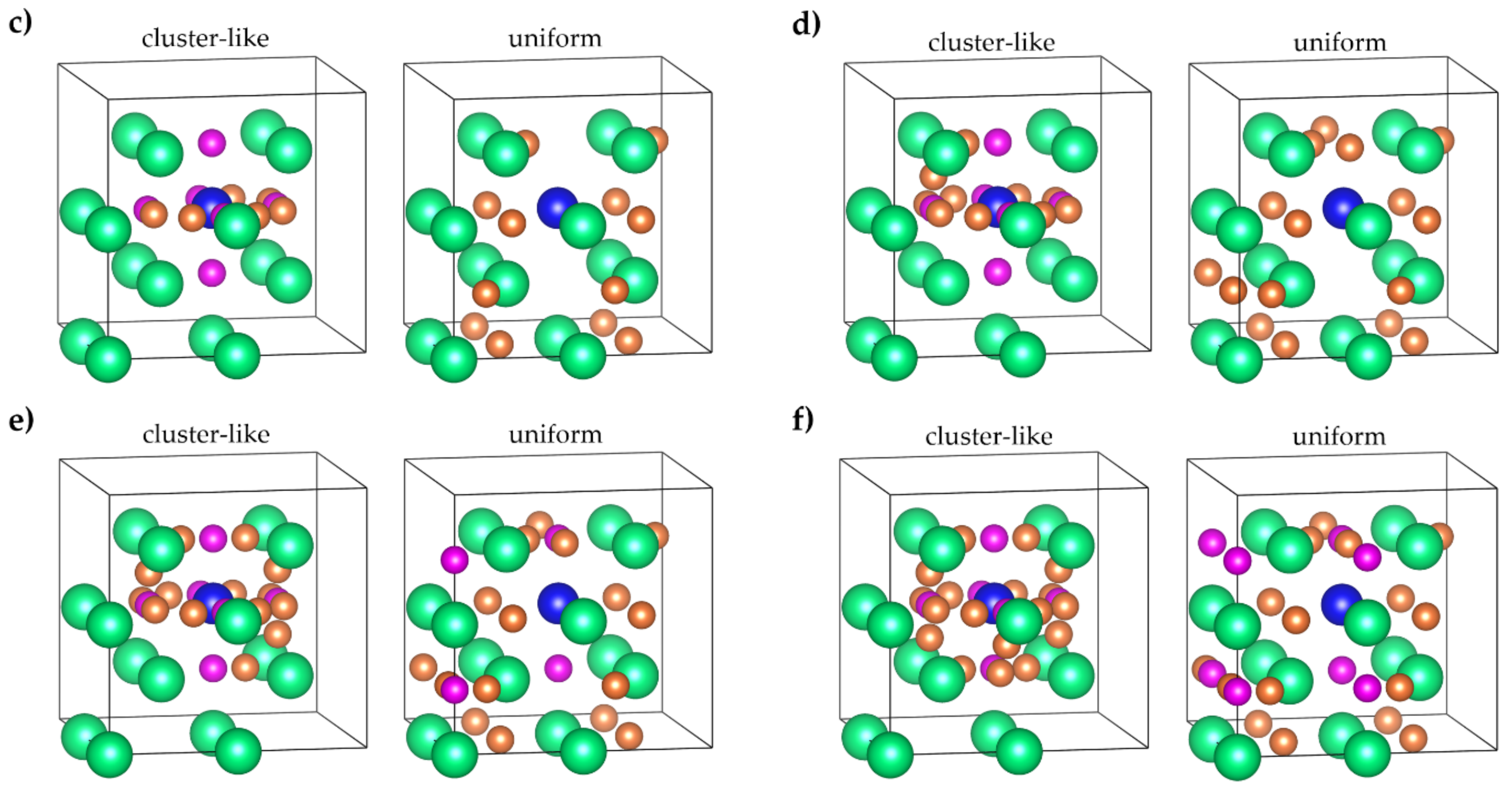



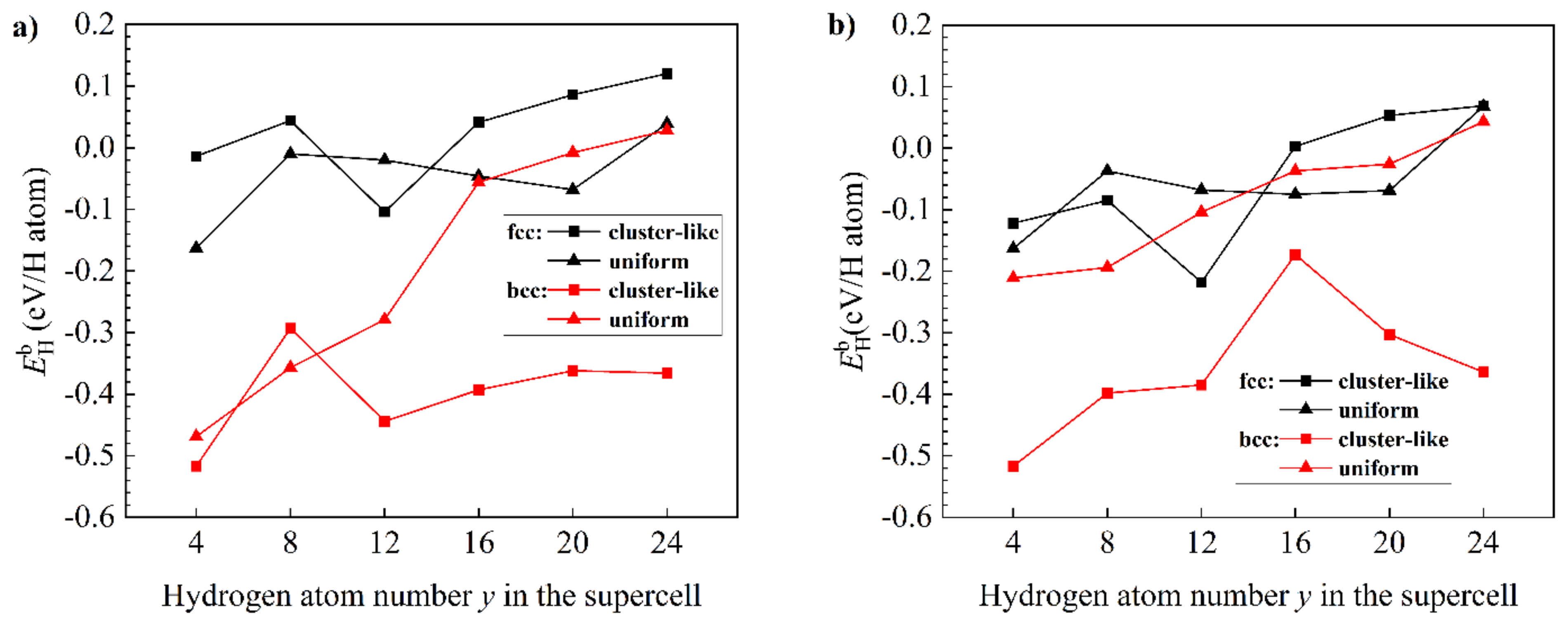
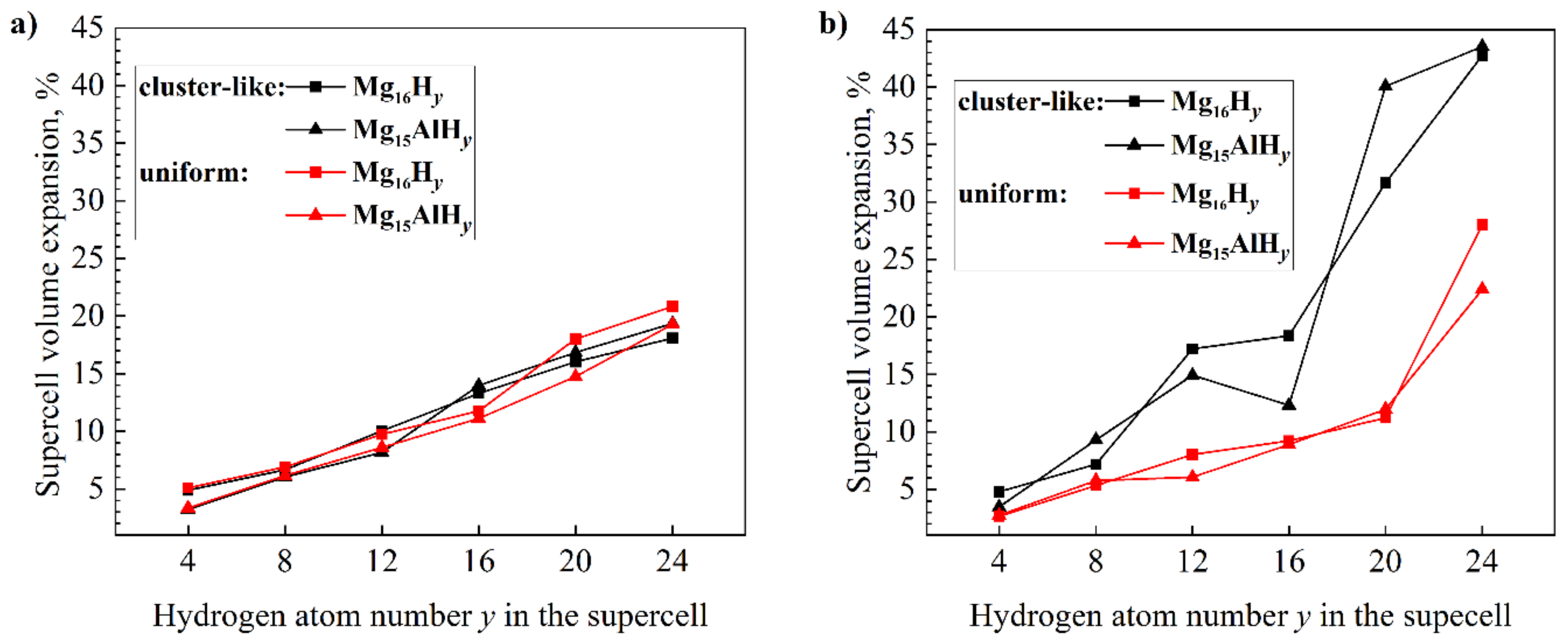

| System | Configuration | Lattice Constants, Å | System | Configuration | Lattice Constants, Å | ||
|---|---|---|---|---|---|---|---|
| fcc | bcc | fcc | bcc | ||||
| Mg16H4 | cluster-like | 4.589 | 3.641 | Mg15AlH4 | cluster-like | 4.550 | 3.615 |
| uniform | 4.592 | 3.616 | uniform | 4.553 | 3.606 | ||
| Mg16H8 | cluster-like | 4.615 | 3.669 | Mg15AlH8 | cluster-like | 4.592 | 3.681 |
| uniform | 4.619 | 3.648 | uniform | 4.593 | 3.641 | ||
| Mg16H12 | cluster-like | 4.663 | 3.780 | Mg15AlH12 | cluster-like | 4.622 | 3.743 |
| uniform | 4.659 | 3.678 | uniform | 4.628 | 3.644 | ||
| Mg16H16 | cluster-like | 4.709 | 3.792 | Mg15AlH16 | cluster-like | 4.703 | 3.715 |
| uniform | 4.687 | 3.692 | uniform | 4.664 | 3.677 | ||
| Mg16H20 | cluster-like | 4.746 | 3.929 | Mg15AlH20 | cluster-like | 4.742 | 3.998 |
| uniform | 4.773 | 3.714 | uniform | 4.714 | 3.711 | ||
| Mg16H24 | cluster-like | 4.774 | 4.037 | Mg15AlH24 | cluster-like | 4.776 | 4.031 |
| uniform | 4.811 | 3.893 | uniform | 4.775 | 3.823 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, J.; Elman, R.; Svyatkin, L.; Kudiiarov, V. Theoretical and Experimental Studies of Al-Impurity Effect on the Hydrogenation Behavior of Mg. Materials 2022, 15, 8126. https://doi.org/10.3390/ma15228126
Lyu J, Elman R, Svyatkin L, Kudiiarov V. Theoretical and Experimental Studies of Al-Impurity Effect on the Hydrogenation Behavior of Mg. Materials. 2022; 15(22):8126. https://doi.org/10.3390/ma15228126
Chicago/Turabian StyleLyu, Jinzhe, Roman Elman, Leonid Svyatkin, and Viktor Kudiiarov. 2022. "Theoretical and Experimental Studies of Al-Impurity Effect on the Hydrogenation Behavior of Mg" Materials 15, no. 22: 8126. https://doi.org/10.3390/ma15228126
APA StyleLyu, J., Elman, R., Svyatkin, L., & Kudiiarov, V. (2022). Theoretical and Experimental Studies of Al-Impurity Effect on the Hydrogenation Behavior of Mg. Materials, 15(22), 8126. https://doi.org/10.3390/ma15228126






