Corrosion of Stirred Electrochemical Nano-Crystalline Hydroxyapatite (HA) Coatings on Ti6Al4V
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structure
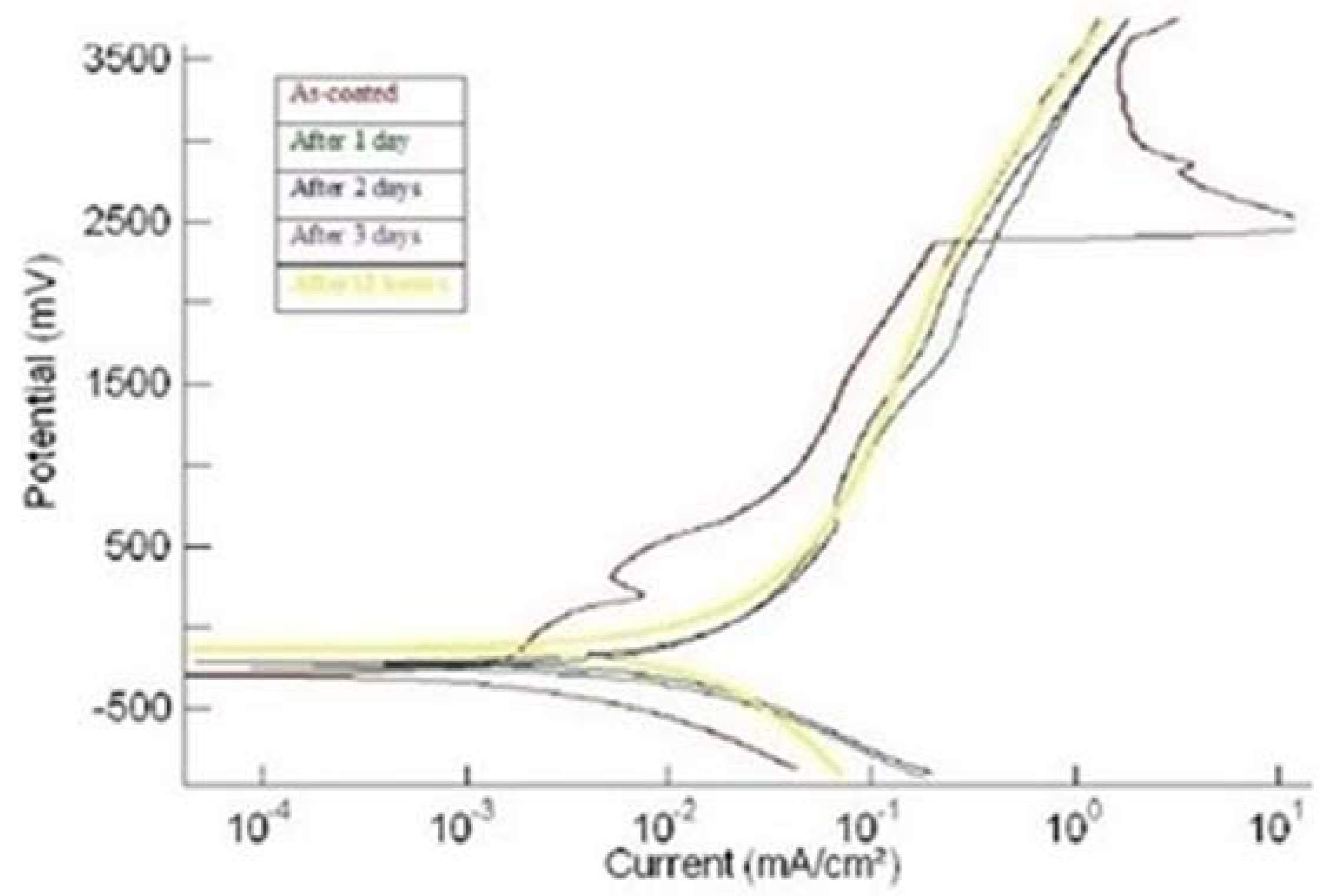
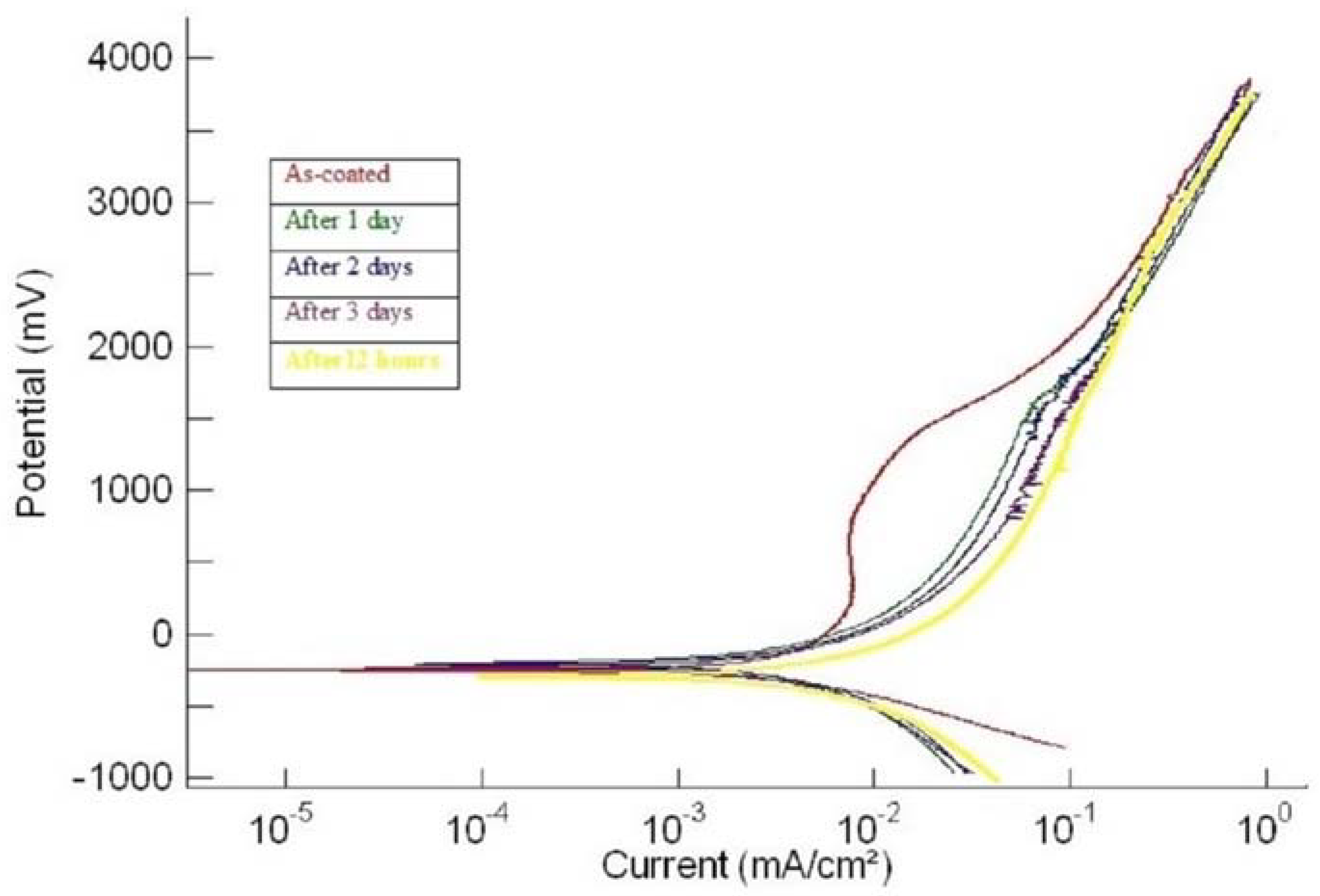
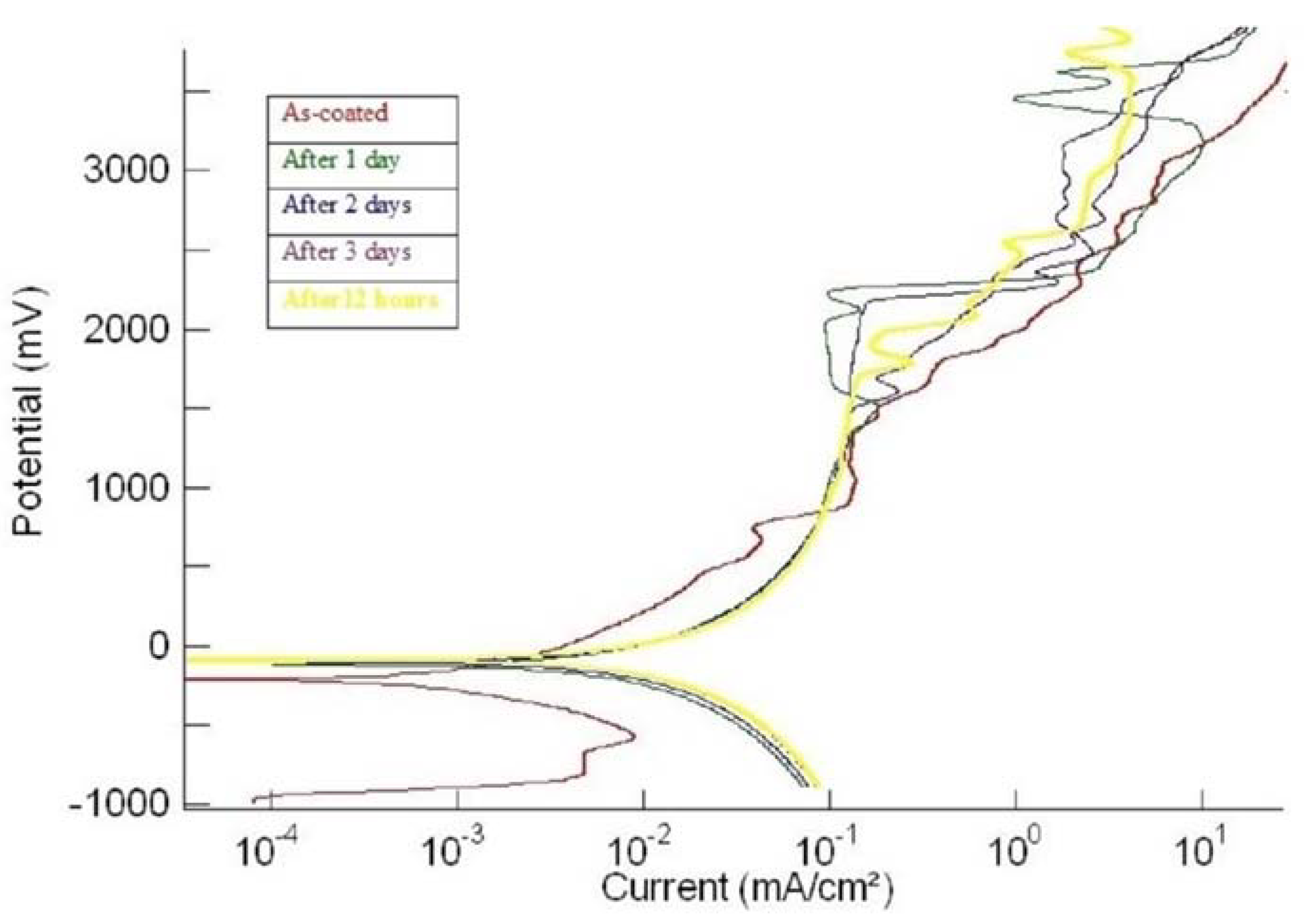
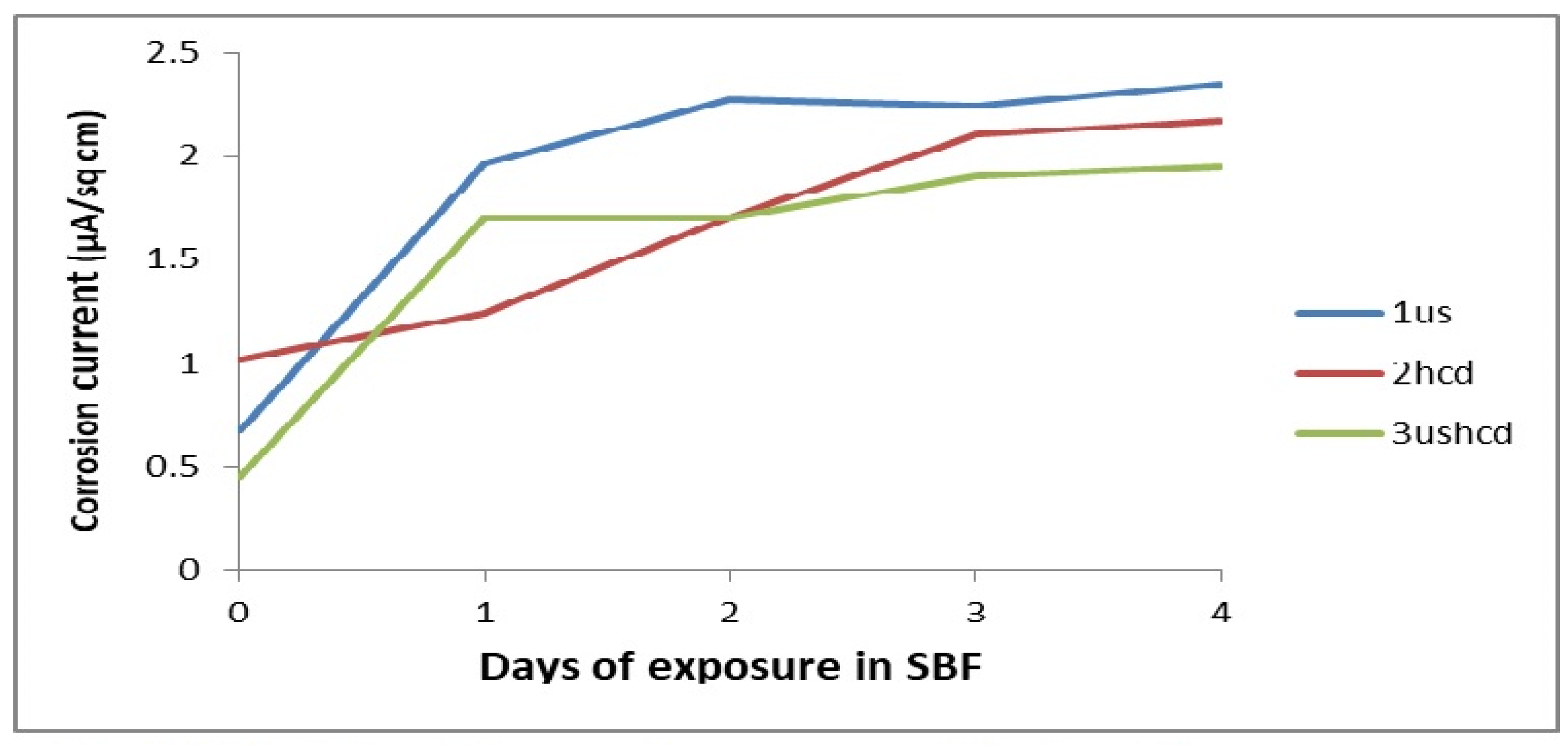
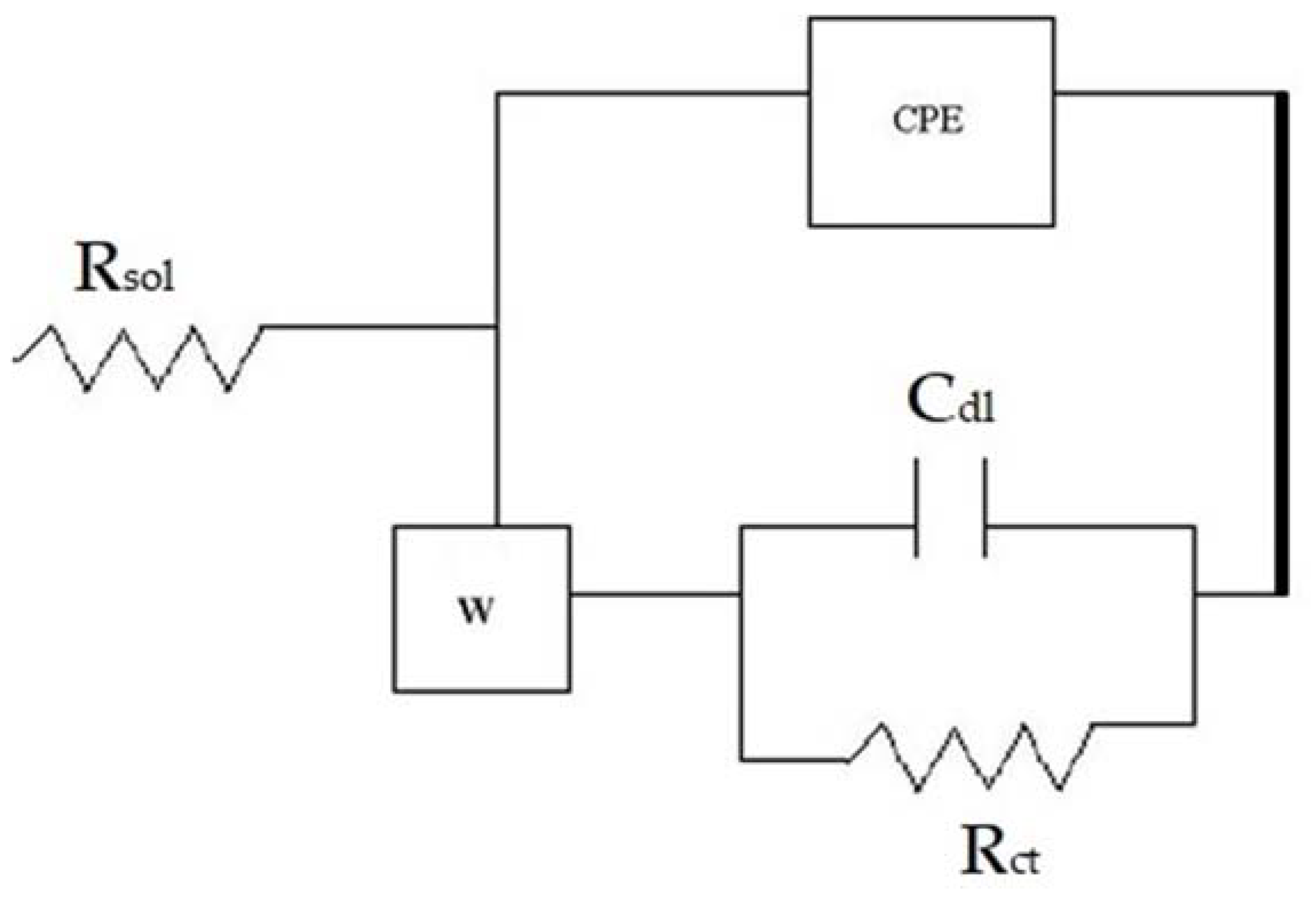
3.2. Corrosion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ishizawa, H.; Ogino, M. Formation and characterization of anodic titanium oxide films containing Ca and P. J. Biomed. Mater. Res. 1995, 29, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Shirkhanzadeh, M. Electrochemical preparation of protective oxide coatings on titanium surgical alloys. J. Mater. Sci. Mater. Med. 1992, 3, 322–325. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Arenas, M.A.; Tate, T.J.; Conde, A.; De Damborena, J. Corrosion behaviour of nitrogen implanted titanium in simulated body fluid. Br. Corr. J. 2000, 35, 232–236. [Google Scholar] [CrossRef]
- Ducheyne, P.; Van Raemdonck, V.; Heughebaert, J.C.; Heughebaert, M. Structural analysis of hydroxyapatite coatings on titanium. Biomaterials 1986, 7, 97–103. [Google Scholar] [CrossRef]
- Bystrova, A.; Dekhtyar, Y.D.; Popov, A.; Coutinho, J.; Bystrov, V. Modified hydroxyapatite structure and properties: Modeling and synchrotron data analysis of modified hydroxyapatite structure. Ferroelectric 2015, 475, 135–147. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1994, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hübner, W.; Blume, A.; Pushnjakova, R.; Dekhtyar, Y.; Hein, H.J. The influence of X-ray radiation on the mineral/organic matrix interaction of bone tissue: An FT-IR microscopic investigation. Int. J. Artif. Organs 2006, 28, 66–73. [Google Scholar] [CrossRef]
- Timimi, Z.; Tammemi, Z.J. Polymer Blends and Nanocomposite Materials Based on Polymethyl Methacrylate (PMMA) for Bone Regeneration and Repair: Characterization and Preparation. J. Sustain. Mater. Process. Manag. 2022, 2, 15–23. [Google Scholar]
- Ban, S.; Maruno, S. Morphology and microstructure of electrochemically deposited calcium phosphates in a modified simulated body fluid. Biomaterials 1998, 19, 1245–1253. [Google Scholar] [CrossRef]
- Moroni, A.; Caja, V.L.; Egger, E.L.; Trinchese, L.; Chao, E.Y. Histomorphometry of hydroxyapatite coated and uncoated porous titanium bone implants. Biomaterials 1994, 15, 926–930. [Google Scholar] [CrossRef]
- Gledhill, H.C.; Turner, I.G.; Doyle, C. In vitro dissolution behaviour of two morphologically different thermally sprayed hydroxyapatite coatings. Biomaterials 2001, 22, 695–700. [Google Scholar] [CrossRef]
- Graβmann, O.; Heimann, R.B. Compositional and microstructural changes of engineered plasma-sprayed hydroxyapatite coatings on Ti6Al4V substrates during incubation in protein-free simulated body fluid. J. Biomed. Mater. Res. 2000, 53, 685–693. [Google Scholar] [CrossRef]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Induction plasma sprayed Sr and Mg doped nano hydroxyapatite coatings on Ti for bone implant. J. Biomed. Mater. Res. 2011, 99B, 258–265. [Google Scholar] [CrossRef]
- Li, L.; Lu, X.; Meng, Y.Z.; Weyant, C.M. Comparison study of biomimetic strontium-doped calcium phosphate coatings by electrochemical deposition and air plasma spray: Morphology, composition and bioactive performance. J. Mater. Sci. Mater. Med. 2012, 23, 2359–2368. [Google Scholar] [CrossRef]
- Kasuga, T.; Kondo, H.; Nogami, M. Apatite formation on TiO2 in simulated body fluid. J. Cryst. Growth 2002, 235, 235–240. [Google Scholar] [CrossRef]
- Jonášová, L.; Müller, F.A.; Helebrant, A.; Strnad, J.; Greil, P. Hydroxyapatite formation on alkali-treated titanium with different content of Na+ in the surface layer. Biomaterials 2002, 23, 3095–3101. [Google Scholar] [CrossRef]
- Montenero, A.; Gnappi, G.; Ferrari, F.; Cesari, M.; Salvioli, E.; Mattogno, L.; Kaciulis, S.; Fini, M. Sol-gel derived hydroxyapatite coatings on titanium substrate. J. Mater. Sci. 2000, 15, 2791–2797. [Google Scholar] [CrossRef]
- Catauro, M.; Verardi, D.; Melisi, D.; Belotti, F.; Mustarelli, P. Novel sol-gel organic-inorganic hybrid materials for drug delivery. J. Appl. Biomater. Biomech. 2010, 8, 42–51. [Google Scholar]
- Farnoush, H.; Mohandesi, J.A.; Fatmehsari, D.H.; Moztarzadeh, F. Modification of electrophoretically deposited nano-hydroxyapatite coatings by wire brushing on Ti–6Al–4V substrates. Ceram. Int. 2012, 38, 4885–4893. [Google Scholar] [CrossRef]
- de Sena, L.A.; de Andrade, M.C.; Rossi, A.M.; de Almeida, S.G. Hydroxyapatite deposition by electrophoresis on titanium sheets with different surface finishing. J. Biomed. Mater. Res. 2002, 60, 1–7. [Google Scholar] [CrossRef] [PubMed]
- You, C.K.; Meng, X.W.; Kwon, T.Y.; Zang, Y.Z.; Ong, J.L.; Kim, S.; Kim, K.H. Development of hydroxyapatite thin film on titanium substrate by electrophoretic deposition. Key Eng. Mater. 2005, 284–286, 901–904. [Google Scholar] [CrossRef]
- Meng, X.; Kwon, T.Y.; Kim, K.H. Different morphology of hydroxyapatite coatings on titanium by electrophoretic deposition. Key Eng. Mater. 2005, 309–311, 639–642. [Google Scholar]
- Ban, S.; Maruno, S. Effect of temperature on electrochemical deposition of calcium phosphate coatings in a simulated body fluid. Biomaterials 1995, 16, 977–981. [Google Scholar] [CrossRef]
- Wang, J.; Layrolle, P.; Stigter, M.; de Groot, K. Biomimetic and electrolytic calcium phosphate coatings on titanium alloy: Physicochemical characteristics and cell attachment. Biomaterials 2004, 25, 583–592. [Google Scholar] [CrossRef]
- Marcelo, H.; Da Silva, P.; Soares, G.D.A.; Elias, C.N.; Best, S.M.; Gibson, I.R.; Di Silvio, L.; Dalby, M.J. In vitro cellular response to titanium electrochemically coated with hydroxyapatite compared to titanium with three different levels of surface roughness. J. Mater. Sci. Mater. Med. 2003, 14, 511–519. [Google Scholar]
- Balasundaram, G.; Sato, M.; Webster, T.J. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials 2006, 27, 2798–2805. [Google Scholar] [CrossRef]
- Pezzatini, S.; Solito, R.; Morbidelli, L.; Lamponi, S.; Boanini, E.; Bigi, A.; Ziche, M. The effect of hydroxyapatite nanocrystals on microvascular endothelial cell viability and functions. J. Biomed. Mater. Res. 2006, 76A, 656–663. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Lanford, W.A. Increased osteoblast adhesion on titanium-coated hydroxylapatite that forms CaTiO3. J. Biomed. Mater. Res. 2003, 67A, 975–980. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Enhanced functions of osteoclast-like cells on nanophase ceramics. Biomaterials 2001, 22, 1327–1333. [Google Scholar] [CrossRef]
- Narayanan, R.; Kim, S.Y.; Kwon, T.Y.; Kim, K.H. Nanocrystalline hydroxyapatite coatings from ultrasonated electrolyte: Preparation, characterization, and osteoblast responses. J. Biomed. Mater. Res. 2008, 87A, 1053–1060. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K.; Kwon, T.Y.; Kim, K.H. Electrochemical nano-grained calcium phosphate coatings on Ti–6Al–4V for biomaterial applications. Scr. Mater. 2007, 56, 229–232. [Google Scholar] [CrossRef]
- Li, T.T.; Ling, L.; Lin, M.C.; Peng, H.K.; Ren, H.T.; Lou, C.W.; Lin, J.H. Recent advances in multifunctional hydroxyapatite coating by electrochemical deposition. J. Mater. Sci. 2020, 5, 6352–6374. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Y.; Liu, L.; Liu, Q.; Ding, H. Effect of ultrasonic cold forging technology as the pretreatment on the corrosion resistance of MAO Ca/P coating on AZ31B Mg alloy. J. Alloy. Comp. 2015, 635, 278–288. [Google Scholar] [CrossRef]
- Fang, H.; Zhou, S.; Qi, X.; Wang, C.; Tian, Y. A multifunctional osteogenic system of ultrasonically spray deposited bone-active coatings on plasma-activated magnesium. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- Fathyunes, L.; Khalil-Allafi, J. Characterization and corrosion behavior of graphene oxide-hydroxyapatite composite coating applied by ultrasound-assisted pulse electrodeposition. Ceram. Int. 2017, 43, 13885–13894. [Google Scholar] [CrossRef]
- Fardi, S.R.; Kkhorsand, H.; Askarnia, R.; Pardehkhorram, R.; Adabifiroozjaei, E. Improvement of biomedical functionality of titanium by ultrasound-assisted electrophoretic deposition of hydroxyapatite-graphene oxide nanocomposites. Ceram. Int. 2020, 46, 18297–18307. [Google Scholar] [CrossRef]
- Sun, J.; Cai, S.; Sun, J.; Shen, K.; Liu, J.; Xu, G. Ultrasonic aqueous synthesis of corrosion resistant hydroxyapatite coating on magnesium alloys for the application of long-term implant. Ultrason. Sonochem. 2019, 58, 104677. [Google Scholar] [CrossRef]
- Lin, M.H.; Chen, Y.C.; Liao, C.C.; Lin, L.W.; Chen, C.F.; Wang, K.K.; Chen, S.T.; Hsueh, Y.H.; Wu, C.H.; Ou, S.F. Improvement in bioactivity and corrosion resistance of Ti by hydroxyapatite deposition using ultrasonic mechanical coating and armoring. Ceram. Int. 2022, 48, 4999–5008. [Google Scholar] [CrossRef]
- Lin, M.H.; Fan, F.Y.; Kuo, C.H.; Lin, L.W.; Wang, K.K.; Chen, C.F.; Wang, Y.H.; Ou, S.F. Nanostructured hydroxyapatite coatings on NiTi shape memory alloys by ultrasonic mechanical coating and armouring. Surf. Coat. Technol. 2022, 431, 127998. [Google Scholar] [CrossRef]
- Cigada, A.; Cabrini, M.; Pedeferri, P. Increasing of the corrosion resistance of the Ti6Al4V alloy by high thickness anodic oxidation. J. Mater. Sci. Mater. Med. 1992, 3, 408–412. [Google Scholar] [CrossRef]
- Yen, S.K.; Lin, C.M. Cathodic reactions of electrolytic hydoxyapatite coating on pure titanium. Mater. Chem. Phys. 2002, 77, 70–76. [Google Scholar] [CrossRef]
- Elzbieta, K.C. Gel-like layer development during formation of thin anodic films on titanium in phosphoric acid solutions. Corr. Sci. 2004, 46, 2487–2502. [Google Scholar]
- Cheng, X.; Sharon, R.G. Corrosion behavior of titanium in the presence of calcium phosphate and serum proteins. Biomaterials 2005, 26, 7350–7356. [Google Scholar] [CrossRef] [PubMed]

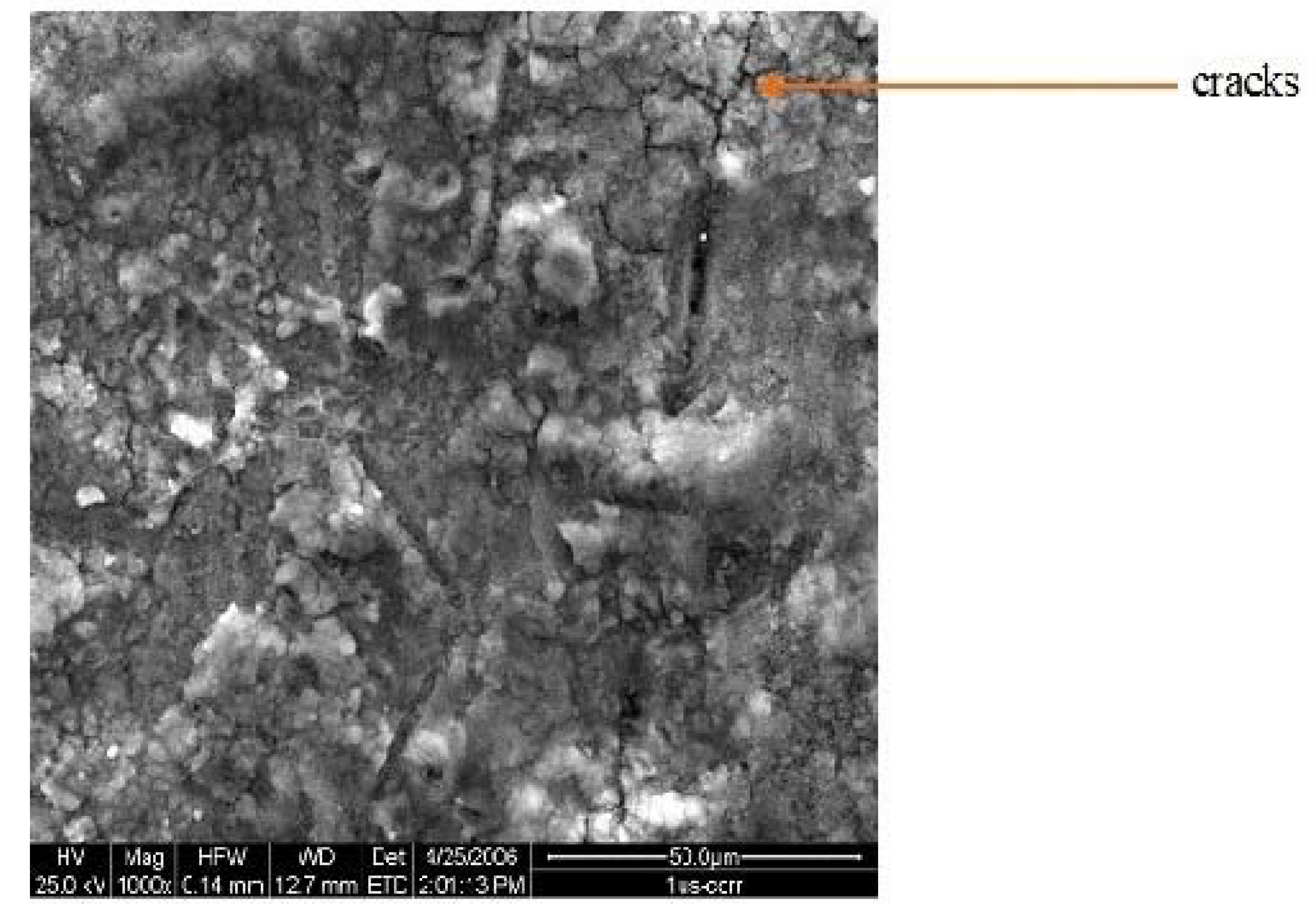
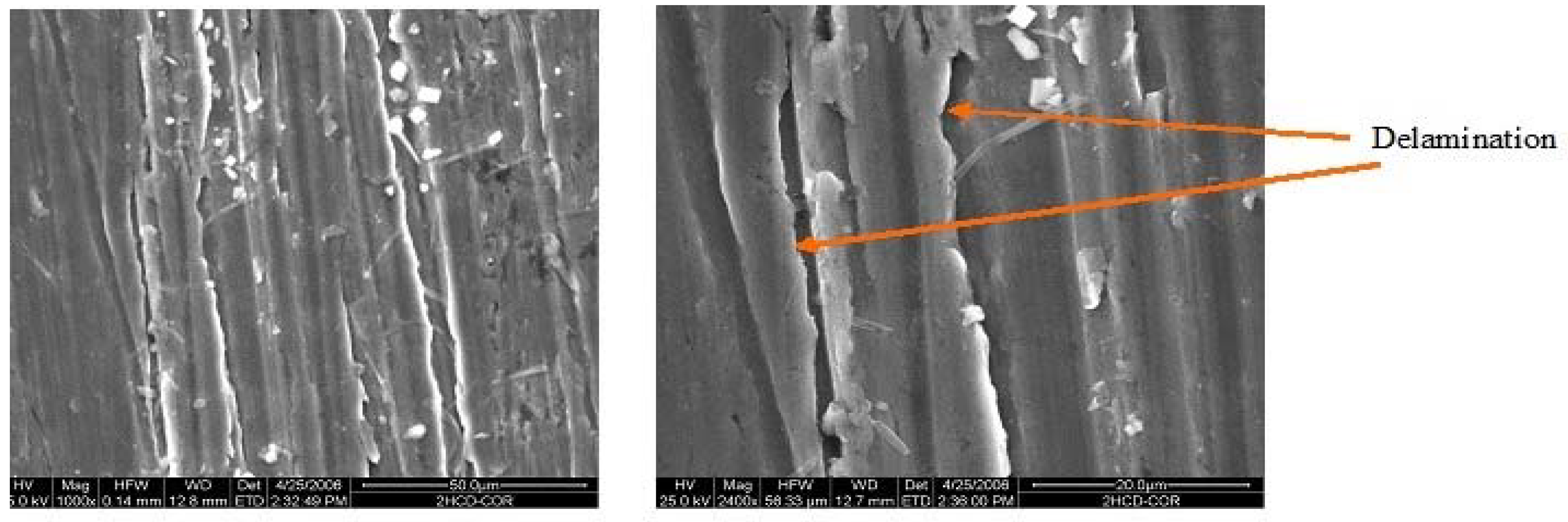
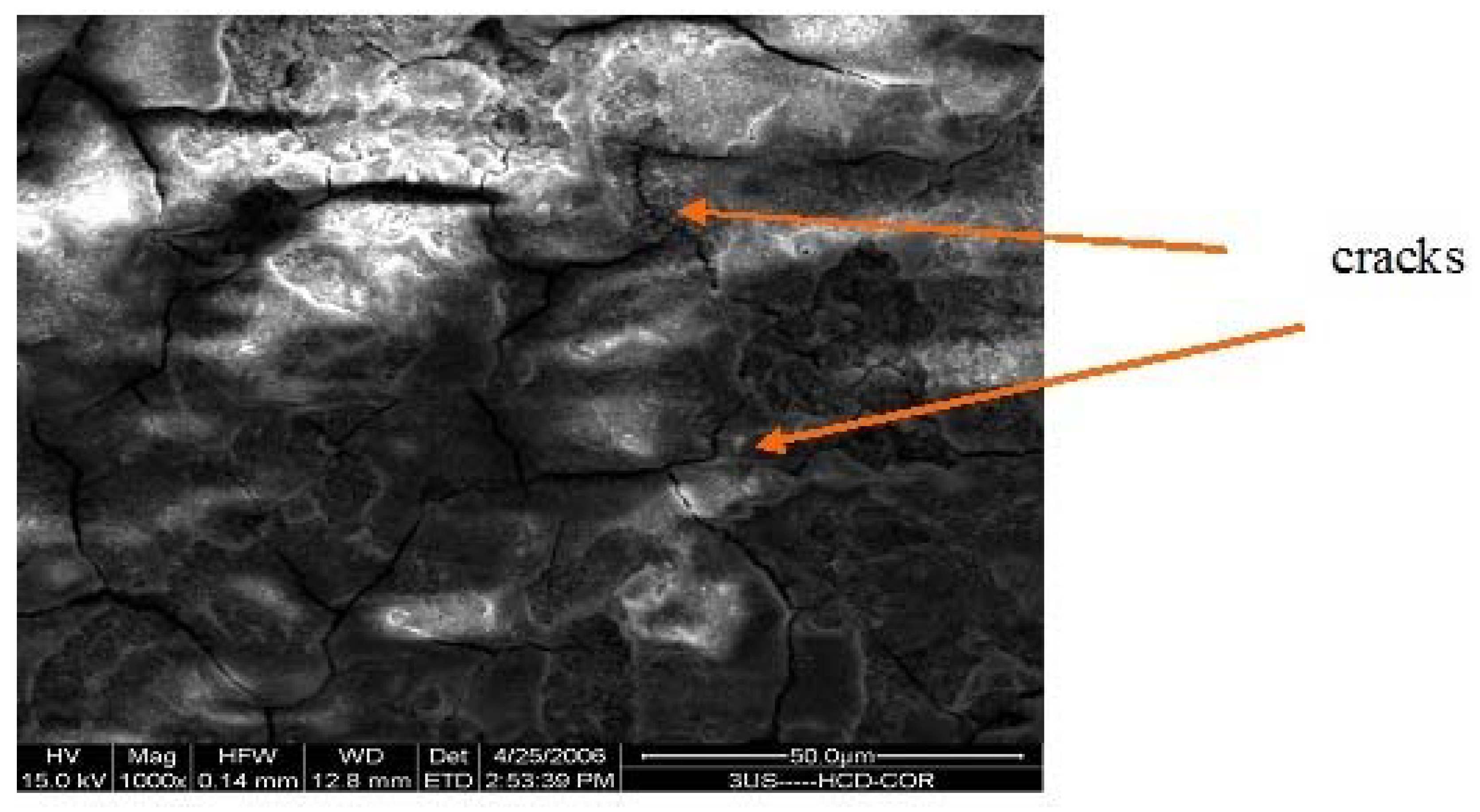
| Electrolyte | Electrolysis Conditions | Further Treatment | Nomenclature |
|---|---|---|---|
| 0.042 M Ca(NO3)2 and 0.025 M (NH4)2HPO4 | 20 mA/cm2 30 min | Ultrasonic agitation | 1us |
| 0.042 M Ca(NO3)2 and 0.025 M (NH4)2HPO4 | 50 mA/cm2 30 min | - | 2hcd |
| 0.042 M Ca(NO3)2 and 0.025 M (NH4)2HPO4 | 50 mA/cm2 30 min | Ultrasonic agitation | 3ushcd |
| Coating | Testing Condition | Ecorr (mV) | icorr (µA/cm2) | Epass (mV) | ipass (µA/cm2) |
|---|---|---|---|---|---|
| 1us | (i) As-plated | −296 | 0.67 | 190–350 | 7.5 |
| (ii) After 1 day | −250 | 1.96 | No passivation | No passivation | |
| (iii) After 2 days | −233 | 2.27 | No passivation | No passivation | |
| (iv) After 3 days | −153 | 2.24 | No passivation | No passivation | |
| (v) After 4 days | −139 | 2.35 | No passivation | No passivation | |
| 2hcd | (i) As-plated | −243 | 1.02 | 250–950 | 7.7 |
| (ii) After 1 day | −186 | 1.24 | No passivation | No passivation | |
| (iii) After 2 days | −223 | 1.7 | No passivation | No passivation | |
| (iv) After 3 days | −290 | 2.10 | No passivation | No passivation | |
| (v) After 4 days | −290 | 2.17 | No passivation | No passivation | |
| 3ushcd | (i) As-plated | −207 | 0.45 | No passivation | No passivation |
| (ii) After 1 day | −112 | 1.7 | No passivation | No passivation | |
| (iii) After 2 days | −95.0 | 1.7 | No passivation | No passivation | |
| (iv) After 3 days | −90.0 | 1.9 | No passivation | No passivation | |
| (v) After 4 days | −95.0 | 1.95 | No passivation | No passivation |
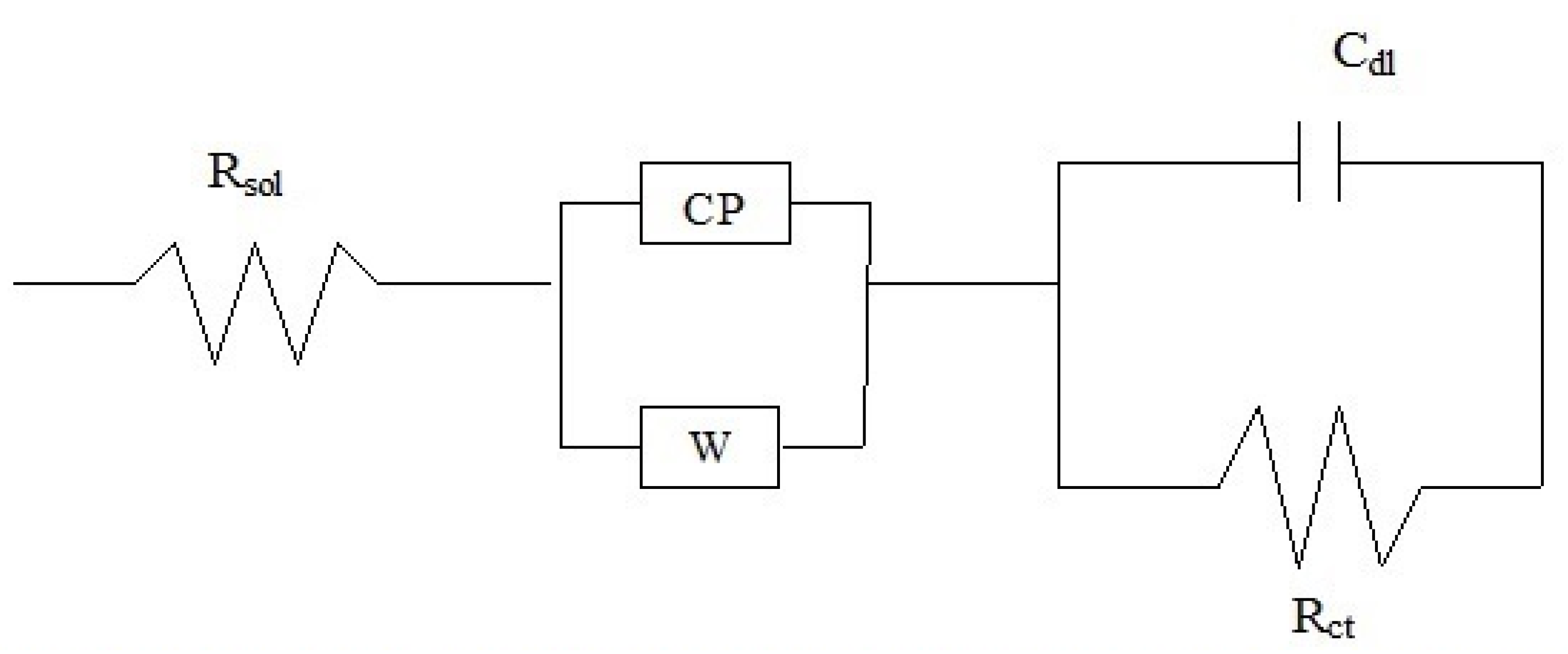
| Coating | Warburg Imp. (Ώ-cm2) | Rct (Ώ-cm2) | Cdl (F) | ZCPE or Total Imp. ‘Z’ (Ώ-cm2) |
|---|---|---|---|---|
| 1us | ||||
| (i) As-plated | 4.9 × 108 | 1.44 × 105 | 2.61 × 10−4 | 8.19 × 105 |
| (ii) After 1 day | 2.6 × 108 | 0.39 × 105 | 2.61 × 10−4 | 5.79 × 105 |
| (iii) After 2 days | 1.5 × 108 | 0.76 × 105 | 2.61 × 10−4 | 5.35 × 105 |
| (iv) After 3 days | 1.42 × 108 | 1.44 × 105 | 2.61 × 10−4 | 15.5 × 105 |
| (v) After 4 days | 1.45 × 108 | 0.54 × 105 | 2.61 × 10−4 | 2.5 × 105 |
| 2hcd | ||||
| (i) As-plated | 4.0 × 105 | 5.42 × 105 | 2.61 × 10−4 | 1.69 × 108 |
| (ii) After 1 day | 2.86 × 105 | 1.61 × 105 | 2.61 × 10−4 | 20.7 × 108 |
| (iii) After 2 days | 2.65 × 105 | 1.67 × 105 | 2.61 × 10−4 | 2.087 × 108 |
| (iv) After 3 days | 2.59 × 105 | 1.94 × 105 | 2.61 × 10−4 | 2.53 × 108 |
| (v) After 4 days | 2.55 × 105 | 1.31 × 105 | 2.61 × 10−4 | 1.49 × 108 |
| 3ushcd | ||||
| (i) As-plated | 89.6 × 105 | 2.85 × 105 | 2.61 × 10−4 | 5.33 × 108 |
| (ii) After 1 day | 2.82 × 105 | 0.76 × 105 | 2.61 × 10−4 | 8.63 × 108 |
| (iii) After 2 days | 29.1 × 105 | 1.17 × 105 | 2.61 × 10−4 | 1.35 × 108 |
| (iv) After 3 days | 2.22 × 105 | 1.17 × 105 | 2.61 × 10−4 | 17.2 × 108 |
| (v) After 4 days | 2.31 × 105 | 1.37 × 105 | 2.61 × 10−4 | 63 × 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramaswamy, N.; Gopalan, V.; Kwon, T.Y. Corrosion of Stirred Electrochemical Nano-Crystalline Hydroxyapatite (HA) Coatings on Ti6Al4V. Materials 2022, 15, 8609. https://doi.org/10.3390/ma15238609
Ramaswamy N, Gopalan V, Kwon TY. Corrosion of Stirred Electrochemical Nano-Crystalline Hydroxyapatite (HA) Coatings on Ti6Al4V. Materials. 2022; 15(23):8609. https://doi.org/10.3390/ma15238609
Chicago/Turabian StyleRamaswamy, Narayanan, Venkatachalam Gopalan, and Tae Yub Kwon. 2022. "Corrosion of Stirred Electrochemical Nano-Crystalline Hydroxyapatite (HA) Coatings on Ti6Al4V" Materials 15, no. 23: 8609. https://doi.org/10.3390/ma15238609
APA StyleRamaswamy, N., Gopalan, V., & Kwon, T. Y. (2022). Corrosion of Stirred Electrochemical Nano-Crystalline Hydroxyapatite (HA) Coatings on Ti6Al4V. Materials, 15(23), 8609. https://doi.org/10.3390/ma15238609






