Photoluminescent Gold/BSA Nanoclusters (AuNC@BSA) as Sensors for Red-Fluorescence Detection of Mycotoxins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AuNC@BSA
2.3. Spectrophotometric Study of AuNC@BSA Interactions with Ligands
3. Results and Discussion
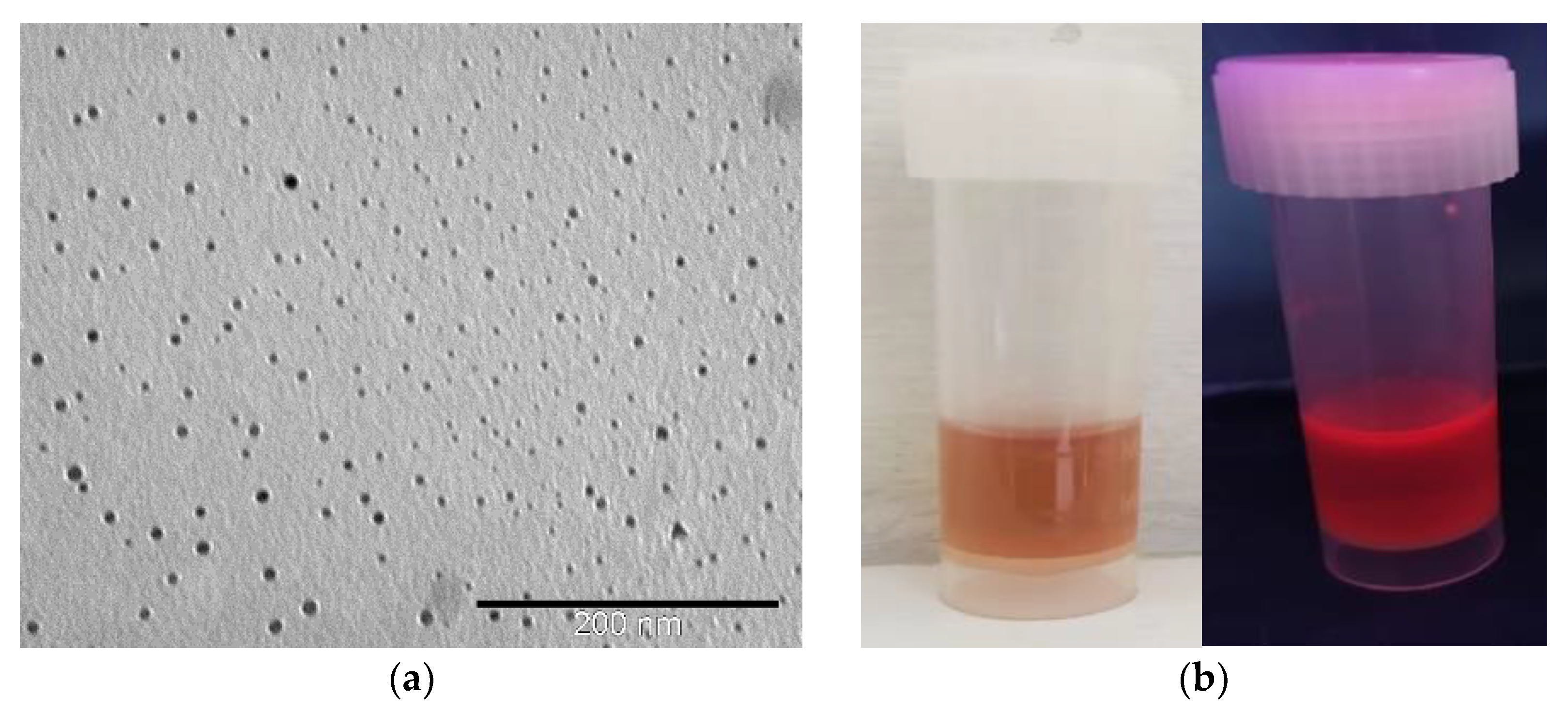
3.1. Characterisation of AuNC@BSA
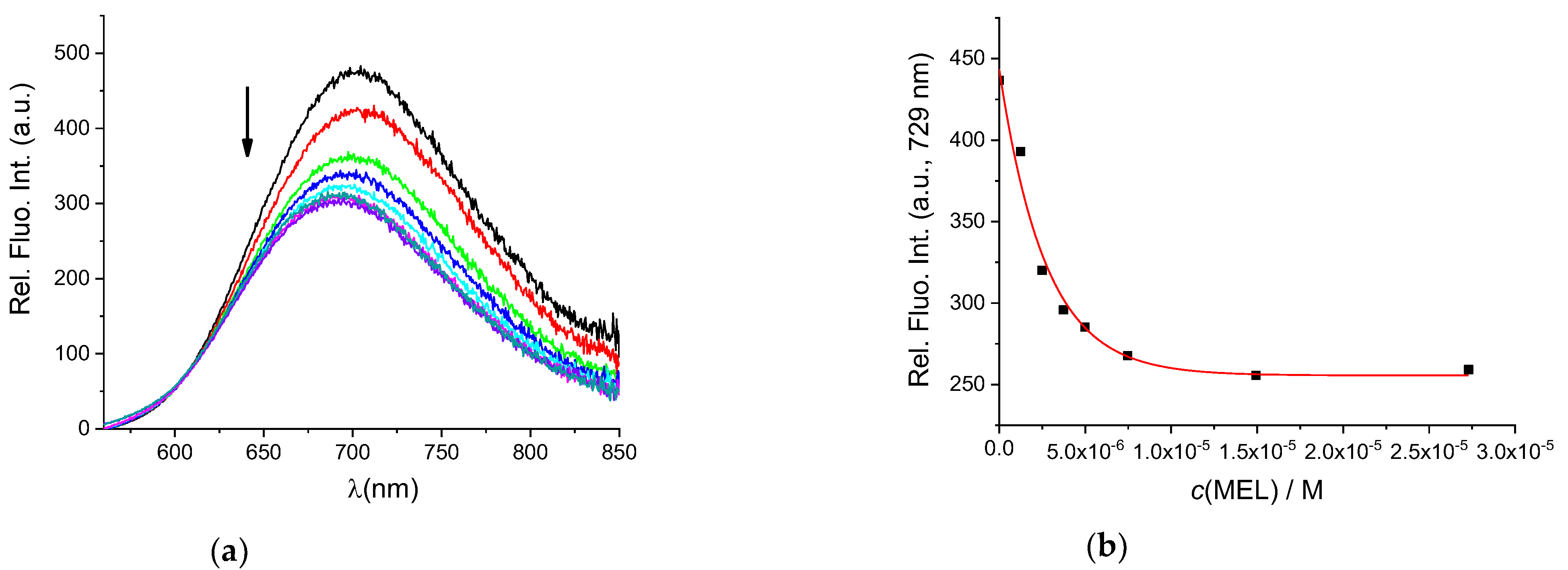
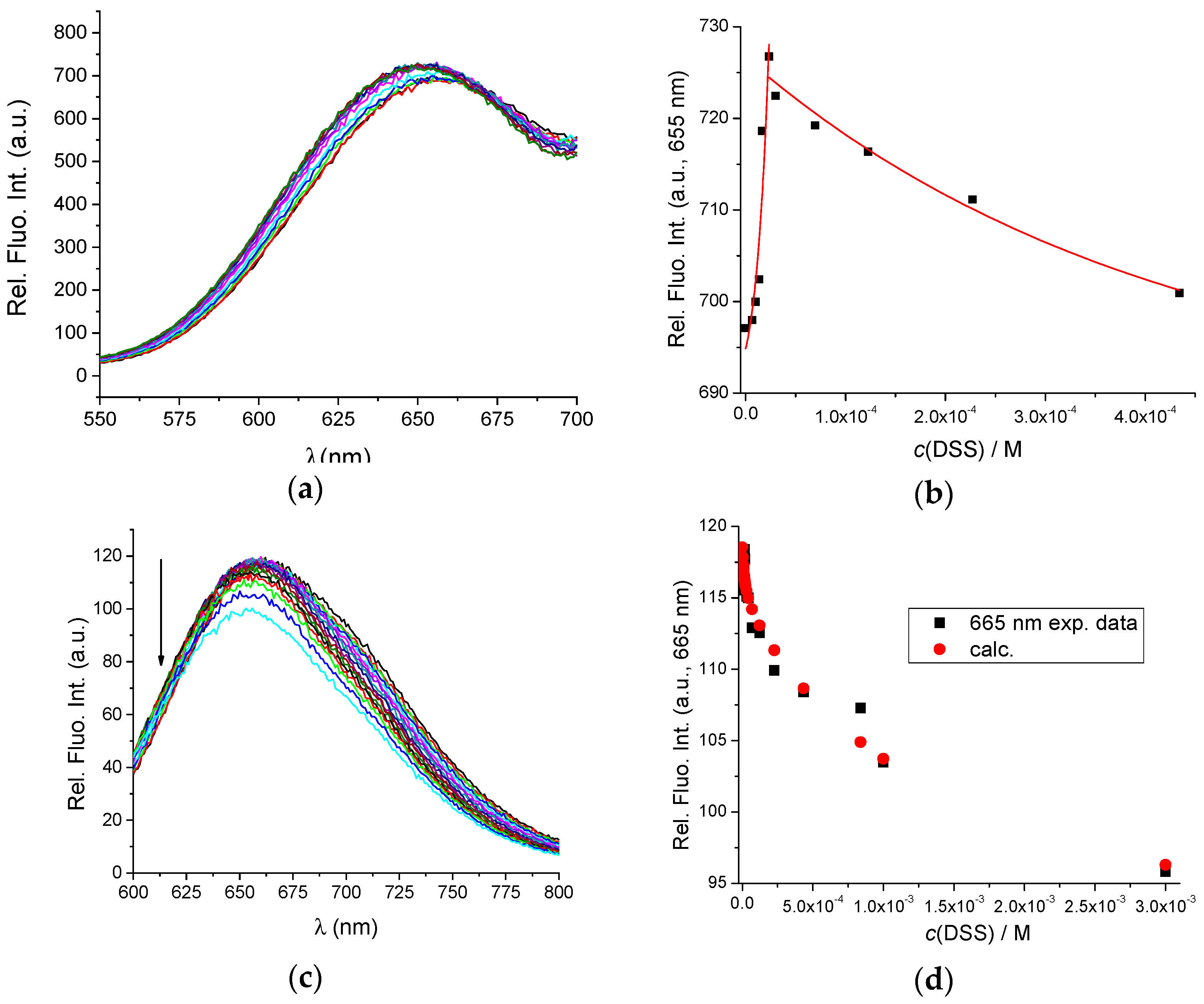
3.2. Interactions of AuNC@BSA with Meloxicam (MEL) and Diclofenac Sodium Salt (DSS)
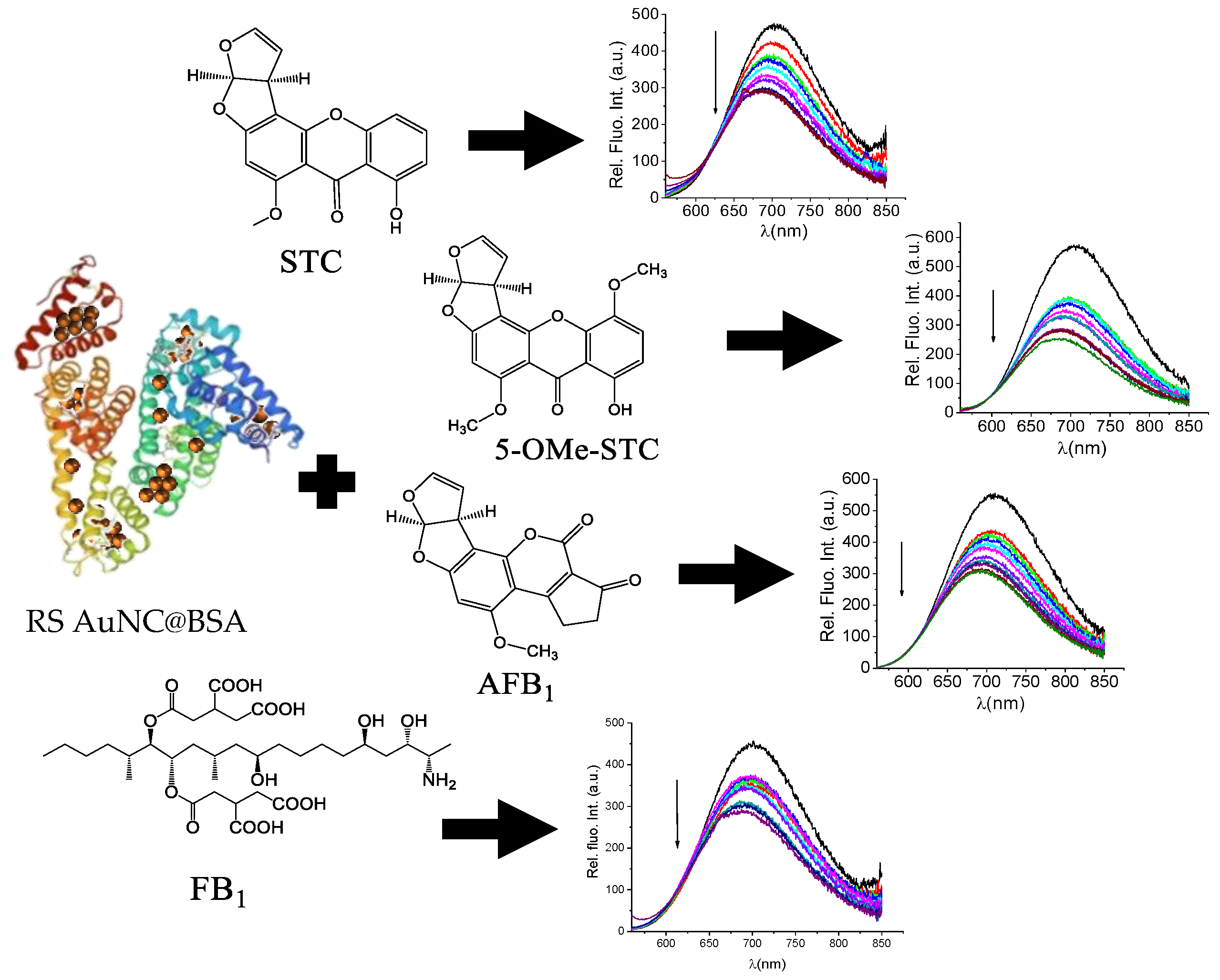
3.3. Detection of Mycotoxins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bastús, N.G.; Comenge, J.; Puntes, V.F. Kinetically Controlled Seeded Growth Synthesis of Citrate-Stabilized Gold Nanoparticles of up to 200 nm: Size Focusing versus Ostwald Ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef]
- Gonzalez, E.; Arbiol, J.; Puntes, V.F. Carving at the Nanoscale: Sequential Galvanic Exchange and Kirkendall Growth at Room Temperature. Science 2011, 334, 1377–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrabalak, S.E.; Chen, J.; Sun, Y.; Lu, X.; Au, L.; Cobley, C.M.; Xia, Y. Gold Nanocages: Synthesis, Properties, and Applications. Acc. Chem. Res. 2008, 41, 1587–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarhini, M.; Benlyamani, I.; Hamdani, S.; Agusti, G.; Fessi, H.; Greige-Gerges, H.; Bentaher, A.; Elaissari, A. Protein-Based Nanoparticle Preparation via Nanoprecipitation Method. Materials 2018, 11, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-L.; Jin, L.-Y.; Dong, Y.-M.; Wu, X.-M.; Li, Z.-Y. Intrinsic Enzyme Mimicking Activity of Gold Nanoclusters upon Visible Light Triggering and Its Application for Colorimetric Trypsin Detection. Biosens. Bioelectron. 2015, 64, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Sajanlal, P.R.; Pradeep, T. A Fifteen Atom Silver Cluster Confined in Bovine Serum Albumin. J. Mater. Chem. 2011, 21, 11205–112012. [Google Scholar] [CrossRef]
- Dutta, D.; Chattopadhyay, A.; Ghosh, S.S. Cationic BSA Templated Au–Ag Bimetallic Nanoclusters As a Theranostic Gene Delivery Vector for HeLa Cancer Cells. ACS Biomater. Sci. Eng. 2016, 2, 2090–2098. [Google Scholar] [CrossRef]
- Yan, L.; Cai, Y.; Zheng, B.; Yuan, H.; Guo, Y.; Xiao, D.; Choi, M.M.F. Microwave-Assisted Synthesis of BSA-Stabilized and HSA-Protected Gold Nanoclusters with Red Emission. J. Mater. Chem. 2012, 22, 1000–1005. [Google Scholar] [CrossRef]
- Lopez, A.; Liu, J. DNA-Templated Fluorescent Gold Nanoclusters Reduced by Good’s Buffer: From Blue-Emitting Seeds to Red and near Infrared Emitters. Can. J. Chem. 2015, 93, 615–620. [Google Scholar] [CrossRef]
- Wen, F.; Dong, Y.; Feng, L.; Wang, S.; Zhang, S.; Zhang, X. Horseradish Peroxidase Functionalized Fluorescent Gold Nanoclusters for Hydrogen Peroxide Sensing. Anal. Chem. 2011, 83, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Tang, Y.; Zhang, L.; Zhao, S. A Label-Free Fluorescent Assay for Free Chlorine in Drinking Water Based on Protein-Stabilized Gold Nanoclusters. Talanta 2015, 132, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qian, S.; Chen, J.; Cai, J.; Wu, S.; Cai, Z. Protein-Templated Gold Nanoclusters Based Sensor for off–on Detection of Ciprofloxacin with a High Selectivity. Talanta 2012, 94, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Hemmateenejad, B.; Shakerizadeh-shirazi, F.; Samari, F. BSA-Modified Gold Nanoclusters for Sensing of Folic Acid. Sens. Actuators B Chem. 2014, 199, 42–46. [Google Scholar] [CrossRef]
- Matulionyte, M.; Dapkute, D.; Budenaite, L.; Jarockyte, G.; Rotomskis, R. Photoluminescent Gold Nanoclusters in Cancer Cells: Cellular Uptake, Toxicity, and Generation of Reactive Oxygen Species. Int. J. Mol. Sci. 2017, 18, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, B.A.; Kubiak-Ossowska, K.; Mulheran, P.A.; Birch, D.J.S.; Chen, Y. Locating the Nucleation Sites for Protein Encapsulated Gold Nanoclusters: A Molecular Dynamics and Fluorescence Study. Phys. Chem. Chem. Phys. 2015, 17, 21935–21941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopu, C.L.; Shanti Krishna, A.; Sreenivasan, K. Fluorimetric Detection of Hypochlorite Using Albumin Stabilized Gold Nanoclusters. Sens. Actuators B Chem. 2015, 209, 798–802. [Google Scholar] [CrossRef]
- Cao, D.; Fan, J.; Qiu, J.; Tu, Y.; Yan, J. Masking Method for Improving Selectivity of Gold Nanoclusters in Fluorescence Determination of Mercury and Copperions. Biosens. Bioelectron. 2013, 42, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Meng, H.; Tu, Y.; Yan, J. A Nanocluster-Based Fluorescent Sensor for Sensitive Hemoglobin Detection. Talanta 2017, 170, 233–237. [Google Scholar] [CrossRef]
- Peng, J.; Su, Y.; Huang, F.-O.; Zuo, O.; Yang, L.; Li, Y.; Zhao, Y.; Qi, L.-W. A Simple and Rapid Fluorescent Approach for Flavonoids Sensor Based on Gold Nanoclusters. J. Colloid Interface Sci. 2019, 539, 175–183. [Google Scholar] [CrossRef]
- Yu, Y.; New, S.Y.; Lin, J.; Su, X.; Tan, Y.N. A Rapid and Quantitative Fluorimetric Method for Protein-Targeting Small Molecule Drug Screening. J. Vis. Exp. 2015, 104, 53261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zohrabi, T.; Hosseinkhani, S. Ternary Nanocomplexes of Metallic Nanoclusters and Recombinant Peptides for Fluorescence Imaging and Enhanced Gene Delivery. Mol. Biotechnol. 2020, 62, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Xu, Y.; Zhao, Y.; Zhong, H.; Luo, X. Fabrication of BSA@AuNC-Based Nanostructures for Cell Fluoresce Imaging and Target Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 8947–8954. [Google Scholar] [CrossRef]
- Masikini, M.; Williams, A.; Sunday, C.; Waryo, T.; Nxusani, E.; Wilson, L.; Qakala, S.; Bilibana, M.; Douman, S.; Jonnas, A.; et al. Label Free Poly(2,5-Dimethoxyaniline)–Multi-Walled Carbon Nanotubes Impedimetric Immunosensor for Fumonisin B1 Detection. Materials 2016, 9, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA. Scientific Opinion on the risk for public and animal health related to the presence of sterigmatocystin in food and feed. EFSA J. 2013, 11, 3254. [Google Scholar] [CrossRef]
- Engelhart, S.; Loock, A.; Skutlarek, D.; Sagunski, H.; Lommel, A.; Färber, H.; Exner, M. Occurrence of toxigenic Aspergillus versicolor isolates and sterigmatocystin in carpet dust from damp indoor environments. Appl. Environ. Microbiol. 2002, 68, 3886–3890. [Google Scholar] [CrossRef] [Green Version]
- Jakšić, D.; Sertć, M.; Kifer, D.; Kocsubè, S.; Mornar Turk, A.; Nigović, B.; Šarkanj, B.; Krska, R.; Sulyok, M.; Šegvić Klarić, M. Fungi and their secondary metabolites in water-damaged indoors after a major flood event in eastern Croatia. Indoor Air 2020, 31, 730–744. [Google Scholar] [CrossRef]
- Wong, J.; Magun, B.; Wood, L. Lung inflammation caused by inhaled toxicants: A review. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1391–1401. [Google Scholar] [CrossRef] [Green Version]
- Tuanny Franco, L.; Mousavi Khaneghah, A.; In Lee, S.H.; Fernandes Oliveira, C.A. Biomonitoring of mycotoxin exposure using urinary biomarker approaches: A review. Toxin Rev. 2019, 40, 383–403. [Google Scholar] [CrossRef]
- IARC. A Review of Human Cancerogens: Aflatoxins. In IARC Monographs on the Evaluation of Carcinogenic Risk to Humans; IARC Monographs; IARC: Lyon, France, 2012; pp. 225–248. [Google Scholar]
- Moon, J.; Kim, G.; Lee, S. A Gold Nanoparticle and Aflatoxin B1-BSA Conjugates Based Lateral Flow Assay Method for the Analysis of Aflatoxin B1. Materials 2012, 5, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.-S.; Skipper, P.L.; Peng, X.; Groopman, J.D.; Chen, J.-S.; Wogan, G.N.; Tannenbaum, S.R. Serum albumin adducts in the molecular epidemiology of aflatoxin carcinogenesis: Correlation with aflatoxin B1 intake and urinary excretion of aflatoxin M1. Carcinogenesis 1988, 9, 1323–1325. [Google Scholar] [CrossRef] [PubMed]
- Majorek, K.A.; Porebski, P.J.; Daval, A.; Zimmerman, M.D.; Jablonska, K.; Stewart, A.J.; Chruszcz, M.; Minor, W. Structural and immunologic characterization of bovine, horse and rabbit serum albumins. Mol. Immunol. 2012, 52, 174–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Wei, S.-C.; Chiu, W.-J.; Cang, J.; Hsu, P.-H.; Huang, C.-C. Nitrite Ion-Induced Fluorescence Quenching of Lumnescent BSA-Au 25 Nanoclusters: Mechanism and Application. Analyst 2014, 139, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Opálková Šišková, A.; Kozma, E.; Opálek, A.; Kroneková, Z.; Kleinová, A.; Nagy, Š.; Kronek, J.; Rydz, J.; Eckstein Andicsová, A. Diclofenac Embedded in Silk Fibroin Fibers as a Drug Delivery System. Materials 2020, 13, 3580. [Google Scholar] [CrossRef]
- Seedher, N.; Bhatia, S. Mechanism of Interaction of the Non-Steroidal Antiinflammatory Drugs Meloxicam and Nimesulide with Serum Albumin. J. Pharm. Biomed. Anal. 2005, 39, 257–262. [Google Scholar] [CrossRef]
- Dutta, S.K.; Basu, S.K.; Sen, K.K. Binding of diclofenac sodium with bovine serum albumin at different temperatures, pH and ionic strengths. Indian J. Exp. Biol. 2006, 44, 123–127. [Google Scholar]
- Maeder, M.; Zuberbuhler, A.D. Nonlinear Least-Squares Fitting of Multivariate Absorption Data. Anal. Chem. 1990, 62, 2220–2224. [Google Scholar] [CrossRef]
- Guan, G.; Zhang, S.-Y.; Cai, Y.; Liu, S.; Bharathi, M.S.; Low, M.; Yu, Y.; Xie, J.; Zheng, Y.; Zhang, Y.-W.; et al. Convenient Purification of Gold Clusters by Co-Precipitation for Improved Sensing of Hydrogen Peroxide, Mercury Ions and Pesticides. Chem. Comm. 2014, 50, 5703–5705. [Google Scholar] [CrossRef]
- Tan, H.; Chen, L.; Ma, L.; Liu, S.; Zhou, H.; Zhang, Y.; Guo, T.; Liu, W.; Dai, H.; Yu, Y. Fluorescence Spectroscopic Investigation of Competitive Interactions between Quercetin and Aflatoxin B1 for Binding to Human Serum Albumin. Toxins 2019, 11, 214. [Google Scholar] [CrossRef]

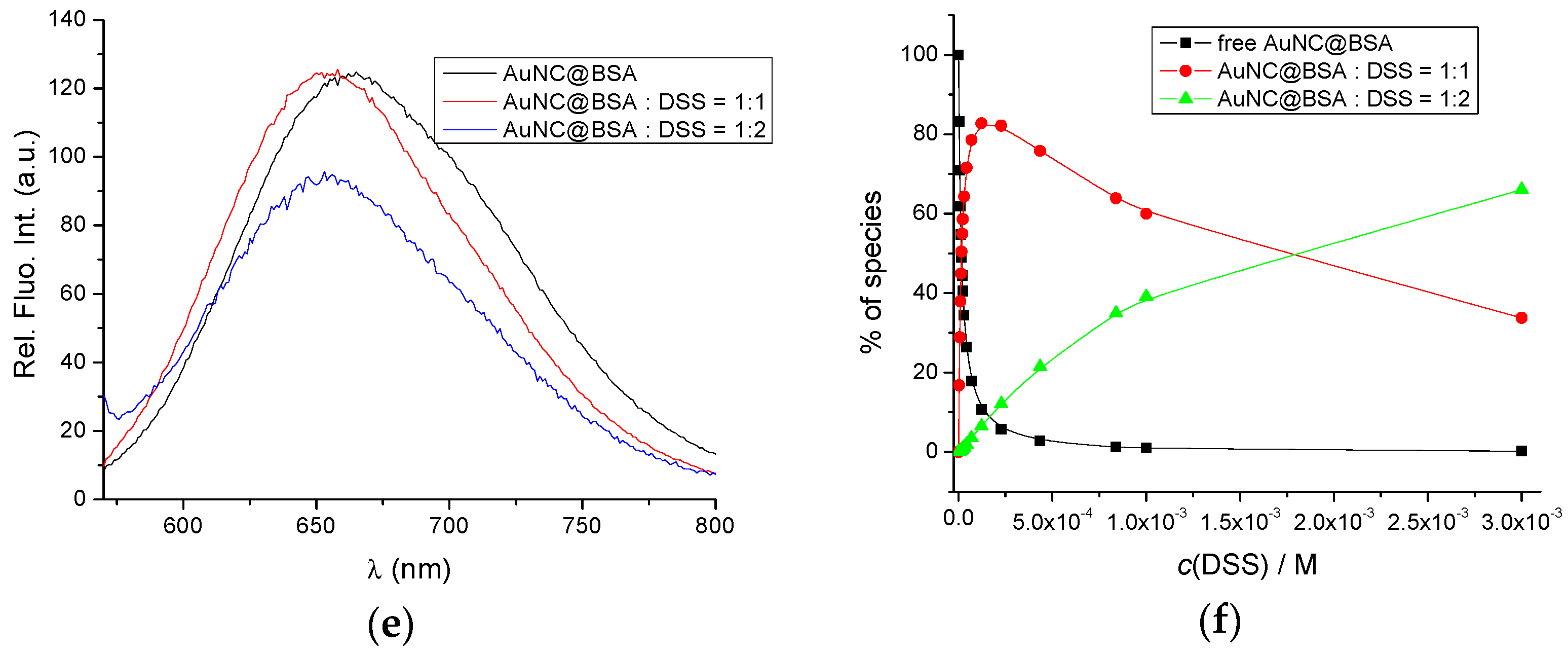
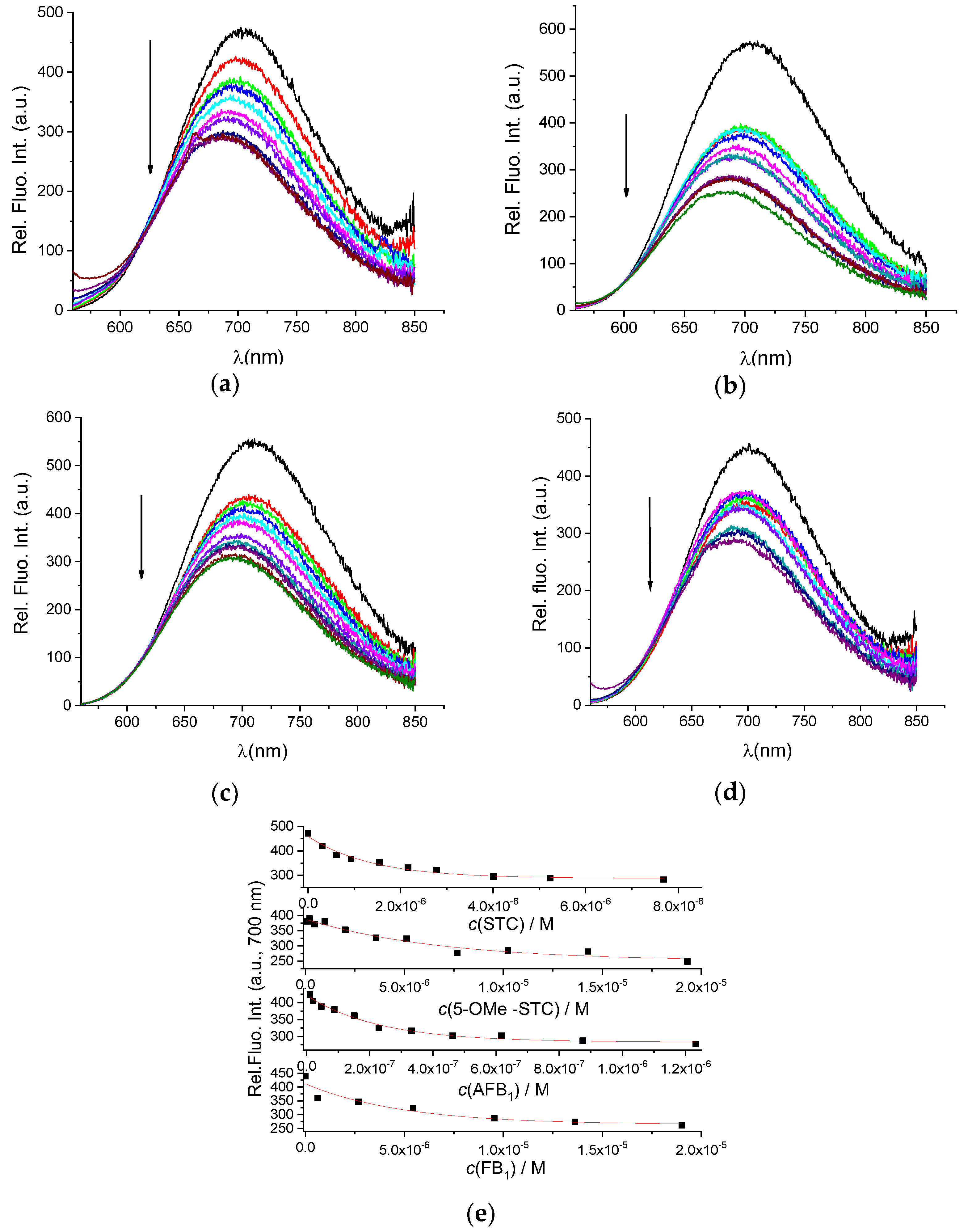
| Ligand | STC | 5-OMe-STC | AFB1 | FB1 | MEL | DSS |
|---|---|---|---|---|---|---|
| K × 105/M−1 | 7.3 ± 0.1 (20) a | 1.5 ± 0.1 | 43.2 ± 0.1 | 2.2 ± 0.1 (8.9) b | 3.7 ± 0.1 | 6.3 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabijanić, I.; Jurković, M.; Jakšić, D.; Piantanida, I. Photoluminescent Gold/BSA Nanoclusters (AuNC@BSA) as Sensors for Red-Fluorescence Detection of Mycotoxins. Materials 2022, 15, 8448. https://doi.org/10.3390/ma15238448
Fabijanić I, Jurković M, Jakšić D, Piantanida I. Photoluminescent Gold/BSA Nanoclusters (AuNC@BSA) as Sensors for Red-Fluorescence Detection of Mycotoxins. Materials. 2022; 15(23):8448. https://doi.org/10.3390/ma15238448
Chicago/Turabian StyleFabijanić, Ivana, Marta Jurković, Daniela Jakšić, and Ivo Piantanida. 2022. "Photoluminescent Gold/BSA Nanoclusters (AuNC@BSA) as Sensors for Red-Fluorescence Detection of Mycotoxins" Materials 15, no. 23: 8448. https://doi.org/10.3390/ma15238448






