Direct Evidence on Effect of Oxygen Dissolution on Thermal and Electrical Conductivity of AlN Ceramics Using Al Solid-State NMR Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
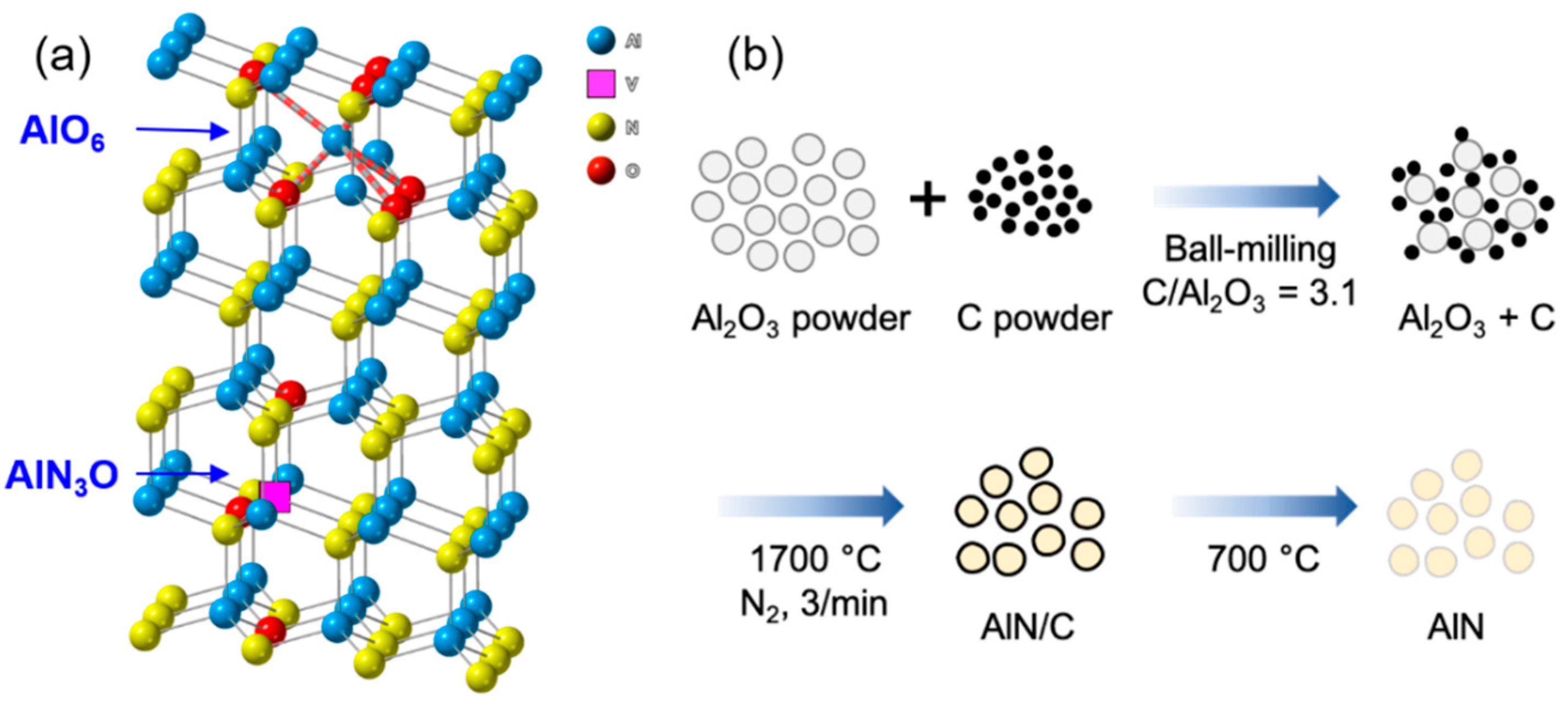
3.1. Synthesis
3.2. 27Al Solid-State NMR
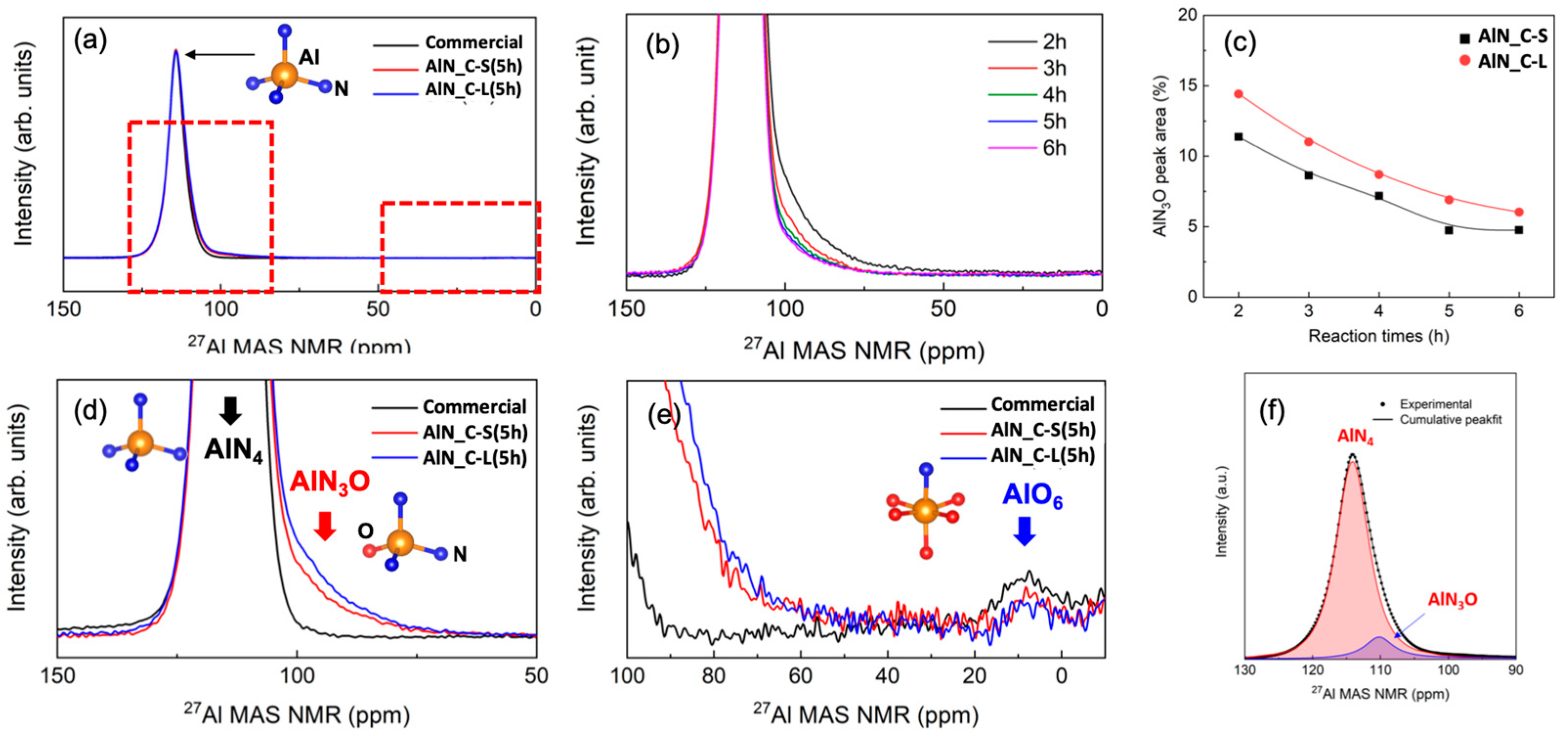
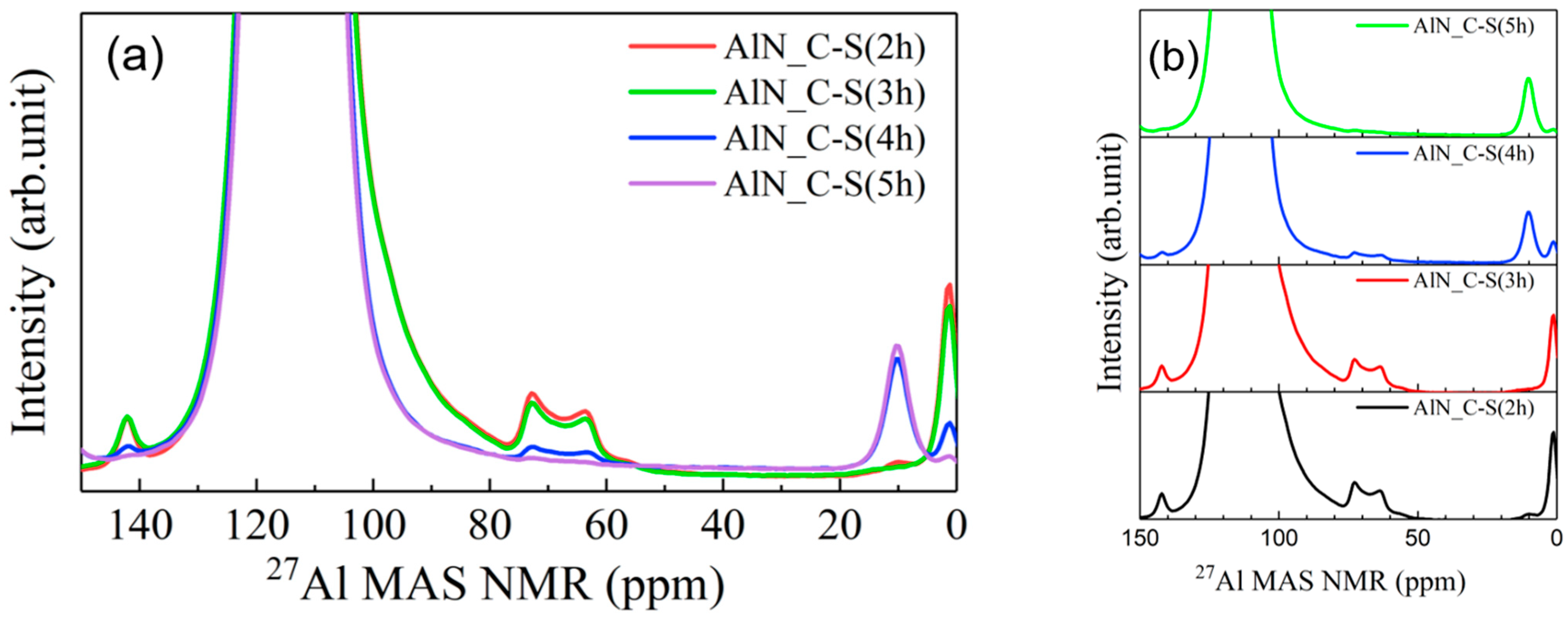
3.2.1. Raw Materials
3.2.2. Sintered Samples
3.3. Thermal Conductivity and Electrical Resistivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prasher, R.S.; Chang, J.-Y.; Sauciuc, I.; Narasimhan, S.; Chau, D.; Chrysler, G.; Myers, A.; Prstic, S.; Hu, C. Nano and Micro Technology-Based Next-Generation Package-Level Cooling Solutions. Intel. Technol. J. 2005, 9, 285–296. [Google Scholar] [CrossRef]
- Semenyuk, V. Thermoelectrics Handbook: Macro to Nano, 1st ed.; Rowe, D.M., Ed.; CRC Taylor and Francis: Boca Raton, FL, USA, 2006; pp. 58-1–58-20. [Google Scholar]
- Pop, E.; Sinha, S.; Goodson, K.E. Heat Generation and Transport in Nanometer-Scale Transistors. Proc. IEEE 2006, 94, 1587–1601. [Google Scholar] [CrossRef]
- Moore, A.L.; Shi, L. Emerging Challenges and Materials for Thermal Management of Electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
- He, Z.; Yan, Y.; Zhang, Z. Thermal Management and Temperature Uniformity Enhancement of Electronic Devices by Micro Heat Sinks: A Review. Energy 2021, 216, 119223. [Google Scholar] [CrossRef]
- Morelli, D.; Beetz, C.; Perry, T. Thermal Conductivity of Synthetic Diamond Films. J. Appl. Phys. 1988, 64, 3063–3066. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, S.; Li, C.; Lv, Y.; Liu, X.; Huang, P.Y.; Broido, D.A.; Lv, B.; Cahill, D.G. High Thermal Conductivity in Isotopically Enriched Cubic Boron Phosphide. Adv. Funct. Mater. 2018, 28, 1805116. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Q.; Lv, Y.; Liu, X.; Wang, X.; Huang, P.Y.; Cahill, D.G.; Lv, B. High Thermal Conductivity in Cubic Boron Arsenide Crystals. Science 2018, 361, 579–581. [Google Scholar] [CrossRef]
- Chen, K.; Song, B.; Ravichandran, N.K.; Zheng, Q.; Chen, X.; Lee, H.; Sun, H.; Li, S.; Udalamatta Gamage, G.A.G.; Tian, F.; et al. Ultrahigh Thermal Conductivity in Isotope-Enriched Cubic Boron Nitride. Science 2020, 367, 555–559. [Google Scholar] [CrossRef]
- Johnsen, F.A.; Rahbek, K. A physical phenomenon and its applications to telegraphy, telephony, etc. J. Inst. Electr. Eng. 1923, 61, 713. [Google Scholar] [CrossRef]
- Slack, G.A. Nonmetallic Crystals with High Thermal Conductivity. J. Phys. Chem. Solids 1973, 34, 321–335. [Google Scholar] [CrossRef]
- Slack, G.A.; Tanzilli, R.A.; Pohl, R.; Vandersande, J. The Intrinsic Thermal Conductivity of AlN. J. Phys. Chem. Solids 1987, 48, 641–647. [Google Scholar] [CrossRef]
- Hofmeister, A.M. Thermal diffusivity and thermal conductivity of single-crystal MgO and Al2O3 and related compounds as a function of temperature. Phys. Chem. Miner. 2014, 41, 361–371. [Google Scholar] [CrossRef]
- Cahill, D.G. Thermal conductivity measurement from 30 to 750 K: The 3ω method. Rev. Sci. Instrum. 1990, 61, 802–808. [Google Scholar] [CrossRef]
- Lee, S.M.; Cahill, D.G. Heat transport in thin dielectric films. J. Appl. Phys. 1997, 81, 2590–2595. [Google Scholar] [CrossRef]
- Koh, Y.R.; Cheng, Z.; Mamun, A.; Bin Hoque, M.S.; Liu, Z.; Bai, T.; Hussain, K.; Liao, M.E.; Li, R.; Gaskins, J.T.; et al. Bulk-like Intrinsic Phonon Thermal Conductivity of Micrometer Thick AlN Films. ACS Appl. Mater. Interfaces 2020, 12, 29443–29450. [Google Scholar] [CrossRef]
- Virkar, A.V.; Jackson, T.B.; Cutler, R.A. Thermodynamic and Kinetic Effects of Oxygen Removal on the Thermal Conductivity of Aluminum Nitride. J. Am. Ceram. Soc. 1989, 72, 2031–2042. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.; Zhang, S.; Wang, Y.; Zhang, Y.; Liu, C.; Zhang, D.; Jia, B.; Guo, D.; Chu, A.; et al. The Quantitative investigation of the lattice oxygen and grain edge oxygen on the thermal conductivity of aluminum nitride ceramics. J. Europ. Ceram. Soc. 2022; in press. [Google Scholar]
- Coëffe-Desvaux, M.; Pradeilles, N.; Marchet, P.; Vandenhende, M.; Joinet, M.; Maître, A. Comparative Study on Electrical Conductivity of CeO2-Doped AlN Ceramics Sintered by Hot-Pressing and Spark Plasma Sintering. Materials 2022, 15, 2399. [Google Scholar] [CrossRef]
- Júnior, A.F.; Shanafield, D.J. Thermal conductivity of polycrystalline aluminum nitride (AlN) ceramics. Cerâmica 2004, 50, 247–253. [Google Scholar] [CrossRef]
- Ganguly, D.; Bera, A.; Hore, R.; Khanra, S.; Maji, P.M.; Kotnees, D.K.; Chattopadhyay, S. Coining the attributes of nano to micro dual hybrid silica-ceramic waste filler based green HNBR composites for triple percolation: Mechanical properties, thermal, and electrical conductivity. Chem. Eng. J. Adv. 2022, 11, 100338. [Google Scholar] [CrossRef]
- Harris, J.H.; Youngman, R.A.; Teller, R.G. on the nature of the oxygen-related defect in aluminum nitride. J. Mater. Res. 1990, 5, 1763–1773. [Google Scholar] [CrossRef]
- Fitzgerald, J.J.; Kohl, S.D.; Piedra, G.P. Observation of Four-Coordinate Aluminum Oxynitride (AlO4-xNx) Environments in AlON Solids by MAS 27Al NMR at 14 T. Chem. Mater. 1994, 6, 1915–1917. [Google Scholar] [CrossRef]
- Jung, W.; Chae, S. 27Al MAS NMR spectroscopic identification of reaction intermediates in the carbothermal reduction and nitridation of alumina. Mater. Chem. Phys. 2010, 123, 610–613. [Google Scholar] [CrossRef]
- Abeles, B. Lattice Thermal Conductivity of Disordered Semiconductor Alloys at High Temperatures. Phys. Rev. 1963, 131, 1906–1911. [Google Scholar] [CrossRef]
- Slack, G.A. Thermal Conductivity of MgO, Al2O3, MgAl2O4, and Fe3O4 Crystals from 3 to 300 K. Phys. Rev. 1962, 126, 427–441. [Google Scholar] [CrossRef]
- Xu, L.R.; Rojo, M.M.; Islam, S.M.; Sood, A.; Vareskic, B.; Katre, A.; Mingo, N.; Goodson, K.E.; Xing, H.G.; Jena, D.; et al. Thermal conductivity of crystalline AlN and the influence of atomic-scale defects. J. Appl. Phys. 2019, 126, 185105. [Google Scholar] [CrossRef]
- Jang, S.A.; Choi, G.M. Electrical conduction in aluminum nitride. J. Am. Ceram. Soc. 1993, 76, 957–960. [Google Scholar] [CrossRef]
- Richards, V.L.; Tien, T.Y.; Pehlke, R.D. High-temperature electrical conductivity of aluminium nitride. J. Mater. Sci. 1987, 22, 3385–3390. [Google Scholar] [CrossRef][Green Version]
- Kim, H.-S.; Chae, J.-M.; Oh, Y.-S.; Kim, H.-T.; Shim, K.-B.; Lee, S.-M. Effects of Carbothermal Reduction on the Thermal and Electrical Conductivities of Aluminum Nitride Ceramics. Ceram. Int. 2010, 36, 2039–2045. [Google Scholar] [CrossRef]
- Yu, D.; Lee, E.; Lee, S.-M.; Kim, J.Y. High-temperature ionic and electronic resistivity of MgO- and Ta2O5- doped aluminum nitride. J. Korean Phys. Soc. 2018, 72, 129–137. [Google Scholar] [CrossRef]
- Lee, E.; Pee, J.-H.; Lee, S.-M.; Kim, J.Y. Enhanced High-Temperature Electrical Resistivity of Aluminum Nitride Obtained by Engineering a Schottky Barrier at Grain Boundaries. J. Korean Phys. Soc. 2020, 77, 673–679. [Google Scholar] [CrossRef]


| Reaction Time | O (wt%/at%) | C (ppm) | AlN3O NMR Peak Area (Raw Material, %) | AlN3O NMR Peak Area (Sintered, %) | |||
|---|---|---|---|---|---|---|---|
| AlN_C-S | AlN_C-L | AlN_C-S | AlN_C-L | AlN_C-S | AlN_C-L | AlN_C-S | |
| 2 h | 1.47/3.77 | 1.57/4.02 | 1691 | 2761 | 11.37 | 14.42 | 10.27 |
| 3 h | 1.22/3.15 | 1.31/3.36 | 1527 | 2416 | 8.63 | 11.01 | 9.86 |
| 4 h | 1.03/2.64 | 1.16/2.97 | 1412 | 2188 | 7.17 | 8.7 | 4.59 |
| 5 h | 0.86/2.20 | 0.99/2.54 | 1087 | 2097 | 4.72 | 6.9 | 2.91 |
| 6 h | 0.84/2.15 | 1.01/2.59 | 1132 | 2072 | 4.75 | 6.04 | 4.29 |
| Commercial | 0.85/2.17 | 280 | 0.0 | - | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, J.-Y.; Ahn, H.; Jeong, M.H.; Lee, E.; Cho, K.; Lee, S.-M.; Shim, W.; Pee, J.-H. Direct Evidence on Effect of Oxygen Dissolution on Thermal and Electrical Conductivity of AlN Ceramics Using Al Solid-State NMR Analysis. Materials 2022, 15, 8125. https://doi.org/10.3390/ma15228125
Kim J, Kim J-Y, Ahn H, Jeong MH, Lee E, Cho K, Lee S-M, Shim W, Pee J-H. Direct Evidence on Effect of Oxygen Dissolution on Thermal and Electrical Conductivity of AlN Ceramics Using Al Solid-State NMR Analysis. Materials. 2022; 15(22):8125. https://doi.org/10.3390/ma15228125
Chicago/Turabian StyleKim, Jaegyeom, Jong-Young Kim, Heewon Ahn, Mu Hyeok Jeong, Eunsil Lee, Keonhee Cho, Sung-Min Lee, Wooyoung Shim, and Jae-Hwan Pee. 2022. "Direct Evidence on Effect of Oxygen Dissolution on Thermal and Electrical Conductivity of AlN Ceramics Using Al Solid-State NMR Analysis" Materials 15, no. 22: 8125. https://doi.org/10.3390/ma15228125
APA StyleKim, J., Kim, J.-Y., Ahn, H., Jeong, M. H., Lee, E., Cho, K., Lee, S.-M., Shim, W., & Pee, J.-H. (2022). Direct Evidence on Effect of Oxygen Dissolution on Thermal and Electrical Conductivity of AlN Ceramics Using Al Solid-State NMR Analysis. Materials, 15(22), 8125. https://doi.org/10.3390/ma15228125






