Corrosion and Wear Behavior of TiO2/TiN Duplex Coatings on Titanium by Plasma Electrolytic Oxidation and Gas Nitriding
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Plasma Discharge Characteristics
3.2. Crystal Structure and Coating Morphology
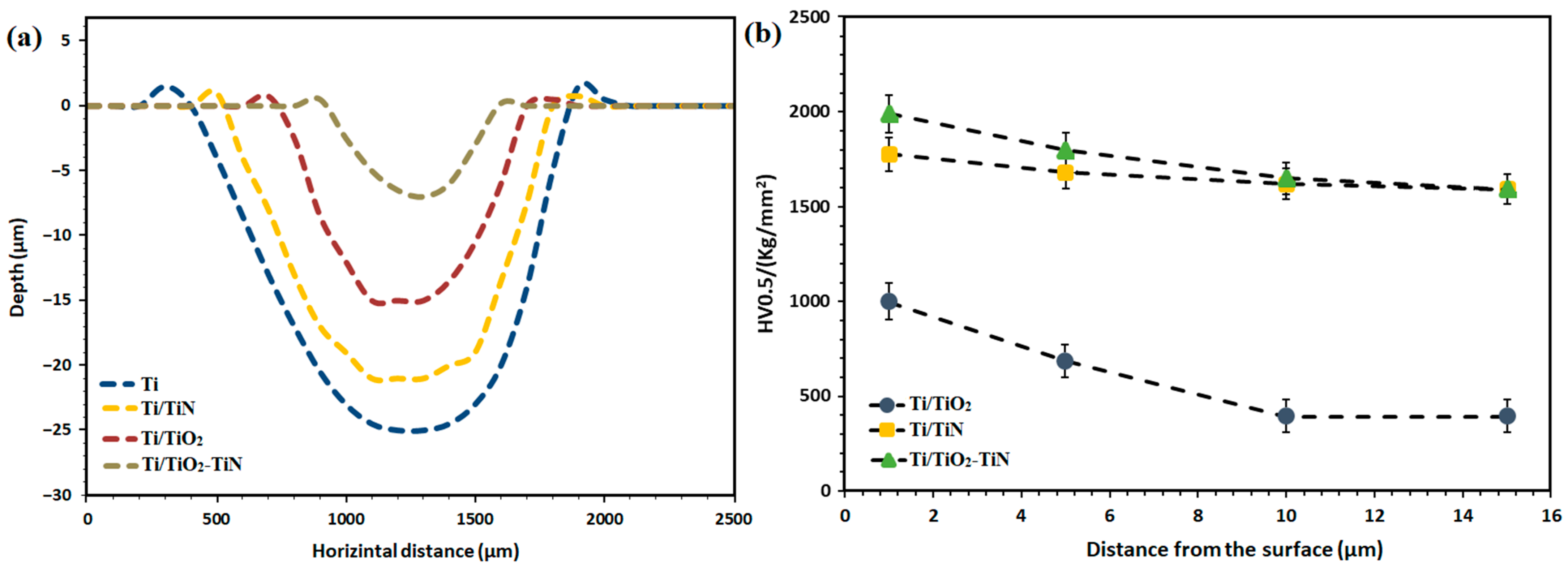
3.3. Wear Property and Micro-Hardness of the Coating
3.4. Electrochemical Behavior of the Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Liao, D.; Zhang, D.; Jiang, X.; Zhao, P.; Xu, R. Friction and wear behavior of graphene-modified titanium alloy micro-arc oxidation coatings. Trans. Indian Inst. Met. 2020, 73, 73–80. [Google Scholar] [CrossRef]
- Shahin, N.; Shamanian, M.; Kharaziha, M. Electrochemical behavior of the double-layer diamond-like carbon/plasma electrolytic oxidation on AZ31 alloy: A comparison of different PEO interlayers. Diam. Relat. Mater. 2022, 130, 109405. [Google Scholar] [CrossRef]
- Bloyce, A.; Morton, P.H.; Bell, T. Surface engineering of titanium and titanium alloys. ASM Handb. 1994, 5, 835–851. [Google Scholar]
- Marinina, G.I.; Vasilyeva, M.S.; Lapina, A.S.; Ustinov, A.Y.; Rudnev, V.S. Electroanalytical properties of metal–oxide electrodes formed by plasma electrolytic oxidation. J. Electroanal. Chem. 2013, 689, 262–268. [Google Scholar] [CrossRef]
- Bakhtiari-Zamani, H.; Saebnoori, E.; Hassannejad, H.; Hassanzadeh-Tabrizi, A. Comparing Morphology and Corrosion Behavior of Nanostructured Coatings Obtained via Plasma Electrolytic Oxidation with Direct and Pulse Currents on Commercial Titanium Substrate. Surf. Eng. Appl. Electrochem. 2019, 55, 667–678. [Google Scholar] [CrossRef]
- Hamrahi, B.; Yarmand, B.; Massoudi, A. Improved in-vitro corrosion performance of titanium using a duplex system of plasma electrolytic oxidation and graphene oxide incorporated silane coatings. Surf. Coatings Technol. 2021, 422, 127558. [Google Scholar] [CrossRef]
- Gabor, R.; Cvrček, L.; Doubková, M.; Nehasil, V.; Hlinka, J.; Unucka, P.; Buřil, M.; Podepřelová, A.; Seidlerová, J.; Bačáková, L. Hybrid coatings for orthopaedic implants formed by physical vapour deposition and microarc oxidation. Mater. Des. 2022, 219, 110811. [Google Scholar] [CrossRef]
- Li, Q.; Liang, J.; Wang, Q. Plasma electrolytic oxidation coatings on lightweight metals. Mod. Surf. Eng. Treat. 2013, 4, 75–99. [Google Scholar]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Daroonparvar, M.; Saebnoori, E.; Chami, A. In vitro degradation behavior, antibacterial activity and cytotoxicity of TiO2-MAO/ZnHA composite coating on Mg alloy for orthopedic implants. Surf. Coat. Technol. 2018, 334, 450–460. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.; Yusof, N.M.; Bakhsheshi-Rad, H.R.; Hamzah, E.; Mardanikivi, T. Deposition of duplex MAO layer/nanostructured titanium dioxide composite coatings on Mg–1% Ca alloy using a combined technique of air plasma spraying and micro arc oxidation. J. Alloys Compd. 2015, 649, 591–605. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.; Yusof, N.M.; Bakhsheshi-Rad, H.R. Fabrication and properties of triplex NiCrAlY/nano Al2O3·13% TiO2/nano TiO2 coatings on a magnesium alloy by atmospheric plasma spraying method. J. Alloys Compd. 2015, 645, 450–466. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ebrahimi-Kahrizsangi, R.; Daroonparvar, M.; Medraj, M. Fabrication and characterization of hydrophobic microarc oxidation/poly-lactic acid duplex coating on biodegradable Mg–Ca alloy for corrosion protection. Vacuum 2016, 125, 185–188. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, S.; Hu, J.; Lin, T. Formation mechanism and corrosion resistance of the hydrophobic coating on anodized magnesium. Corros. Sci. 2016, 111, 334–343. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.; Yusof, N.M.; Bakhsheshi-Rad, H.R. Preparation and corrosion resistance of a nanocomposite plasma electrolytic oxidation coating on Mg-1% Ca alloy formed in aluminate electrolyte containing titania nano-additives. J. Alloys Compd. 2016, 688, 841–857. [Google Scholar] [CrossRef]
- Li, J.; Shi, H.; Liu, F.; Han, E.H. Self-healing epoxy coating based on tung oil-containing microcapsules for corrosion protection. Prog. Org. Coat. 2021, 156, 106236. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Li, H.; Zhang, S.; Zhao, R.; Li, G.; Zhang, R.; Sheng, Y.; Cao, S.; Zhao, Y.; et al. Corrosion resistance and biocompatibility of calcium-containing coatings developed in near-neutral solutions containing phytic acid and phosphoric acid on AZ31B alloy. J. Alloys Compd. 2020, 823, 153721. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, T.; Cheng, Y. A Systematic Study of the Role of Cathodic Polarization and New Findings on the Soft Sparking Phenomenon from Plasma Electrolytic Oxidation of an Al-Cu-Li Alloy. J. Electrochem. Soc. 2022, 169, 071505. [Google Scholar] [CrossRef]
- Narayanan, T.S.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Behzadi, P.; Badr, M.; Zakeri, A. Duplex surface modification of pure Ti via thermal oxidation and gas nitriding: Preparation and electrochemical studies. Ceram. Int. 2022; in press. [Google Scholar] [CrossRef]
- Zhecheva, A.; Sha, W.; Malinov, S.; Long, A. Enhancing the microstructure and properties of titanium alloys through nitriding and other surface engineering methods. Surf. Coat. Technol. 2005, 200, 2192–2207. [Google Scholar] [CrossRef]
- Zukalova, M.; Prochazka, J.; Bastl, Z.; Duchoslav, J.; Rubacek, L.; Havlicek, D.; Kavan, L. Facile conversion of electrospun TiO2 into titanium nitride/oxynitride fibers. Chem. Mater. 2010, 22, 4045–4055. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Khalil-Allafi, J.; Aghaie, E.; Siadati, M.H. Effect of TiO2–Ti and TiO2–TiN composite coatings on corrosion behavior of NiTi alloy. Surf. Interface Anal. 2015, 47, 99–104. [Google Scholar] [CrossRef]
- Alsaran, A.; Purcek, G.; Hacisalihoglu, I.; Vangolu, Y.; Bayrak, Ö.; Karaman, I.; Celik, A. Hydroxyapatite production on ultrafine-grained pure titanium by micro-arc oxidation and hydrothermal treatment. Surf. Coat. Technol. 2011, 205, S537–S542. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Molaei, M.; Nouri, M.; Babaei, K. Review of the role of graphene and its derivatives in enhancing the performance of plasma electrolytic oxidation coatings on titanium and its alloys. Appl. Surf. Sci. Adv. 2021, 6, 100140. [Google Scholar] [CrossRef]
- Sarbishei, S.; Sani, M.A.; Mohammadi, M.R. Effects of alumina nanoparticles concentration on microstructure and corrosion behavior of coatings formed on titanium substrate via PEO process. Ceram. Int. 2016, 42, 8789–8797. [Google Scholar] [CrossRef]
- Abbasi, S.; Mahboob, A.; Bakhtiari Zamani, H.; Bilesan, M.R.; Repo, E.; Hakimi, A. The tribological behavior of nanocrystalline TiO2 coating produced by plasma electrolytic oxidation. J. Nanomater. 2022, 2022, 5675038. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Rameshbabu, N.; Sreekanth, D.; Bose, A.C.; Muthupandi, V.; Subramanian, S. Fabrication and characterization of micro-arc oxidized fluoride containing titania films on Cp Ti. Ceram. Int. 2013, 39, 801–812. [Google Scholar] [CrossRef]
- Niazi, H.; Golestani-Fard, F.; Wang, W.; Shahmiri, M.; Zargar, H.R.; Alfantazi, A.; Bayati, R. Structure–property correlation in EEMAO fabricated TiO2–Al2O3 nanocomposite coatings. ACS Appl. Mater. Interfaces 2014, 6, 5538–5547. [Google Scholar] [CrossRef]
- Xiaohong, W.; Zhaohua, J.; Huiling, L.; Shigang, X.; Xinguo, H. Photo-catalytic activity of titanium dioxide thin films prepared by micro-plasma oxidation method. Thin Solid Films 2003, 441, 130–134. [Google Scholar] [CrossRef]
- Wang, X.; Pakdel, A.; Zhang, J.; Weng, Q.; Zhai, T.; Zhi, C.; Golberg, D.; Bando, Y. Large-surface-area BN nanosheets and their utilization in polymeric composites with improved thermal and dielectric properties. Nanoscale Res. Lett. 2012, 7, 662. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Li, S.; Cui, Y.; Tao, A.; Wang, C.; Li, H.; Zhang, L.; Yu, H.; Jiang, J.; Li, C. Comparison of the osteoblastic activity of low elastic modulus Ti-24Nb-4Zr-8Sn alloy and pure titanium modified by physical and chemical methods. Mater. Sci. Eng. C 2020, 113, 111018. [Google Scholar] [CrossRef] [PubMed]
- Badiger, P.V.; Desai, V.; Ramesh, M.R. Development and characterization of Ti/TiC/TiN coatings by cathodic arc evaporation technique. Trans. Indian Inst. Met. 2017, 70, 2459–2464. [Google Scholar] [CrossRef]
- Zhecheva, A.; Malinov, S.; Sha, W. Microstructure and microhardness of gas nitrided surface layers in Ti–8Al–1Mo–1V and Ti–10V–2Fe–3Al alloys. Surf. Eng. 2005, 21, 269–278. [Google Scholar] [CrossRef]
- Li, H.; Cui, Z.; Li, Z.; Zhu, S.; Yang, X. Effect of gas nitriding treatment on cavitation erosion behavior of commercially pure Ti and Ti−6Al−4V alloy. Surf. Coat. Technol. 2013, 221, 29–36. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Macdonald, D.D.; Matykina, E.; Parfenov, E.V.; Egorkin, V.S.; Curran, J.A.; Troughton, S.C.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Lampke, T.; et al. Review of plasma electrolytic oxidation of titanium substrates: Mechanism, properties, applications and limitations. Appl. Surf. Sci. Adv. 2021, 5, 100121. [Google Scholar] [CrossRef]
- Javidi, M.; Fadaee, H. Plasma electrolytic oxidation of 2024-T3 aluminum alloy and investigation on microstructure and wear behavior. Appl. Surf. Sci. 2013, 286, 212–219. [Google Scholar] [CrossRef]
- Doolabi, D.S.; Ehteshamzadeh, M.; Mirhosseini, S.M. Effect of NaOH on the structure and corrosion performance of alumina and silica PEO coatings on aluminum. J. Mater. Eng. Perform. 2012, 21, 2195–2202. [Google Scholar] [CrossRef]
- Farshid, S.; Kharaziha, M. Micro and nano-enabled approaches to improve the performance of plasma electrolytic oxidation coated magnesium alloys. J. Magnes. Alloy. 2021, 9, 1487–1504. [Google Scholar] [CrossRef]
- Li, H.; Cui, Z.; Li, Z.; Zhu, S.; Yang, X. Surface modification by gas nitriding for improving cavitation erosion resistance of CP-Ti. Appl. Surf. Sci. 2014, 298, 164–170. [Google Scholar] [CrossRef]
- Gao, Y.; Yerokhin, A.; Parfenov, E.; Matthews, A. Application of voltage pulse transient analysis during plasma electrolytic oxidation for assessment of characteristics and corrosion behaviour of Ca- and P-containing coatings on magnesium. Electrochim. Acta 2014, 149, 218–230. [Google Scholar] [CrossRef]
- Shin, K.R.; Kim, Y.S.; Yang, H.W.; Ko, Y.G.; Shin, D.H. In vitro biological response to the oxide layer in pure titanium formed at different current densities by plasma electrolytic oxidation. Appl. Surf. Sci. 2014, 314, 221–227. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T. Effects of Ta content on Young’s modulus and tensile properties of binary Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. A 2004, 371, 283–290. [Google Scholar] [CrossRef]
- Sampatirao, H.; Amruthaluru, S.; Chennampalli, P.; Lingamaneni, R.K.; Nagumothu, R. Fabrication of ceramic coatings on the biodegradable ZM21 magnesium alloy by PEO coupled EPD followed by laser texturing process. J. Magnes. Alloys 2021, 9, 910–926. [Google Scholar] [CrossRef]
- Gao, Y.; Yerokhin, A.; Matthews, A. Effect of current mode on PEO treatment of magnesium in Ca-and P-containing electrolyte and resulting coatings. Appl. Surf. Sci. 2014, 316, 558–567. [Google Scholar] [CrossRef]
- Kucukosman, R.; Sukuroglu, E.E.; Totik, Y.; Sukuroglu, S. Effects of graphene oxide addition on wear behaviour of composite coatings fabricated by plasma electrolytic oxidation (PEO) on AZ91 magnesium alloy. J. Adhes. Sci. Technol. 2021, 35, 242–255. [Google Scholar] [CrossRef]
- Fontana, M. Corrosion Engineering; McGraw-Hill: Singapore, 1987. [Google Scholar]
- Manivasagam, G.; Mudali, U.K.; Asokamani, R.; Raj, B. Corrosion and microstructural aspects of titanium and its alloys as orthopaedic devices. Corros. Rev. 2003, 21, 125–160. [Google Scholar] [CrossRef]
- Bahramian, A.; Raeissi, K.; Hakimizad, A. An investigation of the characteristics of Al2O3/TiO2 PEO nanocomposite coating. Appl. Surf. Sci. 2015, 351, 13–26. [Google Scholar] [CrossRef]
- Molaei, M.; Fattah-Alhosseini, A.; Gashti, S.O. Sodium aluminate concentration effects on microstructure and corrosion behavior of the plasma electrolytic oxidation coatings on pure titanium. Metall. Mater. Trans. A 2018, 49, 368–375. [Google Scholar] [CrossRef]
- Quintero, D.; Galvis, O.; Calderón, J.A.; Castaño, J.G.; Echeverría, F. Effect of electrochemical parameters on the formation of anodic films on commercially pure titanium by plasma electrolytic oxidation. Surf. Coat. Technol. 2014, 258, 1223–1231. [Google Scholar] [CrossRef]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; Ramakrishna, S.; Berto, F. Carbon nanotubes (CNTs)-reinforced magnesium-based matrix composites: A comprehensive review. Materials 2020, 13, 4421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wu, L.; Tang, A.; Ding, X.; Jiang, B.; Atrens, A.; Pan, F. Smart epoxy coating containing zeolites loaded with Ce on a plasma electrolytic oxidation coating on Mg alloy AZ31 for active corrosion protection. Prog. Org. Coat. 2019, 132, 144–147. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Ma, Y.; Song, G.L.; Zheng, D.; Jiang, B.; Atrens, A.; Pan, F. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corros. Sci. 2018, 139, 370–382. [Google Scholar] [CrossRef] [Green Version]
- Weng, W.; Wu, W.; Yu, X.; Sun, M.; Lin, Z.; Ibrahim, M.; Yang, H. Effect of GelMA hydrogel coatings on corrosion resistance and biocompatibility of MAO-Coated Mg alloys. Materials 2020, 13, 3834. [Google Scholar] [CrossRef]
- Egorkin, V.S.; Medvedev, I.M.; Sinebryukhov, S.L.; Vyaliy, I.E.; Gnedenkov, A.S.; Nadaraia, K.V.; Izotov, N.V.; Mashtalyar, D.V.; Gnedenkov, S.V. Atmospheric and marine corrosion of PEO and composite coatings obtained on Al-Cu-Mg aluminum alloy. Materials 2020, 13, 2739. [Google Scholar] [CrossRef]
- Bisztyga-Szklarz, M.; Rząd, E.; Boroń, Ł.; Klimczyk, P.; Polczyk, T.; Łętocha, A.; Rajska, M.; Hebda, M.; Długosz, P. Properties of microplasma coating on AZ91 magnesium alloy prepared from electrolyte with and without the borax addition. Materials 2022, 15, 1354. [Google Scholar] [CrossRef]
- Choi, K.; Kang, S.; Kang, H. Fatigue Behavior of an AM50 die-casting alloy anodized by plasma electrolytic oxidation. Materials 2021, 14, 7795. [Google Scholar] [CrossRef]
- Pezzato, L.; Brunelli, K.; Diodati, S.; Pigato, M.; Bonesso, M.; Dabalà, M. Microstructural and corrosion properties of hydroxyapatite containing PEO coating produced on AZ31 Mg alloy. Materials 2021, 14, 1531. [Google Scholar] [CrossRef]
- Zhang, R.; Zhong, S.; Zeng, L.; Li, H.; Zhao, R.; Zhang, S.; Duan, X.; Huang, J.; Zhao, Y. Novel Mg-incorporated micro-arc oxidation coatings for orthopedic implants application. Materials 2021, 14, 5710. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, W.; Liu, D.; Gao, W.; Chen, J. Preparation and corrosion behavior in marine environment of MAO coatings on magnesium alloy. Materials 2020, 13, 345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Jiang, E.; Wu, L.; Tang, A.; Atrens, A.; Pan, F. Active corrosion protection of phosphate loaded PEO/LDHs composite coatings: SIET study. J. Magnes. Alloy. 2022, 10, 1351–1357. [Google Scholar] [CrossRef]
- Ballam, L.R.; Arab, H.; Bestetti, M.; Franz, S.; Masi, G.; Sola, R.; Donati, L.; Martini, C. Improving the corrosion resistance of wrought ZM21 magnesium alloys by plasma electrolytic oxidation and powder coating. Materials 2021, 14, 2268. [Google Scholar] [CrossRef] [PubMed]
- Korpi, A.G.; Rezaee, S.; Luna, C.; Ţălu, Ş.; Arman, A.; Ahmadpourian, A. Influence of the oxygen partial pressure on the growth and optical properties of RF-sputtered anatase TiO2 thin films. Results Phys. 2017, 7, 3349–3352. [Google Scholar] [CrossRef]
- Zare, M.; Solaymani, S.; Shafiekhani, A.; Kulesza, S.; Ţălu, Ş.; Bramowicz, M. Evolution of rough-surface geometry and crystalline structures of aligned TiO2 nanotubes for photoelectrochemical water splitting. Sci. Rep. 2018, 8, 10870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ţălu, Ş.; Achour, A.; Solaymani, S.; Nikpasand, K.; Dalouji, V.; Sari, A.; Rezaee, S.; Nezafat, N.B. Micromorphology analysis of TiO2 thin films by atomic force microscopy images: The influence of postannealing. Microsc. Res. Tech. 2020, 83, 457–463. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Galimshina, Z.R.; Gil’fanova, G.U.; Alibaeva, E.I.; Danilko, K.V.; Aubakirova, V.R.; Farrakhov, R.G.; Parfenov, E.V.; Valiev, R.Z. Modeling of biological activity of PEO-coated titanium implants with conjugates of cyclic RGD peptide with amino acid bisphosphonates. Materials 2022, 15, 8120. [Google Scholar] [CrossRef]
- Pezzato, L.; Settimi, A.G.; Fanchin, D.; Moschin, E.; Moro, I.; Dabalà, M. Effect of Cu Addition on the corrosion and antifouling properties of PEO coated zinc-aluminized steel. Materials 2022, 15, 7895. [Google Scholar] [CrossRef]
- Schwartz, A.; Kossenko, A.; Zinigrad, M.; Gofer, Y.; Borodianskiy, K.; Sobolev, A. Hydroxyapatite coating on Ti-6Al-7Nb alloy by plasma electrolytic oxidation in salt-based electrolyte. Materials 2022, 15, 7374. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, W.; Liu, H.; Liu, L.; Wang, F.; Qiao, Z. Single dense layer of PEO coating on aluminum fabricated by “chain-like” discharges. Materials 2022, 15, 4635. [Google Scholar] [CrossRef]

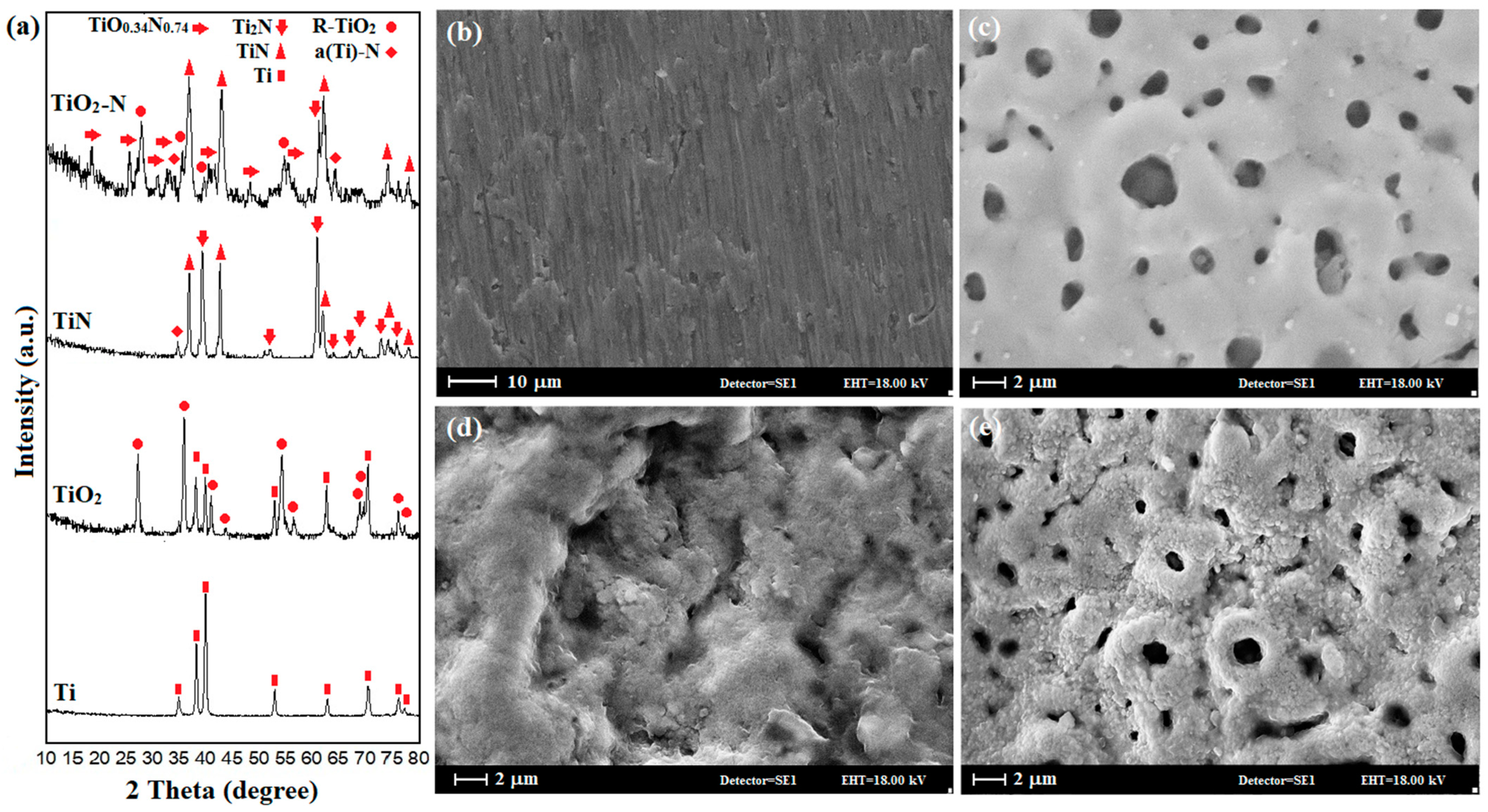
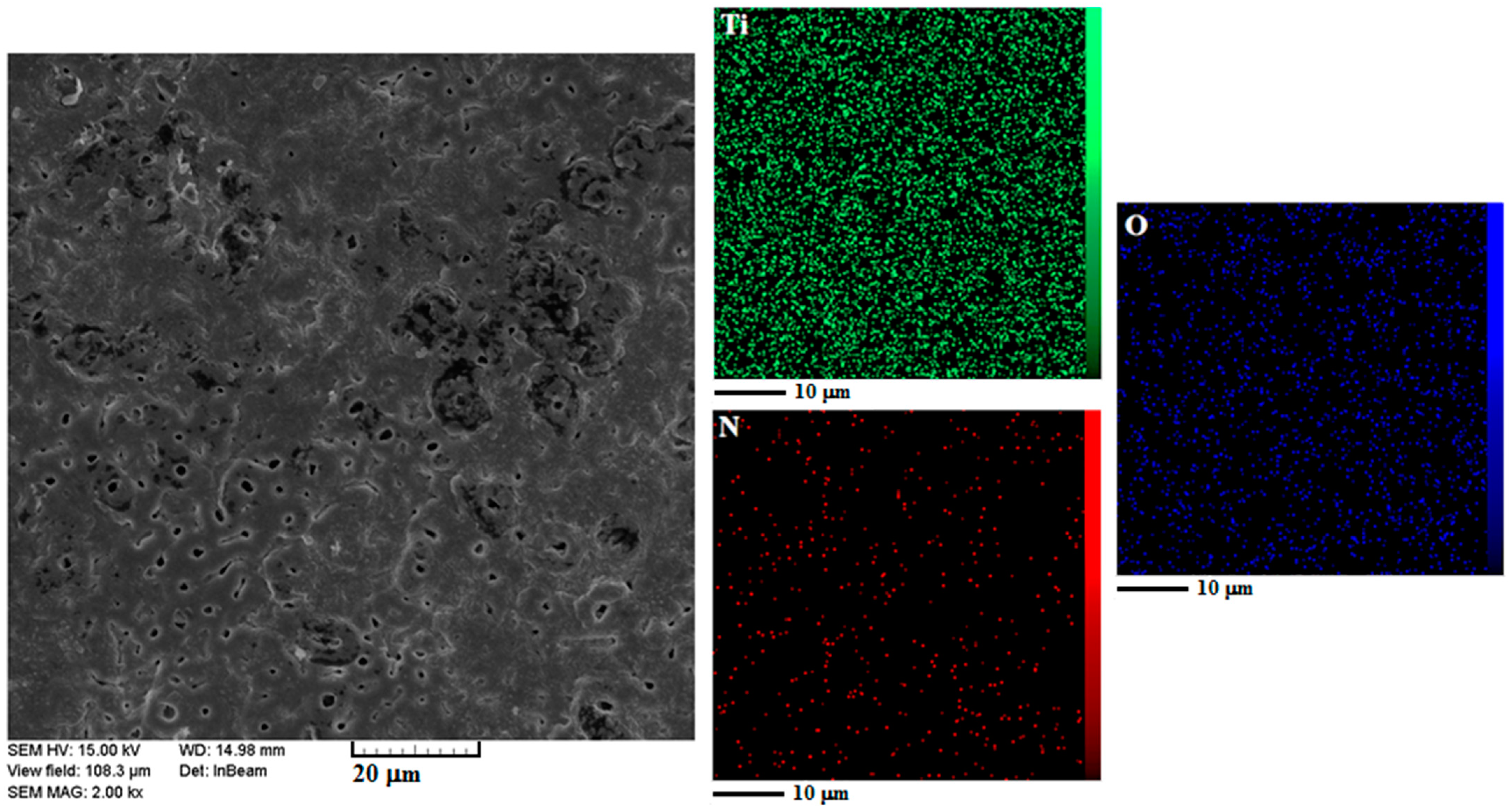

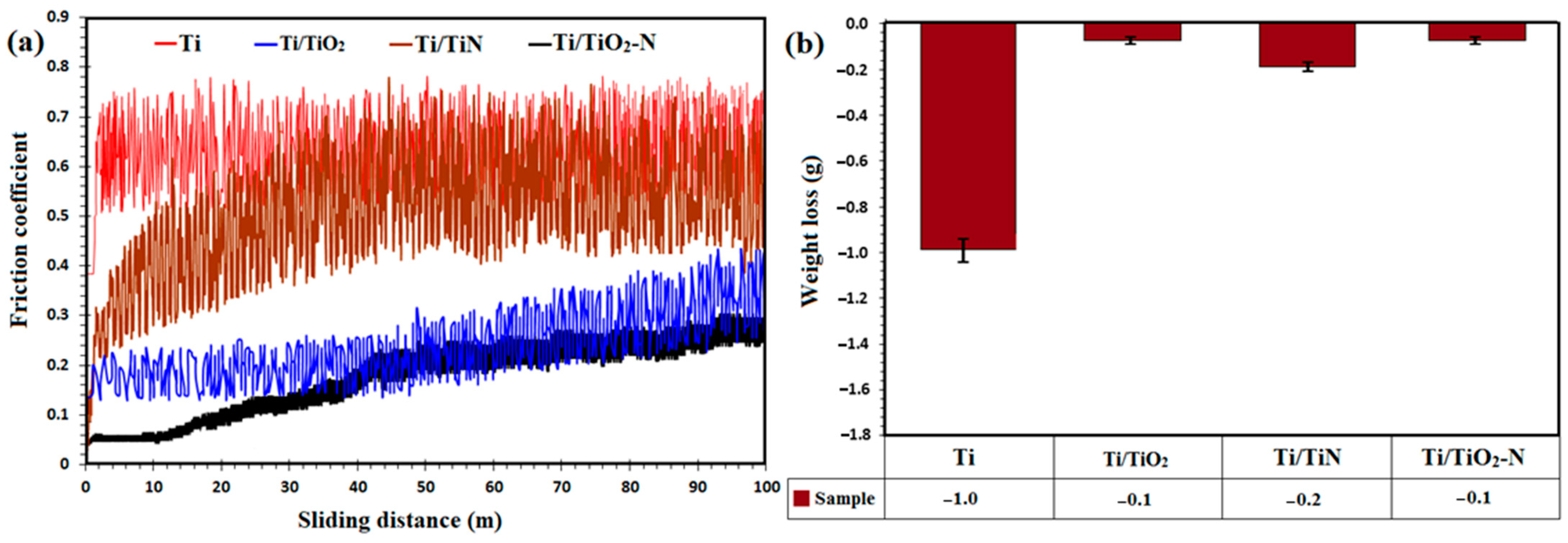
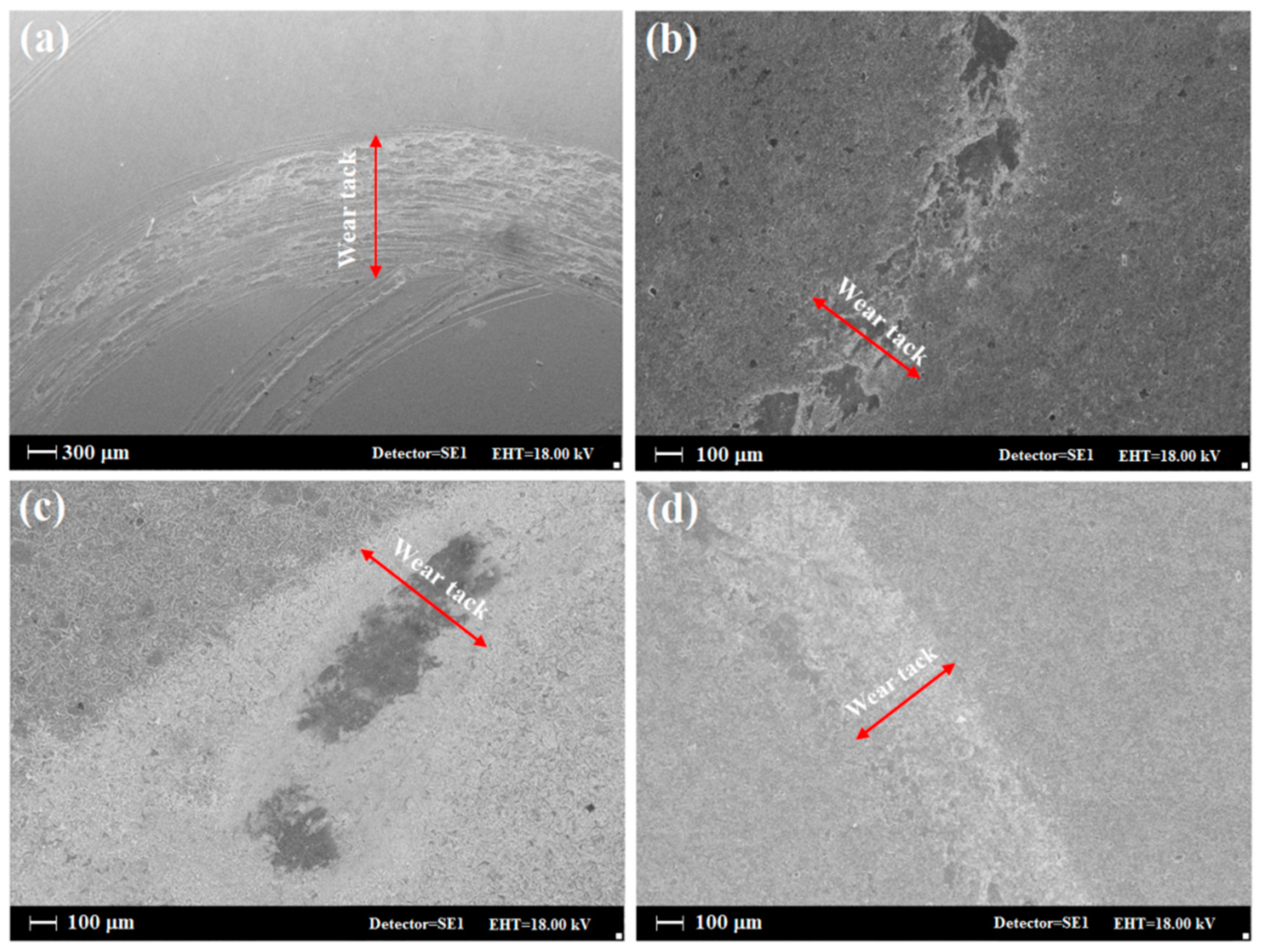

| Sample | FWHM | 2θmax | Average Theoretical Crystallite Size (nm) |
|---|---|---|---|
| (Degree) | (Degree) | ||
| Ti/TiO2 | 0.3444 | 35.91 | 24 |
| Ti/TiN | 0.3444 | 36.11 | 27 |
| Ti/TiO2-N | 0.2952 | 7.36 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhtiari-Zamani, H.; Saebnoori, E.; Bakhsheshi-Rad, H.R.; Berto, F. Corrosion and Wear Behavior of TiO2/TiN Duplex Coatings on Titanium by Plasma Electrolytic Oxidation and Gas Nitriding. Materials 2022, 15, 8300. https://doi.org/10.3390/ma15238300
Bakhtiari-Zamani H, Saebnoori E, Bakhsheshi-Rad HR, Berto F. Corrosion and Wear Behavior of TiO2/TiN Duplex Coatings on Titanium by Plasma Electrolytic Oxidation and Gas Nitriding. Materials. 2022; 15(23):8300. https://doi.org/10.3390/ma15238300
Chicago/Turabian StyleBakhtiari-Zamani, Hassan, Ehsan Saebnoori, Hamid Reza Bakhsheshi-Rad, and Filippo Berto. 2022. "Corrosion and Wear Behavior of TiO2/TiN Duplex Coatings on Titanium by Plasma Electrolytic Oxidation and Gas Nitriding" Materials 15, no. 23: 8300. https://doi.org/10.3390/ma15238300
APA StyleBakhtiari-Zamani, H., Saebnoori, E., Bakhsheshi-Rad, H. R., & Berto, F. (2022). Corrosion and Wear Behavior of TiO2/TiN Duplex Coatings on Titanium by Plasma Electrolytic Oxidation and Gas Nitriding. Materials, 15(23), 8300. https://doi.org/10.3390/ma15238300







