Very Anisotropic 2D Molecular Magnetic Materials Based on Pentagonal Bipyramidal Heptacyanidorhenate(IV)
Abstract
:1. Introduction
2. Results and Discussion
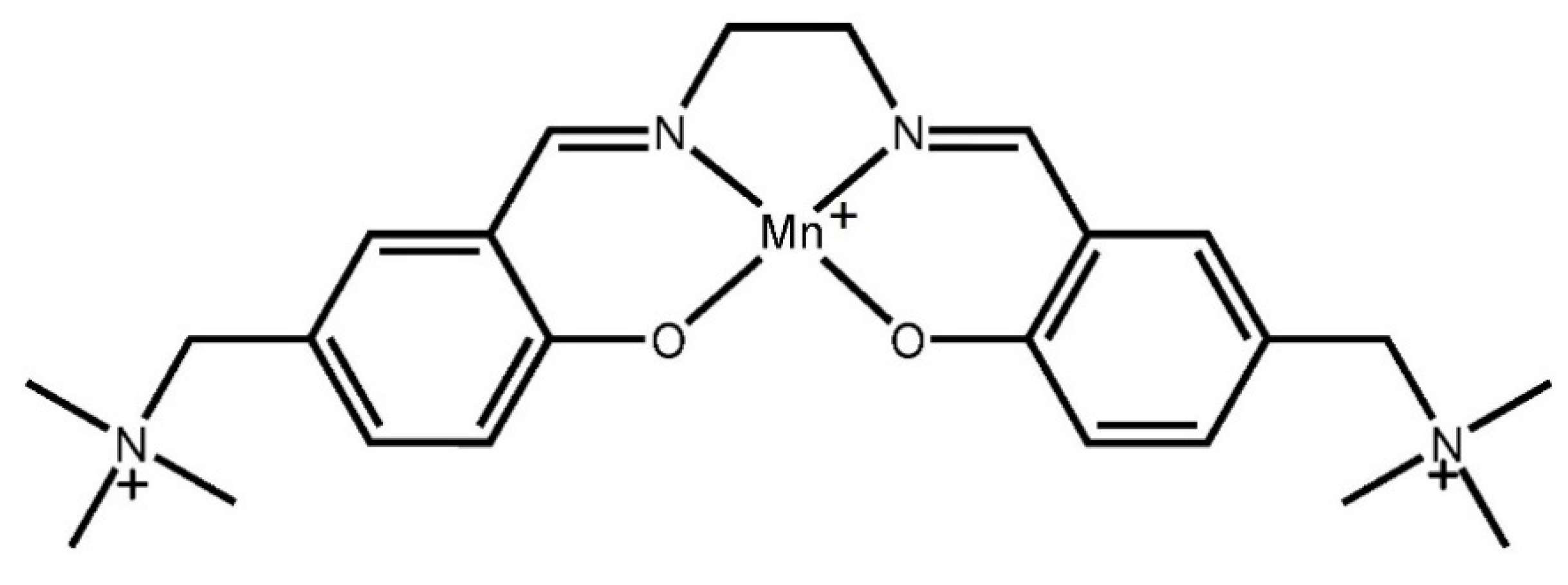
2.1. Synthetic Approach
FT-IR Investigation
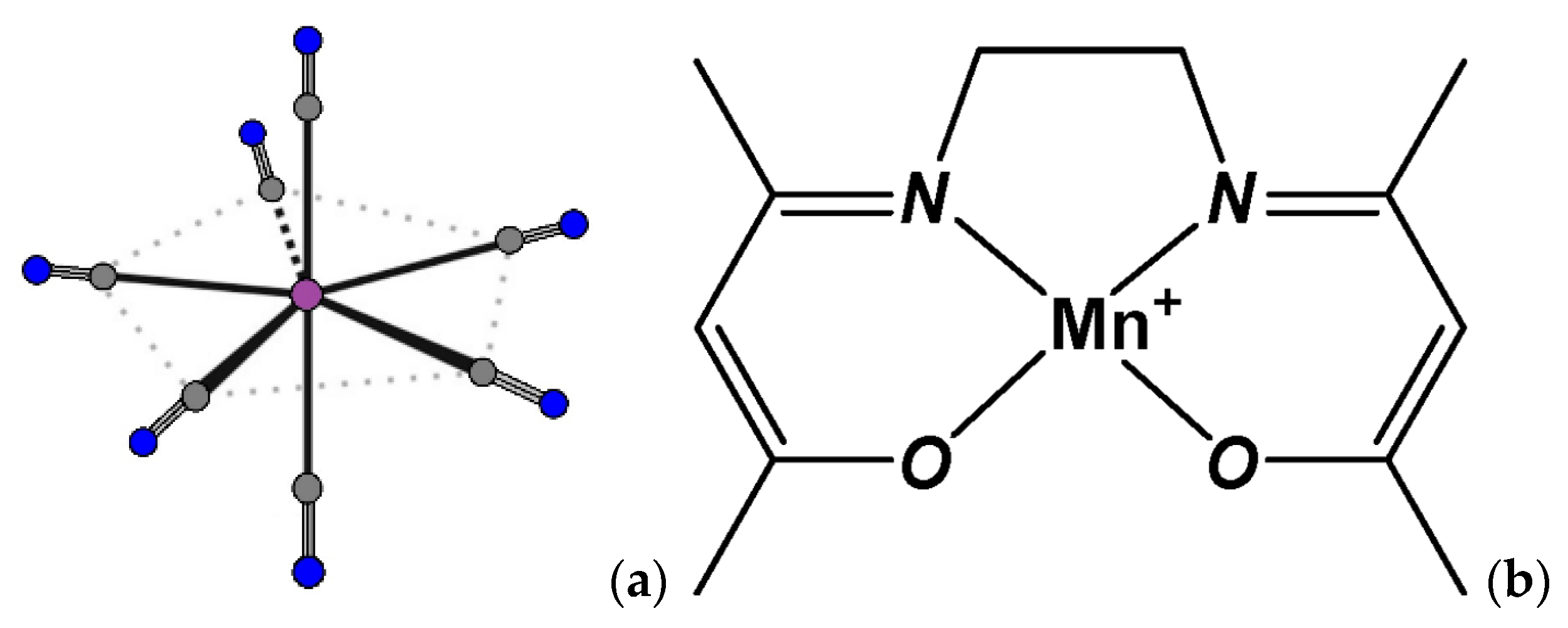
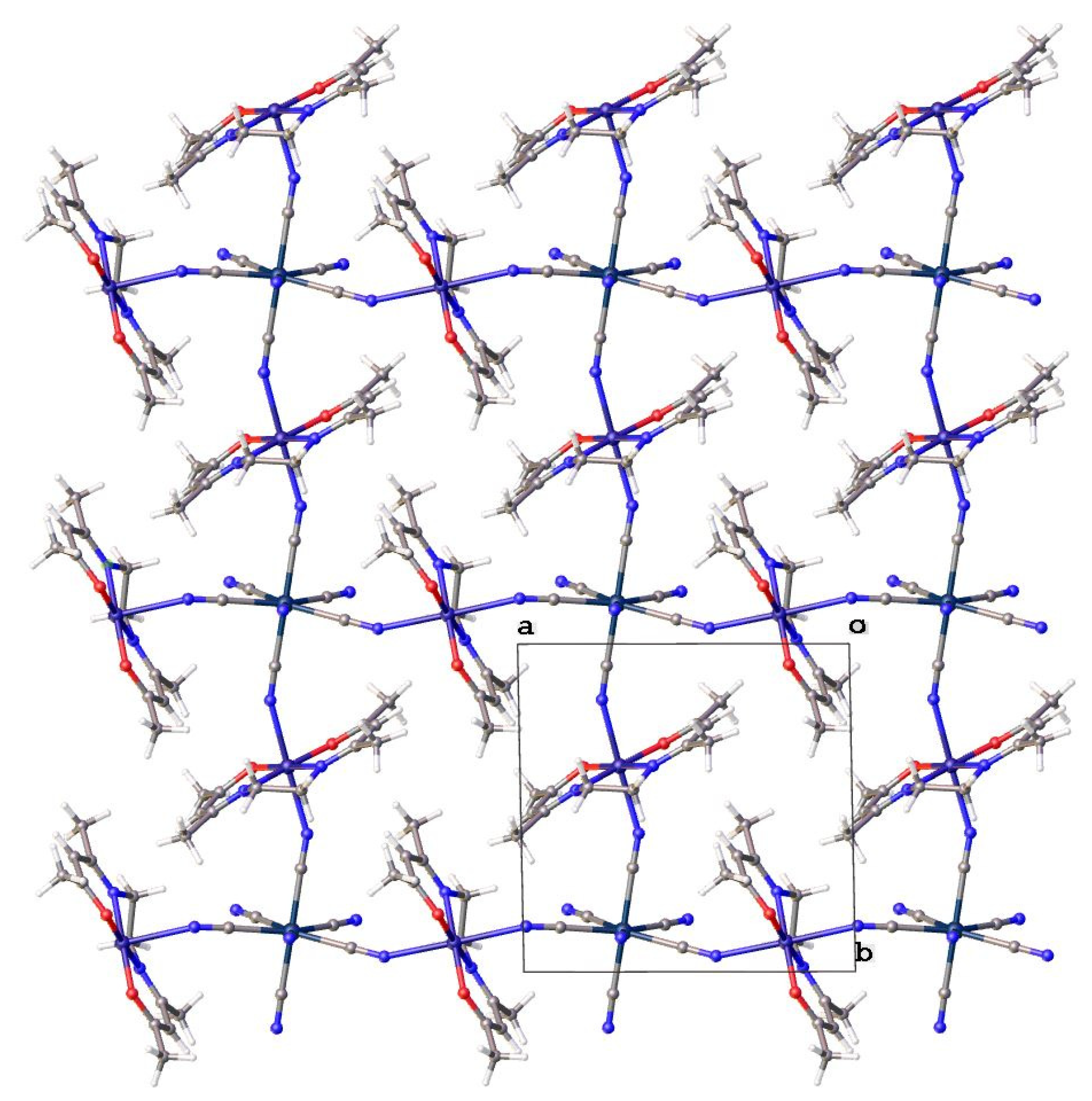
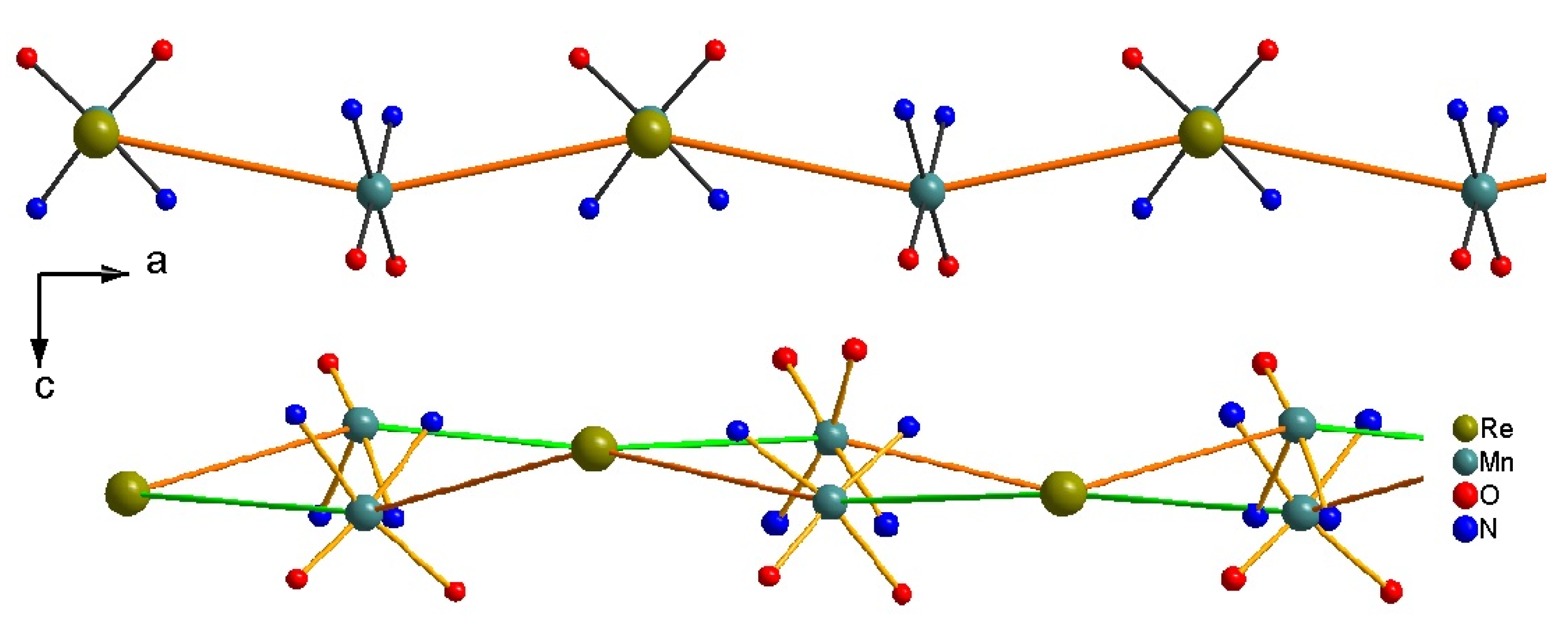
2.2. Crystal Structure Description
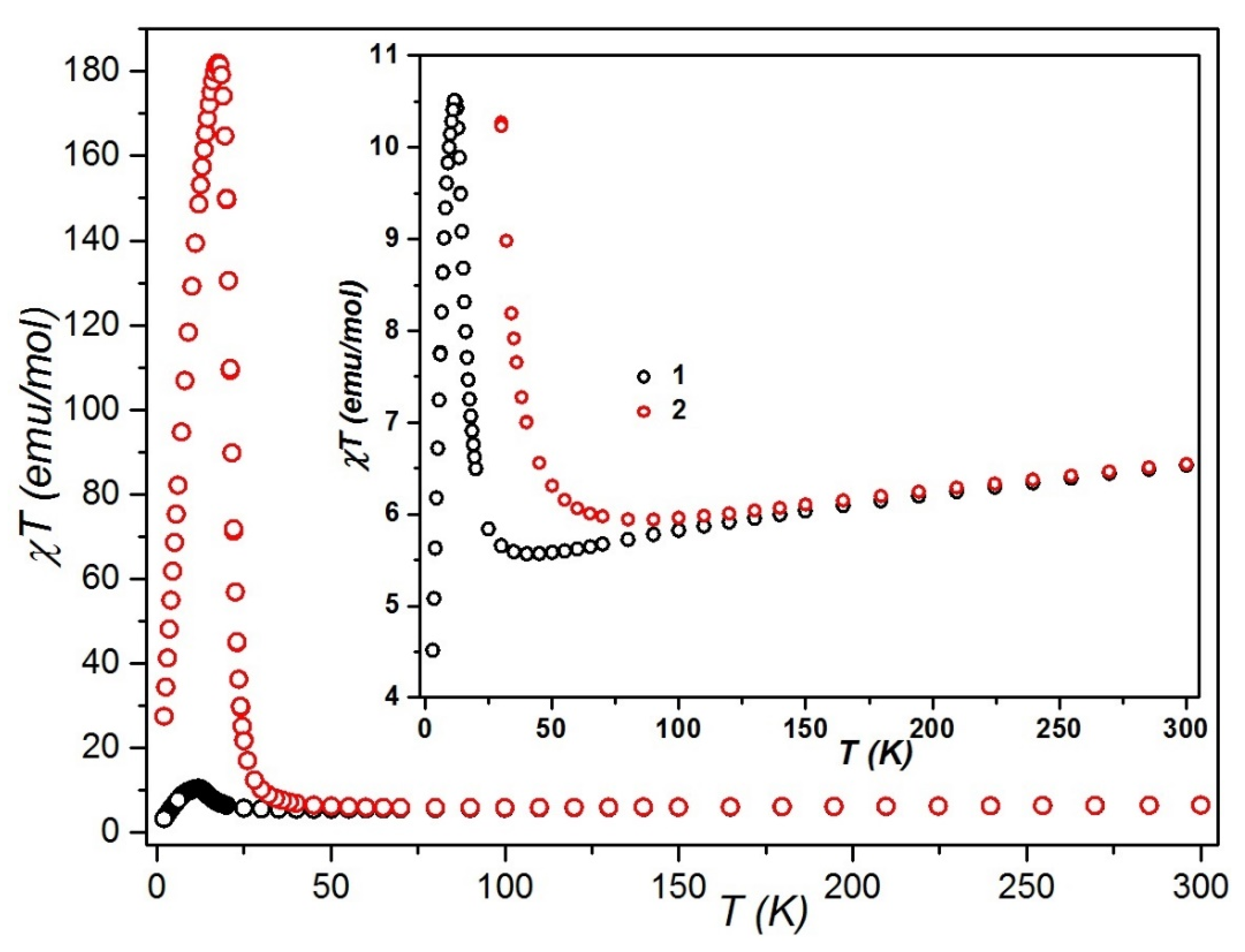
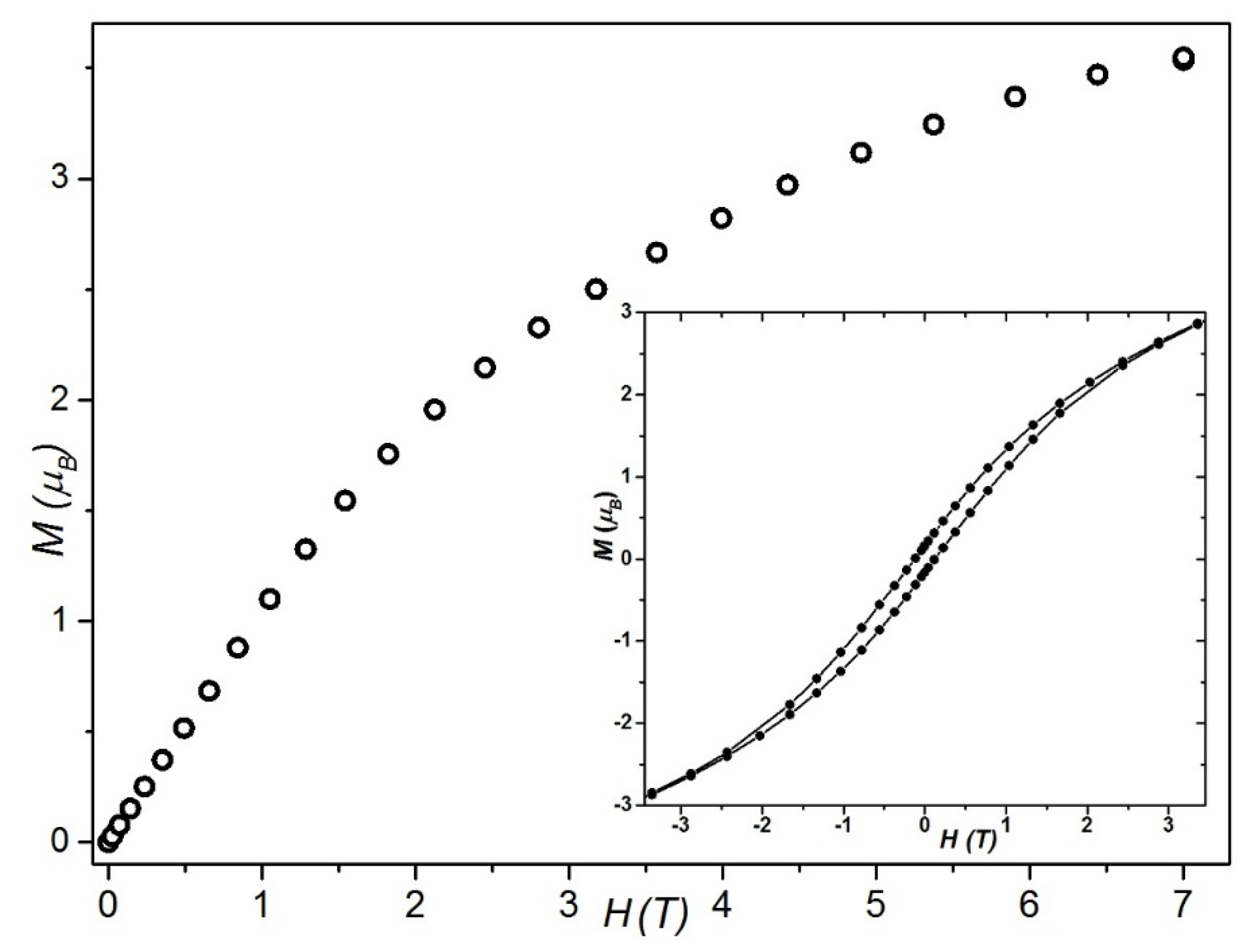
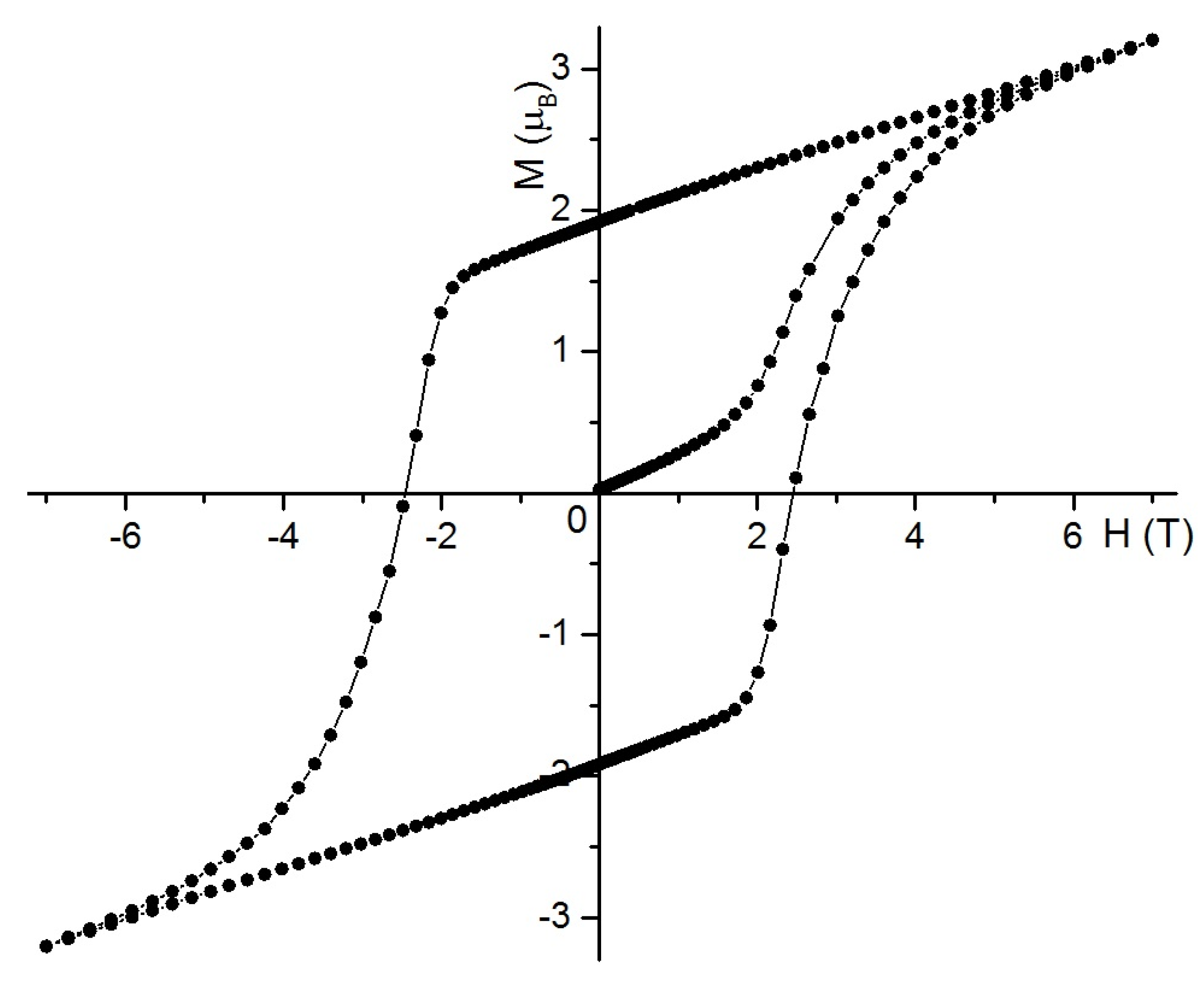
2.3. Magnetic Behavior Investigation
2.3.1. Static Magnetic Studies
2.3.2. Dynamic Magnetic Studies
3. Perspectives
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mironov, V.S. Spin Clusters with Orbitally Degenerate Magnetic Centers as Single-Molecule Nanomagnets with High Blocking Temperatures. Dokl. Phys. Chem. 2004, 397, 154–157. [Google Scholar] [CrossRef]
- Mironov, V.S. New Approaches to the Problem of High-Temperature Single-Molecule Magnets. Dokl. Phys. Chem. 2006, 408, 130–136. [Google Scholar] [CrossRef]
- Freedman, D.E.; Jenkins, D.M.; Iavarone, A.T.; Long, J.R. A Redox-Switchable Single-Molecule Magnet Incorporating [Re(CN)7]3–. J. Am. Chem. Soc. 2008, 130, 2884–2885. [Google Scholar] [CrossRef] [PubMed]
- Zadrozny, J.M.; Freedman, D.E.; Jenkins, D.M.; Harris, T.D.; Iavarone, A.T.; Mathonière, C.; Clérac, R.; Long, J.R. Slow Magnetic Relaxation and Charge-Transfer in Cyano-Bridged Coordination Clusters Incorporating [Re(CN)7]3−/4−. Inorg. Chem. 2010, 49, 8886–8896. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Avendaño, C.; Dunbar, K.R. Molecular Magnetic Materials Based on 4d and 5d Transition Metals. Chem. Soc. Rev. 2011, 40, 3213. [Google Scholar] [CrossRef] [PubMed]
- Dreiser, J.; Pedersen, K.S.; Schnegg, A.; Holldack, K.; Nehrkorn, J.; Sigrist, M.; Tregenna-Piggott, P.; Mutka, H.; Weihe, H.; Mironov, V.S.; et al. Three-Axis Anisotropic Exchange Coupling in the Single-Molecule Magnets NEt4[MnIII2(5-Brsalen)2(MeOH)2MIII(CN)6] (M=Ru, Os). Chem.—A Eur. J. 2013, 19, 3693–3701. [Google Scholar] [CrossRef] [Green Version]
- Qian, K.; Huang, X.-C.; Zhou, C.; You, X.-Z.; Wang, X.-Y.; Dunbar, K.R. A Single-Molecule Magnet Based on Heptacyanomolybdate with the Highest Energy Barrier for a Cyanide Compound. J. Am. Chem. Soc. 2013, 135, 13302–13305. [Google Scholar] [CrossRef]
- Wu, D.-Q.; Shao, D.; Wei, X.-Q.; Shen, F.-X.; Shi, L.; Kempe, D.; Zhang, Y.-Z.; Dunbar, K.R.; Wang, X.-Y. Reversible On–Off Switching of a Single-Molecule Magnet via a Crystal-to-Crystal Chemical Transformation. J. Am. Chem. Soc. 2017, 139, 11714–11717. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, R.; Nakano, M.; Breedlove, B.K.; Yamashita, M. Syntheses, Structures, and Magnetic Properties of Discrete Cyano-Bridged Heterodinuclear Complexes Composed of MnIII(Salen)-Type Complex and MIII(CN)6 Anion (MIII= Fe, Mn, and Cr). Polyhedron 2013, 64, 346–351. [Google Scholar] [CrossRef]
- Shi, L.; Wei, X.; Wang, X.; Wu, D. Research Progress in Molecular Magnetic Materials Based on the [Mo(CN)7]4– Unit. Sci. Sin. Chim. 2020, 50, 1637–1653. [Google Scholar] [CrossRef]
- Coulon, C.; Miyasaka, H.; Clérac, R. Single-Chain Magnets: Theoretical Approach and Experimental Systems BT—Single-Molecule Magnets and Related Phenomena; Winpenny, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 163–206. ISBN 978-3-540-33240-4. [Google Scholar]
- Sun, H.-L.; Wang, Z.-M.; Gao, S. Strategies towards Single-Chain Magnets. Coord. Chem. Rev. 2010, 254, 1081–1100. [Google Scholar] [CrossRef]
- Dhers, S.; Feltham, H.L.C.; Brooker, S. A Toolbox of Building Blocks, Linkers and Crystallisation Methods Used to Generate Single-Chain Magnets. Coord. Chem. Rev. 2015, 296, 24–44. [Google Scholar] [CrossRef]
- Bar, A.K.; Pichon, C.; Sutter, J.-P. Magnetic Anisotropy in Two- to Eight-Coordinated Transition–Metal Complexes: Recent Developments in Molecular Magnetism. Coord. Chem. Rev. 2016, 308, 346–380. [Google Scholar] [CrossRef]
- Coulon, C.; Pianet, V.; Urdampilleta, M.; Clérac, R. Single-Chain Magnets and Related Systems BT—Molecular Nanomagnets and Related Phenomena; Gao, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 143–184. ISBN 978-3-662-45723-8. [Google Scholar]
- Peresypkina, E.V.; Majcher, A.M.; Rams, M.; Vostrikova, K.E. A Single Chain Magnet Involving Hexacyanoosmate. Chem. Commun. 2014, 50, 7150–7153. [Google Scholar] [CrossRef]
- Majcher, A.M.; Pilet, G.; Mironov, V.S.; Vostrikova, K.E. Neutral Low-Dimensional Assemblies of a Mn(III) Schiff Base Complex and Octacyanotungstate(V): Synthesis, Characterization, and Magnetic Properties. Magnetochemistry 2017, 3, 16. [Google Scholar] [CrossRef]
- Vostrikova, K.E. Low-Dimensional Heterometallic Assemblies Involving Orbitally Degenerate Cyanometallate and Displaying Slow Magnetic Dynamics. J. Magn. Magn. Mater. 2018, 459, 71–77. [Google Scholar] [CrossRef]
- Shi, L.; Shao, D.; Wei, X.; Dunbar, K.R.; Wang, X. Enhanced Single-Chain Magnet Behavior via Anisotropic Exchange in a Cyano-Bridged MoIII–MnII Chain. Angew. Chemie Int. Ed. 2020, 59, 10379–10384. [Google Scholar] [CrossRef]
- Charytanowicz, T.; Jankowski, R.; Zychowicz, M.; Chorazy, S.; Sieklucka, B. The Rationalized Pathway from Field-Induced Slow Magnetic Relaxation in CoII–WIV Chains to Single-Chain Magnetism in Isotopological CoII–WV Analogues. Inorg. Chem. Front. 2022, 9, 1152–1170. [Google Scholar] [CrossRef]
- Mironov, V.S.; Chibotaru, L.F.; Ceulemans, A. Mechanism of a Strongly Anisotropic MoIII−CN−MnII Spin−Spin Coupling in Molecular Magnets Based on the [Mo(CN)7]4− Heptacyanometalate: A New Strategy for Single-Molecule Magnets with High Blocking Temperatures. J. Am. Chem. Soc. 2003, 125, 9750–9760. [Google Scholar] [CrossRef] [Green Version]
- Mironov, V.S. Origin of Dissimilar Single-Molecule Magnet Behavior of Three MnII2MoIII Complexes Based on [MoIII(CN)7]4– Heptacyanomolybdate: Interplay of MoIII–CN–MnII Anisotropic Exchange Interactions. Inorg. Chem. 2015, 54, 11339–11355. [Google Scholar] [CrossRef]
- Miyasaka, H.; Madanbashi, T.; Saitoh, A.; Motokawa, N.; Ishikawa, R.; Yamashita, M.; Bahr, S.; Wernsdorfer, W.; Clérac, R. Cyano-Bridged MnIII-MIII Single-Chain Magnets with MIII=CoIII, FeIII, MnIII, and CrIII. Chem.—A Eur. J. 2012, 18, 3942–3954. [Google Scholar] [CrossRef] [PubMed]
- Rams, M.; Peresypkina, E.V.; Mironov, V.S.; Wernsdorfer, W.; Vostrikova, K.E. Magnetic Relaxation of 1D Coordination Polymers (X)2[Mn(Acacen)Fe(CN)6 ], X = Ph4P+, Et4N+. Inorg. Chem. 2014, 53, 10291–10300. [Google Scholar] [CrossRef] [PubMed]
- Ferbinteanu, M.; Miyasaka, H.; Wernsdorfer, W.; Nakata, K.; Sugiura, K.; Yamashita, M.; Coulon, C.; Clérac, R. Single-Chain Magnet (NEt4)[Mn2(5-MeOsalen)2Fe(CN)6] Made of MnIII−FeIII−MnIII Trinuclear Single-Molecule Magnet with an S = 9/2 Spin Ground State. J. Am. Chem. Soc. 2005, 127, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Aguilà, D.; Jeannin, O.; Fourmigué, M.; Jeon, I.-R. MnIII–FeIII Heterometallic Compounds within Hydrogen-Bonded Supramolecular Networks Promoted by an [Fe(CN)5(CNH)]2– Building Block: Structural and Magnetic Properties. Inorg. Chem. 2018, 57, 7892–7903. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-Q.; Qian, K.; Wei, H.-Y.; Wang, X.-Y. A One-Dimensional Magnet Based on [MoIII(CN)7]4–. Inorg. Chem. 2016, 55, 5107–5109. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xia, B.; Wang, Q.-L.; Ma, Y.; Liao, D.-Z.; Tang, J. Slow Magnetic Relaxation Based on the Anisotropic Ising-Type Magnetic Coupling between the MoIII and MnII Centers. Dalt. Trans. 2017, 46, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Sukhikh, T.S.; Wernsdorfer, W.; Vostrikova, K.E. Slow Magnetic Relaxation in Neutral 0D and 1D Assemblies of a Mn(III) Schiff Base Complex and Heptacyanorhenate(IV). Magnetochemistry 2022, 8, 126. [Google Scholar] [CrossRef]
- Samsonenko, D.G.; Paulsen, C.; Lhotel, E.; Mironov, V.S.; Vostrikova, K.E. [MnIII(SchiffBase)]3[ReIV(CN)7], Highly Anisotropic 3D Coordination Framework: Synthesis, Crystal Structure, Magnetic Investigations, and Theoretical Analysis. Inorg. Chem. 2014, 53, 10217–10231. [Google Scholar] [CrossRef] [PubMed]
- David, J.; Mendizábal, F.; Arratia-Pérez, R. Electronic Structure and Molecular Properties of the Heptacyanorhenate [Re(CN)7]3– and [Re(CN)7]4– Complexes. J. Phys. Chem. A 2006, 110, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Peresypkina, E.V.; Samsonenko, D.G.; Vostrikova, K.E. Heterobimetallic Coordination Polymers Involving 3d Metal Complexes and Heavier Transition Metals Cyanometallates. J. Solid State Chem. 2015, 224, 107–114. [Google Scholar] [CrossRef]
- Miyasaka, H.; Okawa, H.; Miyazaki, A.; Enoki, T. Synthesis, Crystal and Network Structures, and Magnetic Properties of a Hybrid Layered Compound: [K(18-Cr)(2-PrOH)2][{Mn(Acacen)}2{Fe(CN)6}] (18-Cr = 18-Crown-6-Ether, Acacen = N, N ‘-ethylenebis(acetylacetonylideneiminate)). Inorg. Chem. 1998, 37, 4878–4883. [Google Scholar] [CrossRef]
- Kou, H.-Z.; Ni, Z.-H.; Zhou, B.C.; Wang, R.-J. A Cyano-Bridged Molecule-Based Magnet Containing Manganese(III) Schiff Base and Octacyanotungstate(V) Building Blocks. Inorg. Chem. Commun. 2004, 7, 1150–1153. [Google Scholar] [CrossRef]
- Miyasaka, H.; Saitoh, A.; Abe, S. Magnetic Assemblies Based on Mn(III) Salen Analogues. Coord. Chem. Rev. 2007, 251, 2622–2664. [Google Scholar] [CrossRef]
- Bennett, M.V.V.; Long, J.R.R. New Cyanometalate Building Units: Synthesis and Characterization of [Re(CN)7]3– and [Re(CN)8]3–. J. Am. Chem. Soc. 2003, 125, 2394–2395. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, H.; Julve, M.; Yamashita, M.; Clérac, R. Slow Dynamics of the Magnetization in One-Dimensional Coordination Polymers: Single-Chain Magnets. Inorg. Chem. 2009, 48, 3420–3437. [Google Scholar] [CrossRef]
- Perlepe, P.; Oyarzabal, I.; Mailman, A.; Yquel, M.; Platunov, M.; Dovgaliuk, I.; Rouzières, M.; Négrier, P.; Mondieig, D.; Suturina, E.A.; et al. Metal-organic magnets with large coercivity and ordering temperatures up to 242 °C. Science 2020, 370, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, X.; Meihaus, K.R.; Meng, X.; Zhang, Y.; Li, L.; Liu, J.-L.; Pedersen, K.S.; Keller, L.; Shi, W.; et al. Coercive Fields Above 6 T in Two Cobalt(II)–Radical Chain Compounds. Angew. Chemie Int. Ed. 2020, 59, 10610–10618. [Google Scholar] [CrossRef]
- Sakamoto, F.; Sumiya, T.; Fujita, M.; Tada, T.; Tan, X.S.; Suzuki, E.; Okura, I.; Fujii, Y. T-Site Selective Photocleavage of DNA by Cationic Schiff Base Complex of Manganese(III). Chem. Lett. 1998, 27, 1127–1128. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno Pineda, E.; Wernsdorfer, W.; Vostrikova, K.E. Very Anisotropic 2D Molecular Magnetic Materials Based on Pentagonal Bipyramidal Heptacyanidorhenate(IV). Materials 2022, 15, 8324. https://doi.org/10.3390/ma15238324
Moreno Pineda E, Wernsdorfer W, Vostrikova KE. Very Anisotropic 2D Molecular Magnetic Materials Based on Pentagonal Bipyramidal Heptacyanidorhenate(IV). Materials. 2022; 15(23):8324. https://doi.org/10.3390/ma15238324
Chicago/Turabian StyleMoreno Pineda, Eufemio, Wolfgang Wernsdorfer, and Kira E. Vostrikova. 2022. "Very Anisotropic 2D Molecular Magnetic Materials Based on Pentagonal Bipyramidal Heptacyanidorhenate(IV)" Materials 15, no. 23: 8324. https://doi.org/10.3390/ma15238324






