Influence of Cellulose Nanofibers on the Behavior of Pickering Emulsions. Part 1. Microscopy and Startup Flow Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
3.1. Morphological Observations
3.1.1. Polarized Optical Microscopy
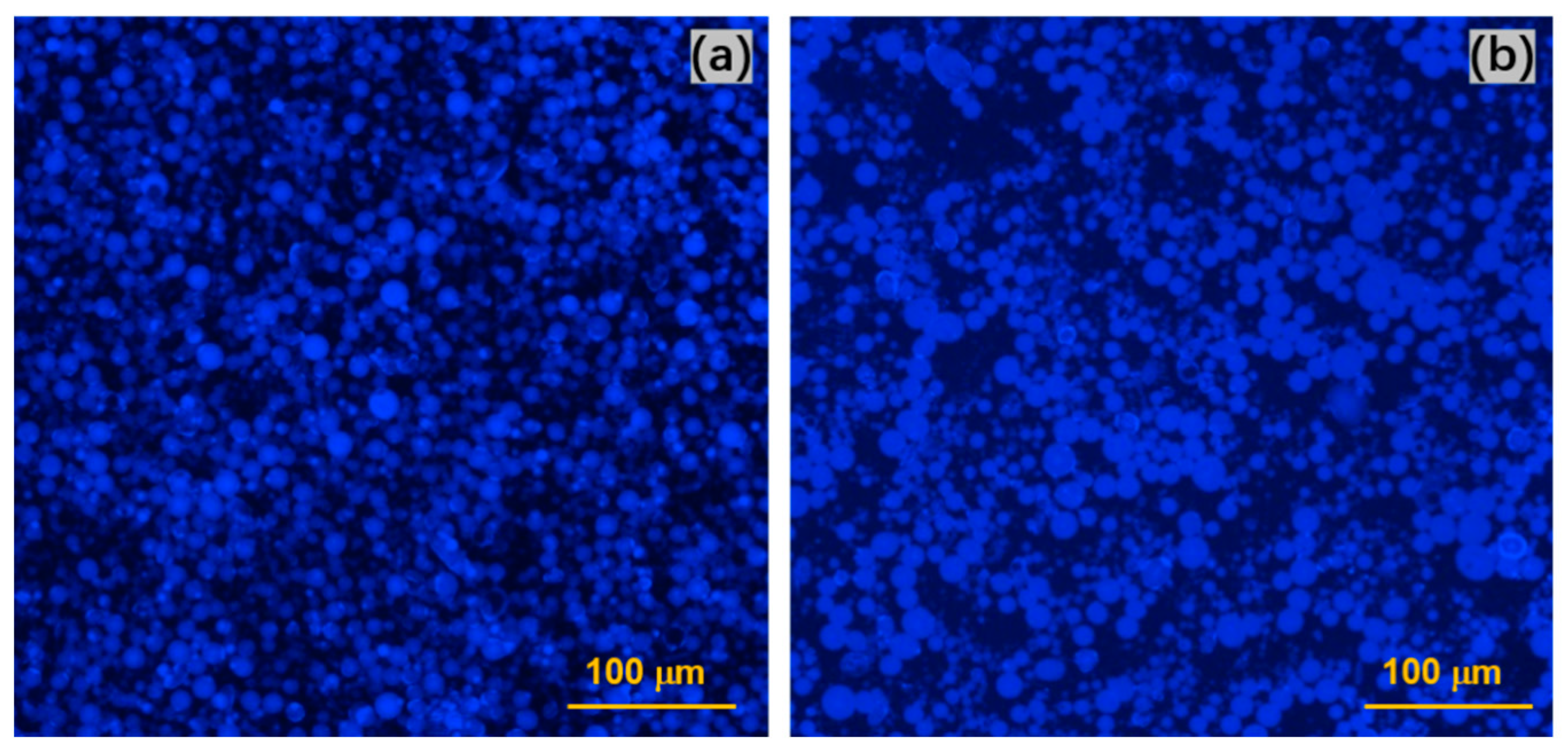
3.1.2. Fluorescence Analysis on CNF Distribution
3.1.3. SEM Analysis on CNF Droplets
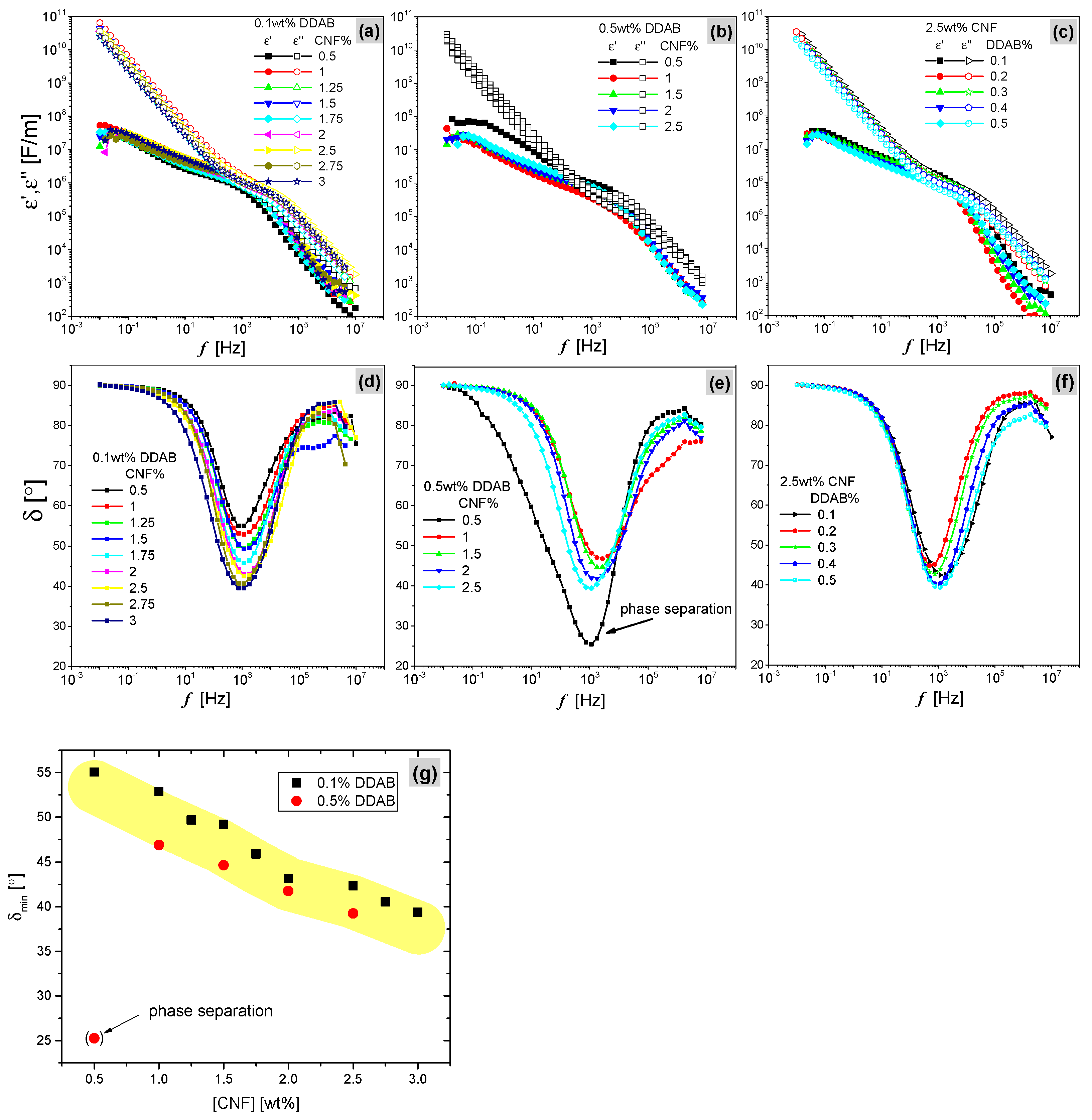
3.2. Dielectric Spectroscopy
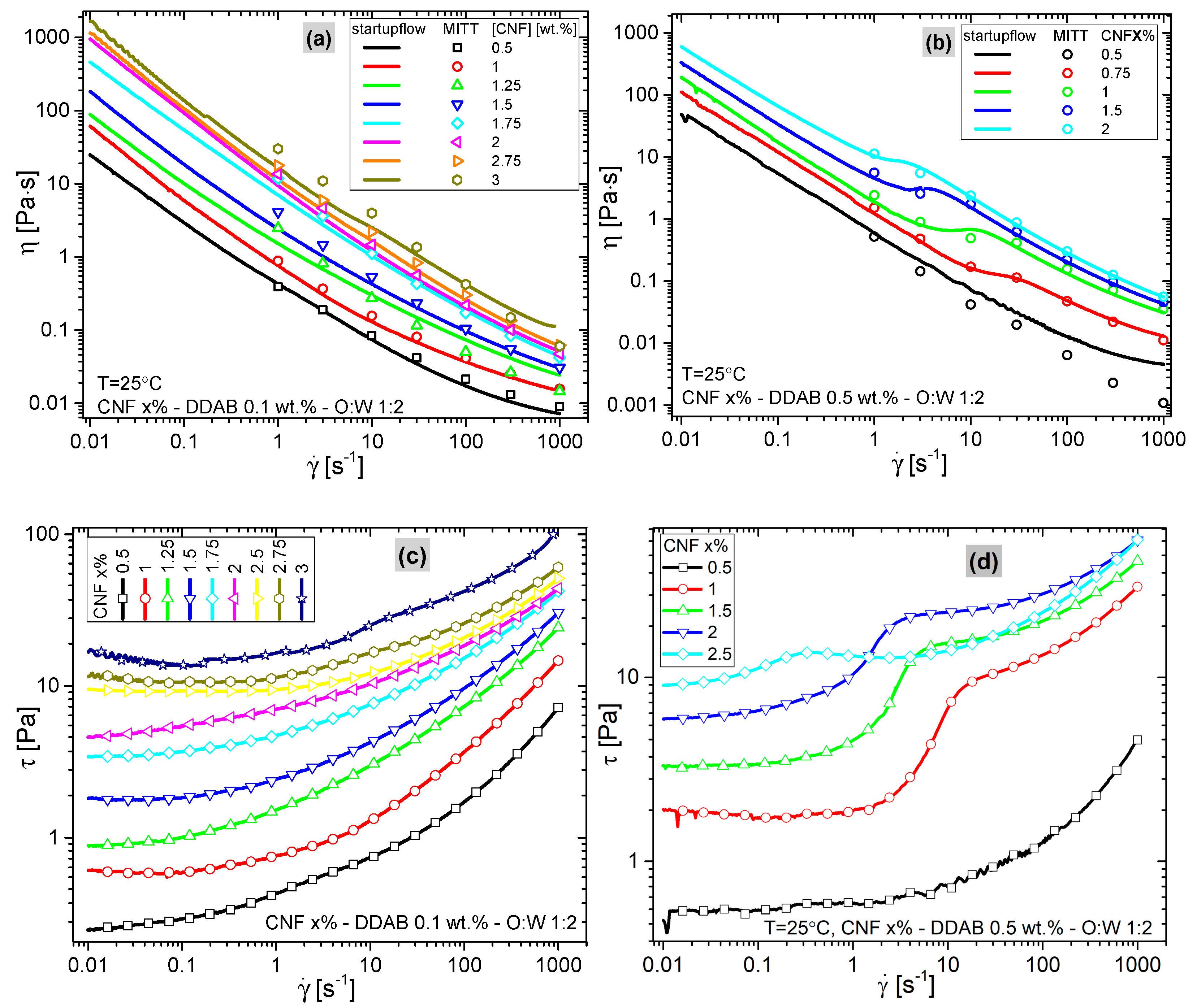
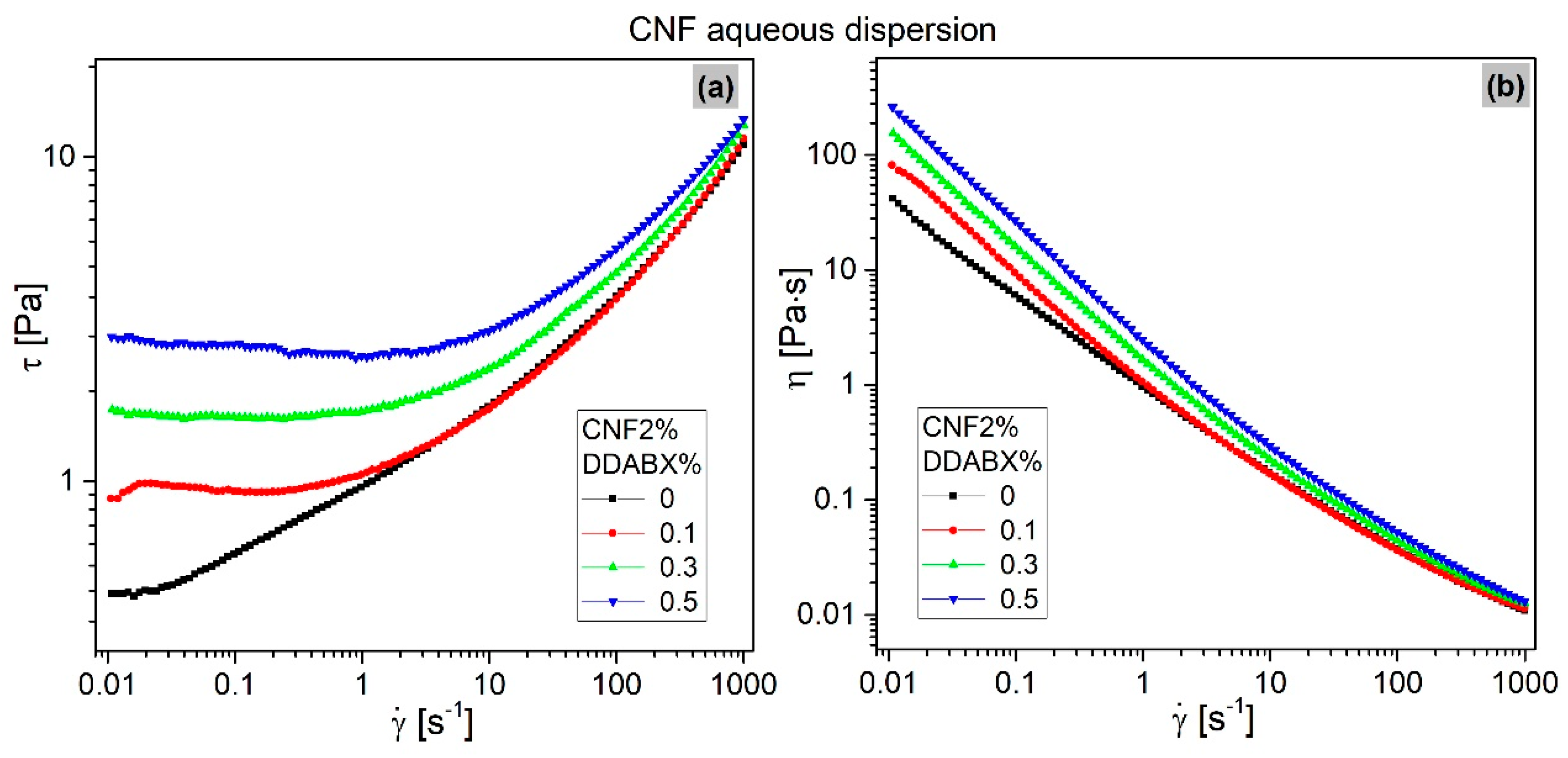
3.3. Rheology
3.3.1. Deformation-Induced Morphology Changes
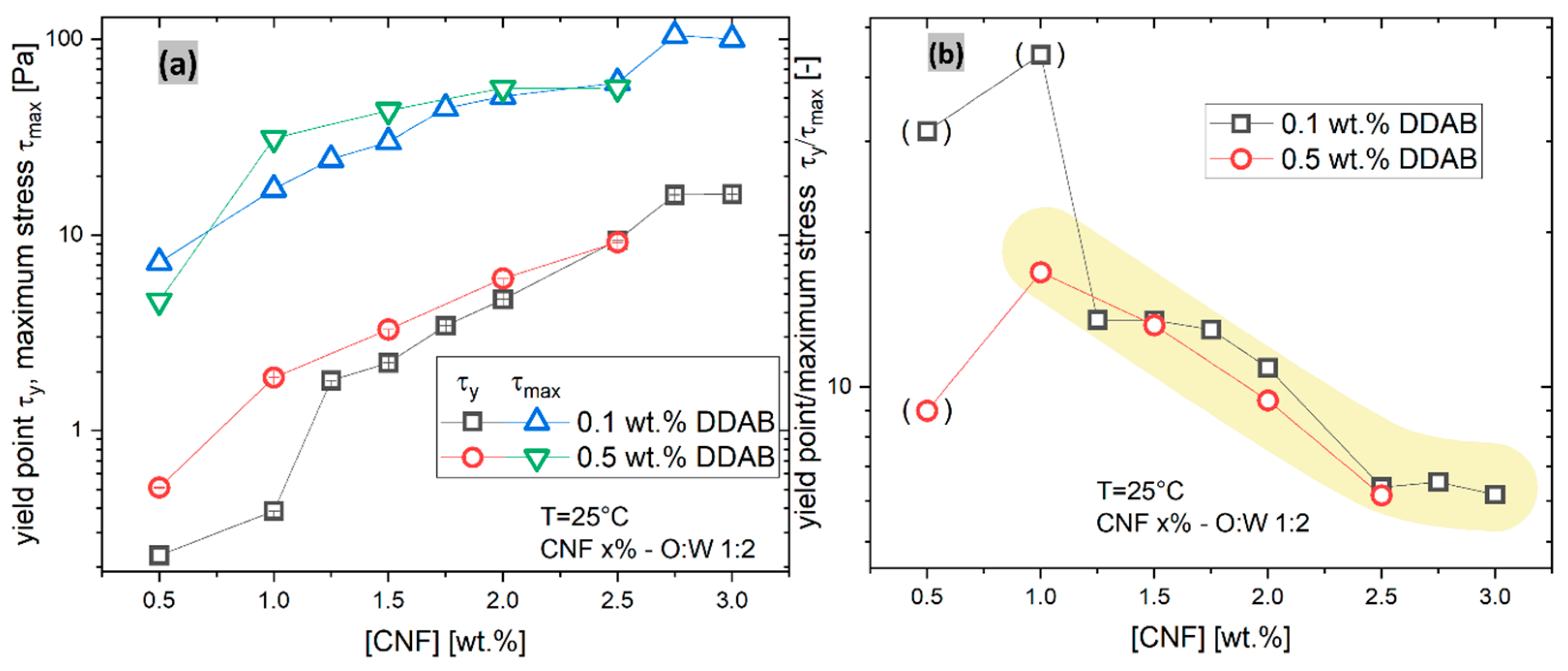
3.3.2. Startup Flows
- 1.
- The maximum stress was directly determined as the maximum stress recorded (always found at a shear rate of = 1000 s−1). Higher shear rates destroyed the sample and were therefore not included in the paper.
- 2.
- The yield stress was determined using a yield-stress modified Carreau–Yasuda model [64]where denotes the characteristic shear rate, a denotes the sharpness of the transition in a regular viscosity function, n denotes the slope of the viscosity function at a high shear rate (n = 0→dlogη/dlog = −1), and denotes the yield stress. As the contribution of dominates the viscosity function at low , the other parameters were not evaluated, owing to their high dependence on each other. For the 0.5% DDAB sample series with the kink in the data, the fitted yield stress values were checked with extreme care and found within the range that one would determine manually from visually extrapolating the data towards = 0.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siqueira, G.; Kokkinis, D.; Libanori, R.; Hausmann, M.K.; Gladman, A.S.; Neels, A.; Tingaut, P.; Zimmermann, T.; Lewis, J.A.; Studart, A.R. Cellulose nanocrystal inks for 3d printing of textured cellular architectures. Adv. Funct. Mater. 2017, 27, 1604619. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Holbery, J.; Li, K. A technique for production of nanocrystalline cellulose with a narrow size distribution. Cellulose 2009, 16, 455–465. [Google Scholar] [CrossRef]
- Rampinelli, G.; Di Landro, L.; Fujii, T. Characterization of biomaterials based on microfibrillated cellulose with different modifications. J. Reinf. Plast. Compos. 2009, 29, 1793–1803. [Google Scholar] [CrossRef]
- Syverud, K.; Stenius, P. Strength and barrier properties of MFC films. Cellulose 2008, 16, 75–85. [Google Scholar] [CrossRef]
- Iwatake, A.; Nogi, M.; Yano, H. Cellulose nanofiber-reinforced polylactic acid. Compos. Sci. Technol. 2008, 68, 2103–2106. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr Polym 2019, 219, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Halib, N.; Ahmad, I.; Grassi, M.; Grassi, G. The remarkable three-dimensional network structure of bacterial cellulose for tissue engineering applications. Int. J. Pharm. 2019, 566, 631–640. [Google Scholar] [CrossRef]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104 Pt A, 97–106. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Azin, M.; Ashori, A. Production of bacterial cellulose using different carbon sources and culture media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.X.; Wu, Y.H.; He, K.H.; Li, Y.; Luo, X.G.; Li, B.; Wang, C.T.; Liu, S.L. Flexible cellulose nanofibrils as novel pickering stabilizers: The emulsifying property and packing behavior. Food Hydrocoll. 2019, 88, 180–189. [Google Scholar] [CrossRef]
- Capron, I.; Cathala, B. Surfactant-free high internal phase emulsions stabilized by cellulose nanocrystals. Biomacromolecules 2013, 14, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, S.; Li, Y.; Forth, J.; Wang, D.; Russell, T.P. Liquid tubule formation and stabilization using cellulose nanocrystal surfactants. Angew. Chem. Int. Ed. Engl. 2017, 56, 12594–12598. [Google Scholar] [CrossRef] [PubMed]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Li, M.C.; Wu, Q.; Moon, R.J.; Hubbe, M.A.; Bortner, M.J. Rheological aspects of cellulose nanomaterials: Governing factors and emerging applications. Adv. Mater. 2021, 33, 2006052. [Google Scholar] [CrossRef]
- Du, L.; Zhong, T.; Wolcott, M.P.; Zhang, Y.; Qi, C.; Zhao, B.; Wang, J.; Yu, Z. Dispersing and stabilizing cellulose nanoparticles in acrylic resin dispersions with unreduced transparency and changed rheological property. Cellulose 2018, 25, 2435–2450. [Google Scholar] [CrossRef]
- Li, M.-C.; Wu, Q.; Song, K.; Lee, S.; Qing, Y.; Wu, Y. Cellulose nanoparticles: Structure–morphology–rheology relationships. ACS Sustain. Chem. Eng. 2015, 3, 821–832. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathala, B.; Capron, I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 2013, 9, 952–959. [Google Scholar] [CrossRef]
- Nomena, E.M.; van der Vaart, M.; Voudouris, P.; Velikov, K.P. Rheology of oil-in-water emulsions stabilised by native cellulose microfibrils in primary plant cells dispersions. Food Struct. 2021, 30, 100239. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, Y.; Chen, W.; Liu, Y.; Wang, Q.; Li, J.; Yu, H. Homogeneous dispersion of cellulose nanofibers in waterborne acrylic coatings with improved properties and unreduced transparency. ACS Sustain. Chem. Eng. 2016, 4, 3766–3772. [Google Scholar] [CrossRef]
- Kalia, S.; Boufi, S.; Celli, A.; Kango, S. Nanofibrillated cellulose: Surface modification and potential applications. Colloid Polym. Sci. 2013, 292, 5–31. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhu, G.M.; Dong, B.Q.; Wang, F.; Tang, J.; Stadler, F.J.; Yang, G.H.; Hong, S.X.; Xing, F. Interfacial jamming reinforced Pickering emulgel for arbitrary architected nanocomposite with connected nanomaterial matrix. Nat. Commun. 2021, 12, 111. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants—Similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Hong, J.S.; Ruhs, P.A.; Fischer, P. Localization of clay particles at the oil-water interface in the presence of surfactants. Rheol. Acta 2015, 54, 725–734. [Google Scholar] [CrossRef]
- Gelot, A.; Friesen, W.; Hamza, H.A. Emulsification of oil and water in the presence of finely divided solids and surface-active agents. Colloids Surf. 1984, 12, 271–303. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Effects of oil type and aqueous phase composition on oil-water mixtures containing particles of intermediate hydrophobicity. Phys. Chem. Chem. Phys. 2000, 2, 2959–2967. [Google Scholar] [CrossRef]
- Hong, J.S.; Fischer, P. Bulk and interfacial rheology of emulsions stabilized with clay particles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 316–326. [Google Scholar] [CrossRef]
- Whitby, C.P.; Fornasiero, D.; Ralston, J. Effect of oil soluble surfactant in emulsions stabilised by clay particles. J. Colloid Interface Sci. 2008, 323, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, S.; Koch, C.R.; Bhattacharjee, S.; Sadrzadeh, M. Dielectric behavior of oil–water emulsions during phase separation probed by electrical impedance spectroscopy. Sens. Actuators B Chem. 2017, 243, 460–464. [Google Scholar] [CrossRef]
- Jiang, Q.; Sun, N.; Kumar, P.; Li, Q.; Liu, B.; Li, A.; Wang, W.; Gao, Z. Real-time analysis of the stability of oil-in-water pickering emulsion by electrochemical impedance spectroscopy. Molecules 2020, 25, 2904. [Google Scholar] [CrossRef]
- de Oliveira, H.P.; de Melo, C.P. b Use of electrical impedance spectroscopy as a practical method of investigating the formation of aggregates in aqueous solutions of dyes and surfactants. J. Phys. Chem. B 2011, 115, 6903–6908. [Google Scholar] [CrossRef]
- Ghasemi, S.; Darestani, M.T.; Abdollahi, Z.; Hawkett, B.S.; Comes, V.G. Electrical impedance spectroscopy for determining critical micelle concentration of ionic emulsifiers. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 195–203. [Google Scholar] [CrossRef]
- Klein, R.J.; Zhang, S.; Dou, S.; Jones, B.H.; Colby, R.H.; Runt, J. Modeling electrode polarization in dielectric spectroscopy: Ion mobility and mobile ion concentration of single-ion polymer electrolytes. J. Chem. Phys. 2006, 124, 144903. [Google Scholar] [CrossRef]
- Sjöblom, J.; Skodvin, T.; Jakobsen, T.; Dukhin, S.S. Dielectric spectroscopy and emulsions. A theoretical and experimental approach. J. Dispers. Sci. Technol. 1994, 15, 401–421. [Google Scholar] [CrossRef]
- Sen, S.; Boyd, R.H. Dielectric relaxation in amorphous linear aliphatic copolyesters. Eur. Polym. J. 2008, 44, 3280–3287. [Google Scholar] [CrossRef]
- Boyle, M.H. The electrical-properties of heterogeneous mixtures containing an oriented spheroidal dispersed phase. Colloid Polym. Sci. 1985, 263, 51–57. [Google Scholar] [CrossRef]
- Hill, R.M.; Cooper, J. Characterization of water-in-oil emulsions by means of dielectric-spectroscopy. J. Mater. Sci. 1992, 27, 4818–4827. [Google Scholar] [CrossRef]
- Skodvin, T.; Sjoblom, J. Models for the dielectric properties of flocculated w/o-emulsions. J. Colloid Interface Sci. 1996, 182, 190–198. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Ilyin, S.O. Morphology and rheology of heavy crude oil/water emulsions stabilized by microfibrillated cellulose. Energy Fuels 2021, 35, 6527–6540. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, J.G.; Su, Y.Q.; Xu, Z.H.; Li, M.C.; Ying, R.F.; Wu, J.Q. Preparation and properties of microfibrillated cellulose with different carboxyethyl content. Carbohydr. Polym. 2019, 206, 616–624. [Google Scholar] [CrossRef]
- Ougiya, H.; Watanabe, K.; Morinaga, Y.; Yoshinaga, F. Emulsion-stabilizing effect of bacterial cellulose. Biosci. Biotechnol. Biochem. 1997, 61, 1541–1545. [Google Scholar] [CrossRef] [Green Version]
- Winuprasith, T.; Suphantharika, M. Properties and stability of oil-in-water emulsions stabilized by microfibrillated cellulose from mangosteen rind. Food Hydrocoll. 2015, 43, 690–699. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef]
- Lu, Y.; Qian, X.L.; Xie, W.Y.; Zhang, W.T.; Huang, J.; Wu, D.F. Rheology of the sesame oil-in-water emulsions stabilized by cellulose nanofibers. Food Hydrocoll. 2019, 94, 114–127. [Google Scholar] [CrossRef]
- Costa, C.; Mira, I.; Benjamins, J.-W.; Lindman, B.; Edlund, H.; Norgren, M. Interfacial activity and emulsion stabilization of dissolved cellulose. J. Mol. Liq. 2019, 292, 111325. [Google Scholar] [CrossRef]
- Kim, J.-H.; Shim, B.S.; Kim, H.S.; Lee, Y.-J.; Min, S.-K.; Jang, D.; Abas, Z.; Kim, J. Review of nanocellulose for sustainable future materials. Int. J. Precis. Eng. Manuf.-Green Technol. 2015, 2, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Zembyla, M.; Lazidis, A.; Murray, B.S.; Sarkar, A. Water-in-oil pickering emulsions stabilized by synergistic particle-particle interactions. Langmuir 2019, 35, 13078–13089. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.L.R.; Gomes, A.; Cangussu, L.B.; Cunha, R.L.; de Oliveira, L.S.; Franca, A.S. Stabilization mechanisms of O/W emulsions by cellulose nanocrystals and sunflower protein. Food Res. Int. 2022, 152, 110930. [Google Scholar] [CrossRef]
- Stadler, F.J.; Cui, S.M.; Hashmi, S.; Handschuh-Wang, S.; Li, W.Q.; Wang, S.C.; Yan, Z.C.; Zhu, G.M. Multiple interval thixotropic test (miTT)-an advanced tool for the rheological characterization of emulsions and other colloidal systems. Rheol. Acta 2022, 61, 229–242. [Google Scholar] [CrossRef]
- Matos, M.; Marefati, A.; Bordes, R.; Gutierrez, G.; Rayner, M. Combined emulsifying capacity of polysaccharide particles of different size and shape. Carbohydr. Polym. 2017, 169, 127–138. [Google Scholar] [CrossRef]
- Zhou, H.; Lv, S.; Liu, J.; Tan, Y.; Muriel Mundo, J.L.; Bai, L.; Rojas, O.J.; Mclements, D.J. Modulation of physicochemical characteristics of Pickering emulsions: Utilization of nanocellulose- and nanochitin-coated lipid droplet blends. J. Agric. Food Chem. 2020, 68, 603–611. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, L.L.; Wang, B.J.; Sui, X.F.; Zhong, Y.; Zhang, L.P.; Mao, Z.P.; Xu, H. Cellulose-rich oleogels prepared with an emulsion-templated approach. Food Hydrocoll. 2018, 77, 460–464. [Google Scholar] [CrossRef]
- Patel, I.; Woodcock, J.; Beams, R.; Stranick, S.J.; Nieuwendaal, R.; Gilman, J.W.; Mulenos, M.R.; Sayes, C.M.; Salari, M.; DeLoid, G.; et al. Fluorescently labeled cellulose nanofibers for environmental health and safety studies. Nanomaterials 2021, 11, 1015. [Google Scholar] [CrossRef]
- Pan, X.D.; McKinley, G.H. Characteristics of electrorheological responses in an emulsion system. J. Colloid Interface Sci. 1997, 195, 101–113. [Google Scholar] [CrossRef]
- Richert, R.; Wagner, H. The dielectric modulus: Relaxation versus retardation. Solid State Ion. 1998, 105, 167–173. [Google Scholar] [CrossRef]
- Valentini, L.; Bittolo Bon, S.; Cardinali, M.; Fortunati, E.; Kenny, J.M. Cellulose nanocrystals thin films as gate dielectric for flexible organic field-effect transistors. Mater. Lett. 2014, 126, 55–58. [Google Scholar] [CrossRef]
- Niu, F.; Li, M.; Huang, Q.; Zhang, X.; Pan, W.; Yang, J.; Li, J. The characteristic and dispersion stability of nanocellulose produced by mixed acid hydrolysis and ultrasonic assistance. Carbohydr. Polym. 2017, 165, 197–204. [Google Scholar] [CrossRef]
- Skodvin, T.; Sjoblom, J.; Saeten, J.O.; Warnheim, T.; Gestblom, B. A time-domain dielectric-spectroscopy study of some model emulsions and liquid margarines. Colloids Surf. A Physicochem. Eng. Asp. 1994, 83, 75–82. [Google Scholar] [CrossRef]
- Nomena, E.M.; Remijn, C.; Rogier, F.; van der Vaart, M.; Voudouris, P.; Velikov, K.P. Unravelling the mechanism of stabilization and microstructure of oil-in-water emulsions by native cellulose microfibrils in primary plant cells dispersions. ACS Appl. Bio. Mater. 2018, 1, 1440–1447. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xu, C.; Huang, J.; Wu, D.; Lv, Q. Rheological properties of nanocrystalline cellulose suspensions. Carbohydr. Polym. 2017, 157, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Chen, Y.; Shi, C.; Ye, Y.; Abid, M.; Jabbar, S.; Wang, P.; Zeng, X.; Wu, T. Rheological properties of an amorphous cellulose suspension. Food Hydrocoll. 2014, 39, 27–33. [Google Scholar] [CrossRef]
- Yasuda, K.; Armstrong, R.C.; Cohen, R.E. Shear flow properties of concentrated solutions of linear and star branched polystyrenes. Rheol. Acta 1981, 20, 163–178. [Google Scholar] [CrossRef]
- Chanamai, R.; Mclements, D.J. Dependence of creaming and rheology of monodisperse oil-in-water emulsions on droplet size and concentration. Colloids Surf. A Physicochem. Eng. Asp. 2000, 172, 79–86. [Google Scholar] [CrossRef]
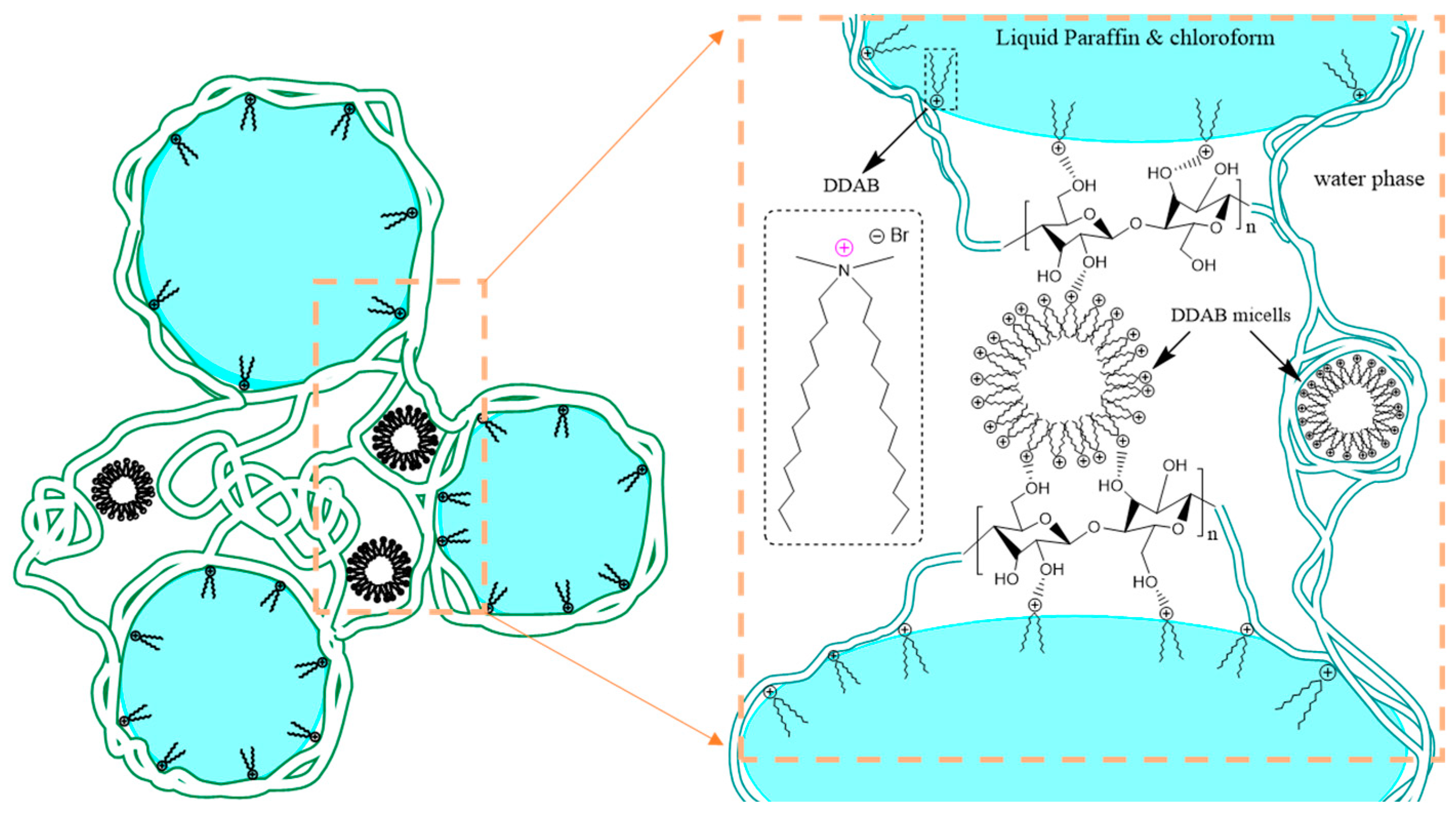
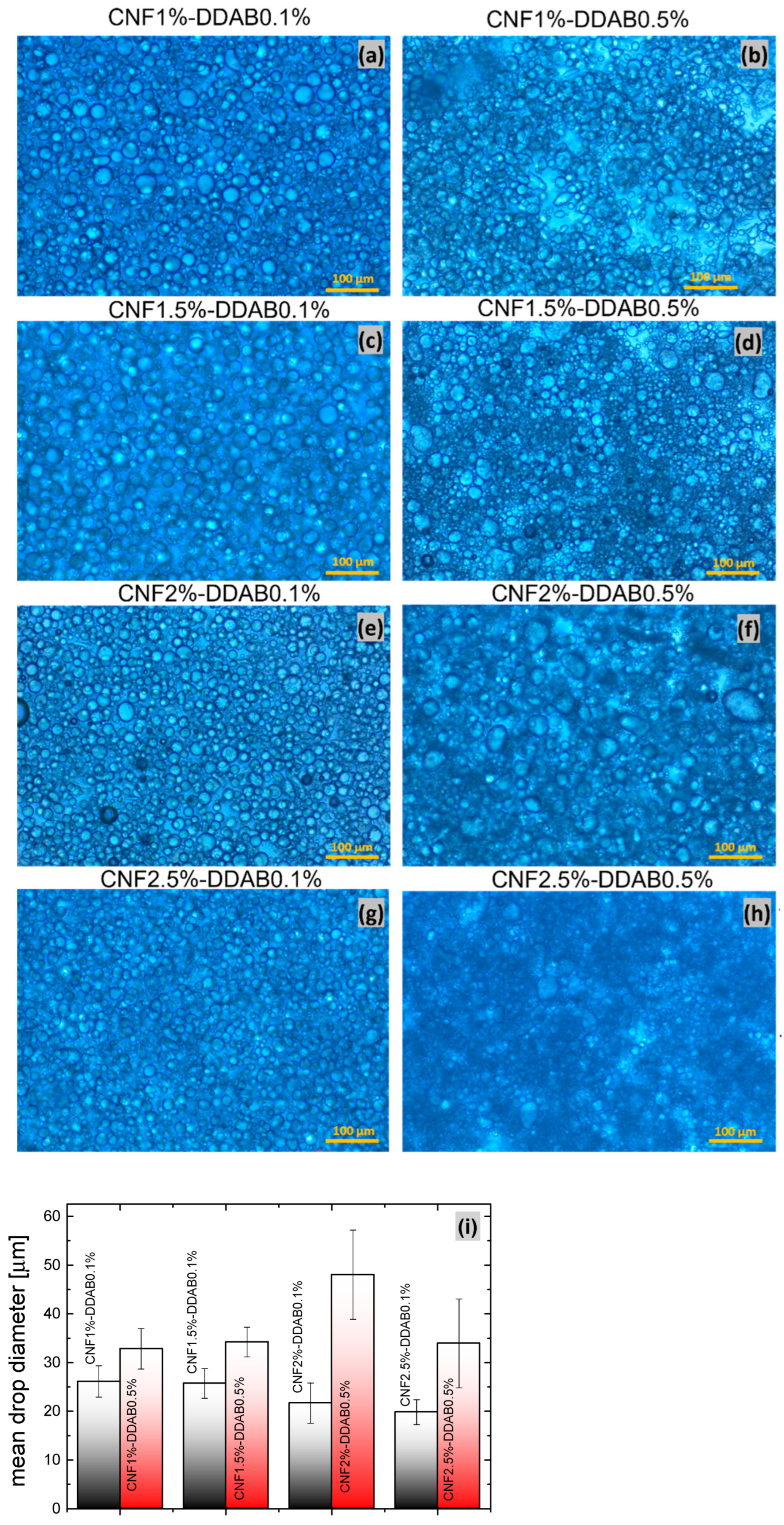
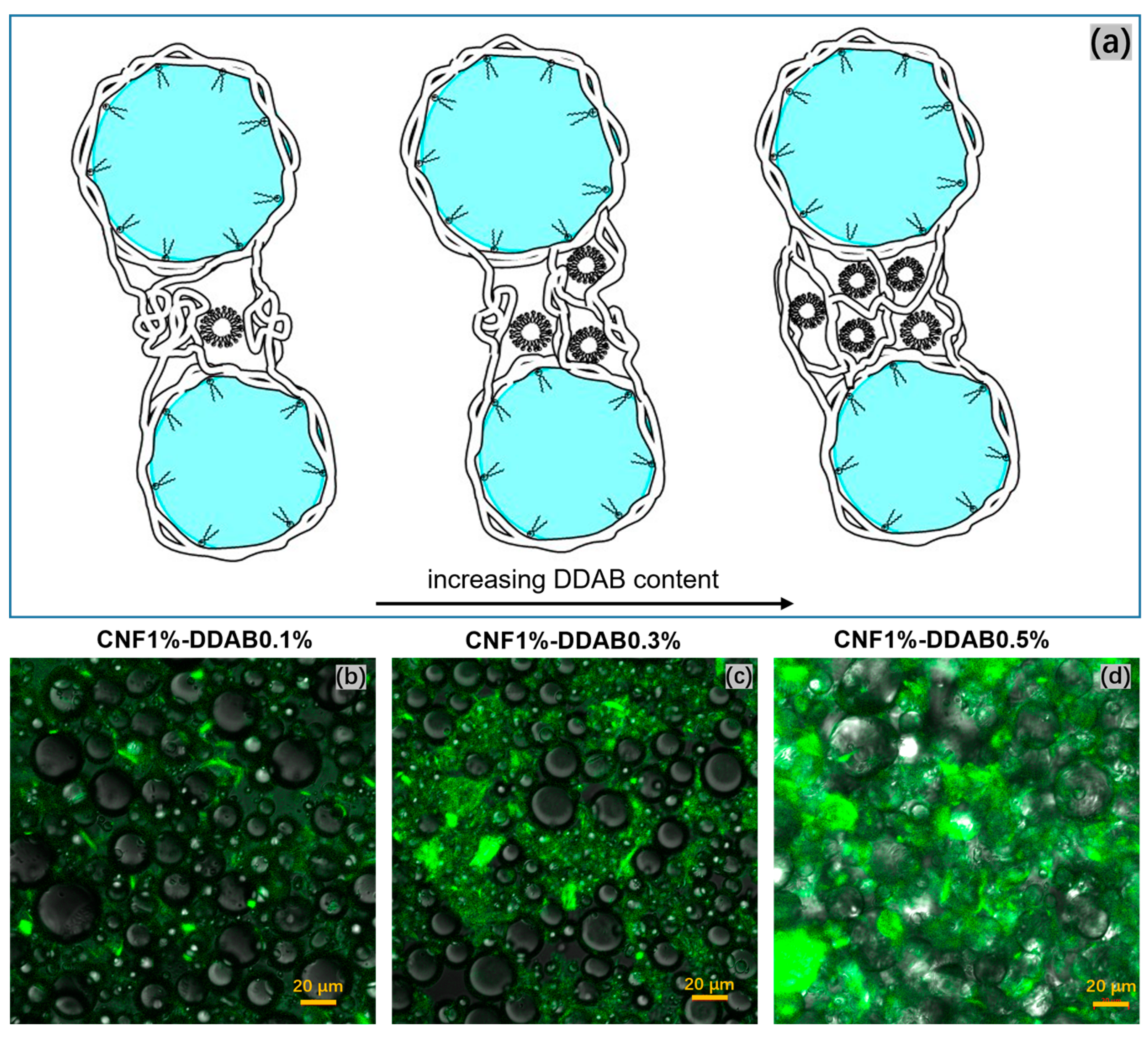
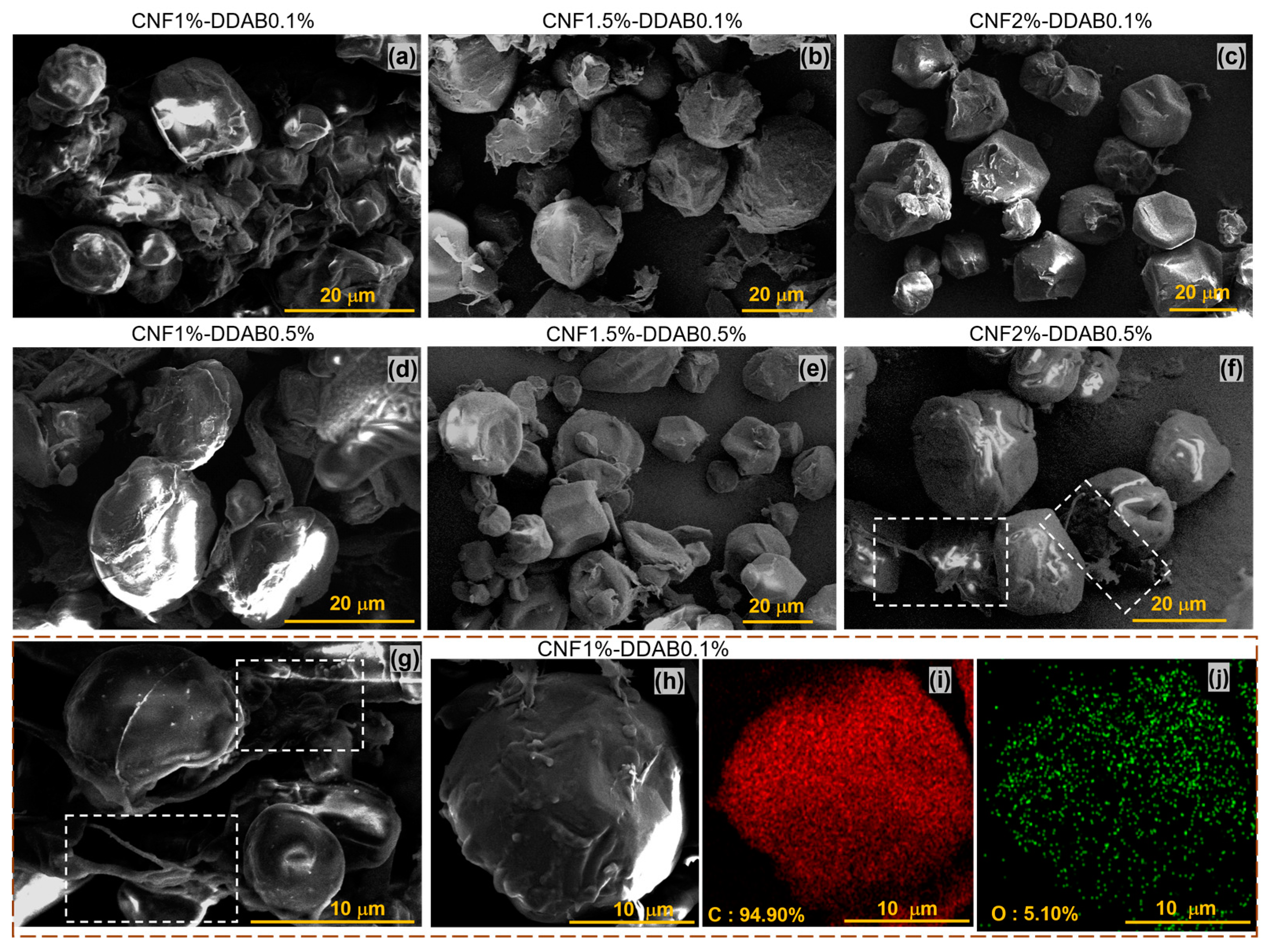

| Sample | CNF (wt.%) | DDAB (wt.%) | O:W Ratio |
|---|---|---|---|
| CNF 0.5%-DDAB 0.1% | 0.5 | 0.1 | 1:2 |
| CNF 1%-DDAB 0.1% | 1 | ||
| CNF 1.25%-DDAB 0.1% | 1.25 | ||
| CNF 1.5%-DDAB 0.1% | 1.5 | ||
| CNF 1.75%-DDAB 0.1% | 1.75 | ||
| CNF 2%-DDAB 0.1% | 2 | ||
| CNF 2.5%-DDAB 0.1% * | 2.5 | ||
| CNF 2.75%-DDAB 0.1% | 2.75 | ||
| CNF 3%-DDAB 0.1% | 3 | ||
| CNF 0.5%-DDAB 0.5% | 0.5 | 0.5 | 1:2 |
| CNF 1%-DDAB 0.5% | 1 | ||
| CNF 1.5%-DDAB 0.5% | 1.5 | ||
| CNF 2%-DDAB 0.5% | 2 | ||
| CNF 2.5%-DDAB 0.5% § | 2.5 | ||
| CNF 3%-DDAB 0.5% | 3 | ||
| CNF 2.5%-DDAB 0.1% * | 2.5 | 0.1 | 1:2 |
| CNF 2.5%-DDAB 0.2% | 0.2 | ||
| CNF 2.5%-DDAB 0.3% | 0.3 | ||
| CNF 2.5%-DDAB 0.4% | 0.4 | ||
| CNF 2.5%-DDAB 0.5% § | 0.5 |
| CNF (wt.%) | DDAB (wt.%) | (°) | (Hz) | | | (°) | |ε*|·106 (F/m) | |σ*|·10−3 (S/m) | |z*| (Ohm) |
|---|---|---|---|---|---|---|---|
| 0.5 | 0.1 | 55.01 | 916.6 | 34.99 | 1.29 | 0.54 | 7.21 |
| 1 | 52.87 | 1175.92 | 37.13 | 1.99 | 1.28 | 2.05 | |
| 1.25 | 49.39 | 1188.99 | 40.61 | 1.20 | 0.77 | 3.32 | |
| 1.5 | 49.27 | 1379.02 | 40.72 | 1.29 | 0.82 | 3.85 | |
| 1.75 | 45.76 | 1203.48 | 44.24 | 1.08 | 1.07 | 2.21 | |
| 2 | 42.91 | 1430.01 | 47.09 | 1.36 | 0.87 | 3.65 | |
| 2.5 | 42.47 | 1412.81 | 47.53 | 2.16 | 0.90 | 3.54 | |
| 2.75 | 40.66 | 1063.07 | 49.39 | 1.80 | 0.75 | 4.23 | |
| 3 | 39.46 | 1099.05 | 50.54 | 1.94 | 0.81 | 3.93 | |
| 0.5 | 0.5 | 25.44 | 1078.02 | 64.56 | 1.10 | 0.70 | 6.28 |
| 1 | 46.74 | 2596.59 | 43.26 | 0.32 | 0.49 | 5.30 | |
| 1.5 | 44.59 | 2204.97 | 45.41 | 0.71 | 0.70 | 2.96 | |
| 2 | 41.77 | 1552.12 | 48.23 | 0.72 | 0.71 | 3.94 | |
| 2.5 | 39.40 | 994.74 | 50.60 | 0.96 | 0.62 | 3.97 | |
| 2.5 | 0.1 | 42.47 | 1175.92 | 47.53 | 2.16 | 0.90 | 2.29 |
| 0.2 | 44.82 | 494.67 | 45.18 | 2.39 | 0.65 | 4.91 | |
| 0.3 | 42.72 | 764.70 | 47.28 | 2.18 | 0.91 | 3.65 | |
| 0.4 | 40.28 | 770.09 | 49.72 | 1.72 | 0.72 | 2.71 | |
| 0.5 | 39.40 | 1159.51 | 50.60 | 0.96 | 0.62 | 3.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, S.-M.; Hashmi, S.; Li, W.-Q.; Handschuh-Wang, S.; Zhu, C.-T.; Wang, S.-C.; Yang, P.-P.; Huang, Y.-F.; Zhu, G.-M.; Stadler, F.J. Influence of Cellulose Nanofibers on the Behavior of Pickering Emulsions. Part 1. Microscopy and Startup Flow Test. Materials 2022, 15, 8285. https://doi.org/10.3390/ma15238285
Cui S-M, Hashmi S, Li W-Q, Handschuh-Wang S, Zhu C-T, Wang S-C, Yang P-P, Huang Y-F, Zhu G-M, Stadler FJ. Influence of Cellulose Nanofibers on the Behavior of Pickering Emulsions. Part 1. Microscopy and Startup Flow Test. Materials. 2022; 15(23):8285. https://doi.org/10.3390/ma15238285
Chicago/Turabian StyleCui, Shu-Ming, Saud Hashmi, Wen-Qiang Li, Stephan Handschuh-Wang, Cheng-Tian Zhu, Shi-Chang Wang, Pian-Pian Yang, Yan-Fei Huang, Guang-Ming Zhu, and Florian J. Stadler. 2022. "Influence of Cellulose Nanofibers on the Behavior of Pickering Emulsions. Part 1. Microscopy and Startup Flow Test" Materials 15, no. 23: 8285. https://doi.org/10.3390/ma15238285







