Low Core Losses of Fe-Based Soft Magnetic Composites with an Zn-O-Si Insulating Layer Obtained by Coupling Synergistic Photodecomposition
Abstract
:1. Introduction
2. Experimental
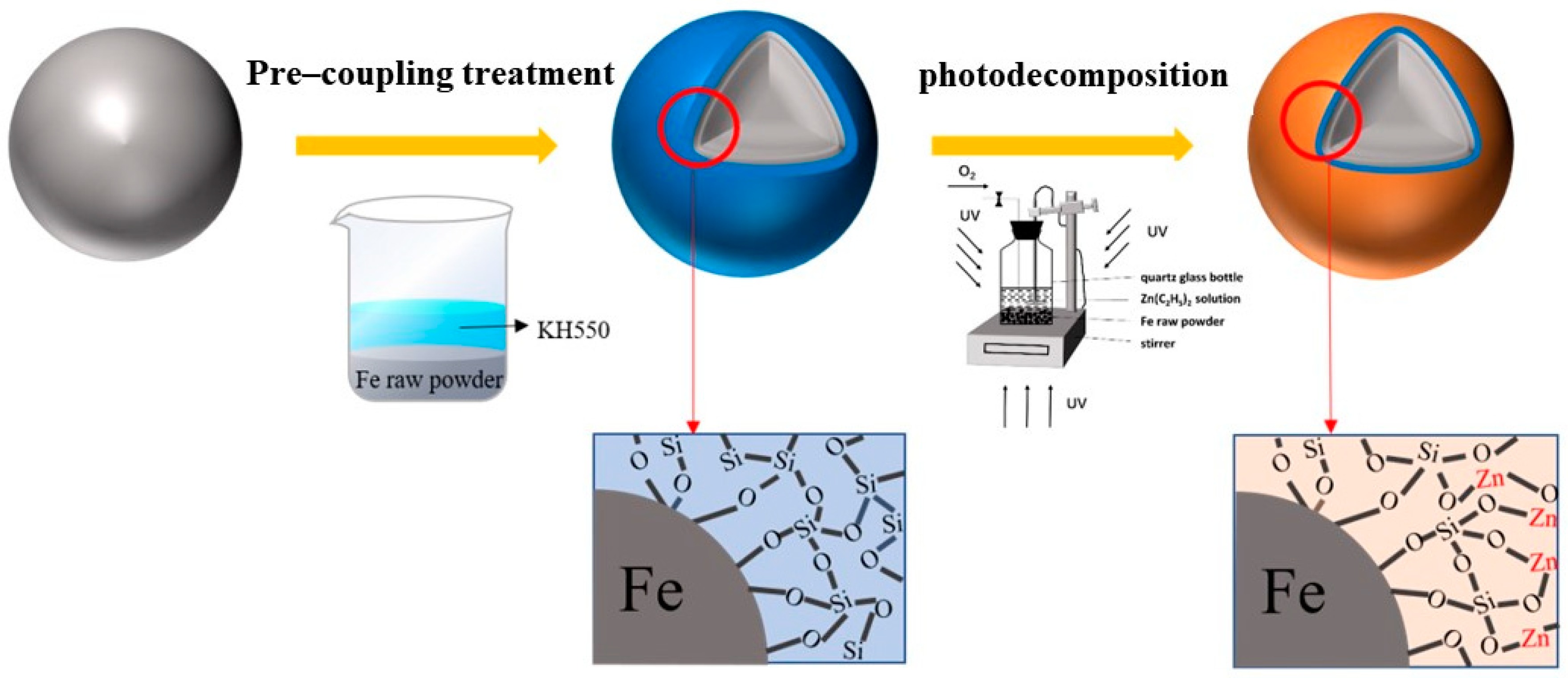
2.1. Procedure
2.2. Characterization
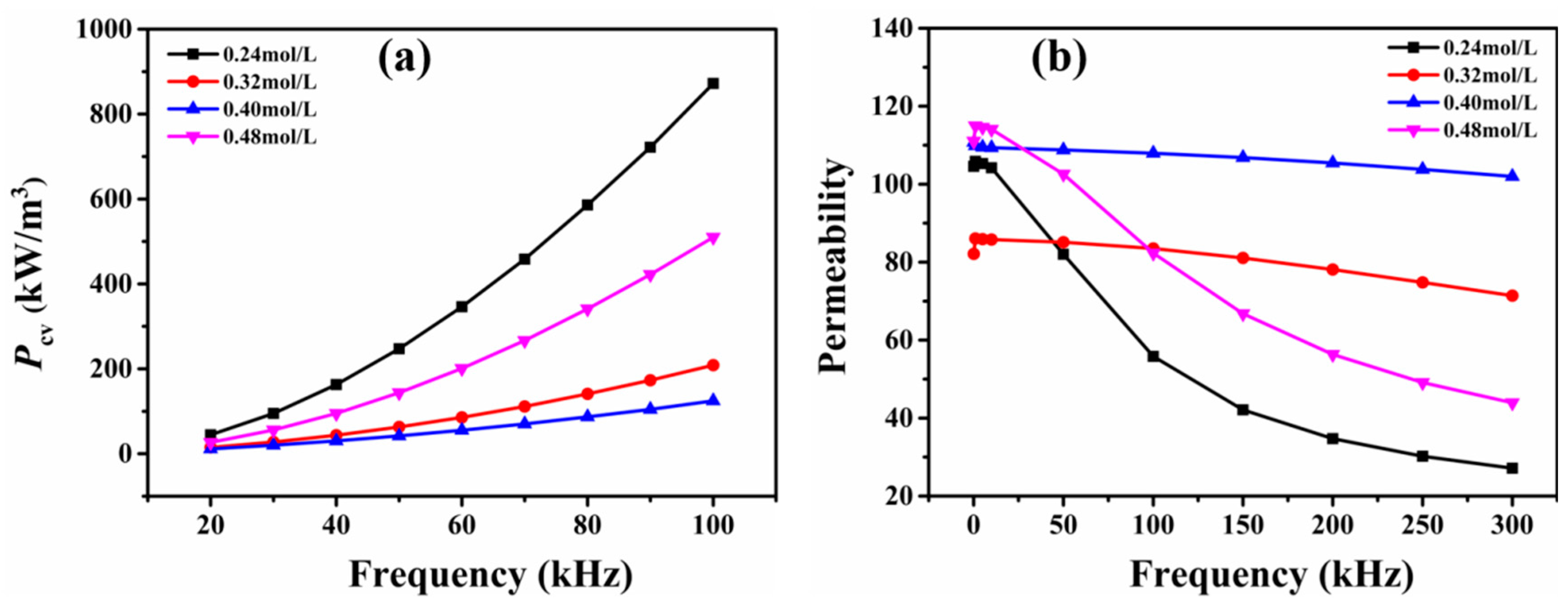
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schoppa, A.; Delarbre, P. Soft Magnetic Powder Composites and Potential Applications in Modern Electric Machines and Devices. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Wu, S.; Fan, J.L.; Liu, J.X.; Gao, H.X.; Zhang, D.H.; Sun, A.Z. Synthesis and Magnetic Properties of Soft Magnetic Composites Based on Silicone Resin-Coated Iron Powders. J. Supercond. Nov. Magn. 2018, 31, 587–595. [Google Scholar] [CrossRef]
- Azimi-Roeen, G.; Kashani-Bozorg, S.F.; Nosko, M.; Nagy, S.; Matko, I. Formation of Al/(Al13Fe4 + Al2O3) Nano-composites via Mechanical Alloying and Friction Stir Processing. J. Mater. Eng. Perform. 2018, 27, 471–482. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Vasil’eva, E.A.; Smenova, I.V.; Baskevich, A.S.; Danilenko, I.A.; Konstantinova, T.E.; Danilov, F.I. Electrodeposition of Fe and Composite Fe/ZrO2 Coatings from a Methanesulfonate Bath. Surf. Eng. Appl. Electrochem. 2015, 51, 66–75. [Google Scholar] [CrossRef]
- Taghvaei, A.H.; Ebrahimi, A.; Gheisari, K.; Janghorban, K. Analysis of the magnetic losses in iron-based soft magnetic composites with MgO insulation produced by sol-gel method. J. Magn. Magn. Mater. 2010, 322, 3748–3754. [Google Scholar] [CrossRef]
- Li, J.X.; Yu, J.; Li, W.C.; Che, S.L.; Zheng, J.W.; Qiao, L.; Ying, Y. The preparation and magnetic performance of the iron-based soft magnetic composites with the Fe@Fe3O4 powder of in situ surface oxidation. J. Magn. Magn. Mater. 2018, 454, 103–109. [Google Scholar] [CrossRef]
- Nie, W.; Yu, T.; Wang, Z.G.; Wei, X.W. High-performance core-shell-type FeSiCr@MnZn soft magnetic composites for high-frequency applications. J. Alloys Compd. 2021, 864, 158215. [Google Scholar] [CrossRef]
- Guo, R.D.; Wang, S.M.; Yu, Z.; Sun, K.; Jiang, X.N.; Wu, G.H.; Wu, C.J.; Lan, Z.W. FeSiCr@NiZn SMCs with ultra-low core losses, high resistivity for high frequency applications. J. Alloys Compd. 2020, 830, 154736. [Google Scholar] [CrossRef]
- Zheng, J.W.; Zheng, H.D.; Lei, J.; Qiao, L.; Ying, Y.; Cai, W.; Li, W.C.; Yu, J.; Liu, Y.H.; Huang, X.L.; et al. Structure and magnetic properties of Fe-based soft magnetic composites with an Li-Al-O insulation layer obtained by hydrothermal synthesis. J. Alloys Compd. 2020, 816, 152617. [Google Scholar] [CrossRef]
- Chen, J.C.; Guo, H.S.; Zhong, X.X.; Fu, J.Y.; Huang, S.J.; Xie, X.H.; Li, L.Z. Microstructural and magnetic properties of core-shell FeSiAl composites with Ni0.4Zn0.45Co0.15Fe2O4 layer by sol-gel method. J. Magn. Magn. Mater. 2021, 529, 167774. [Google Scholar] [CrossRef]
- Luo, Z.; Fan, X.; Feng, B.; Yang, Z.; Chen, D.; Jiang, S.; Wang, J.; Wu, Z.; Liu, X.; Li, G.; et al. Highly enhancing electromagnetic properties in Fe-Si/MnO-SiO2 soft magnetic composites by improving coating uniformity. Adv. Powder Technol. 2021, 32, 4846–4856. [Google Scholar] [CrossRef]
- Shi, X.Y.; Chen, X.Y.; Wan, K.; Zhang, B.W.; Duan, P.T.; Zhang, H.; Zeng, X.D.; Liu, W.; Su, H.L.; Zou, Z.Q.; et al. Enhanced magnetic and mechanical properties of gas atomized Fe-Si-Al soft magnetic composites through adhesive insulation. J. Magn. Magn. Mater. 2021, 534, 168040. [Google Scholar] [CrossRef]
- Chan, L.W.; Liu, X.H.; Heng, P.W.S. Liquid phase coating to produce controlled-release alginate microspheres. J. Microencapsul. 2005, 22, 891–900. [Google Scholar] [CrossRef]
- Lei, J.; Zheng, J.W.; Zheng, H.D.; Qiao, L.; Ying, Y.; Cai, W.; Li, W.C.; Yu, J.; Lin, M.; Che, S.L. Effects of heat treatment and lubricant on magnetic properties of iron-based soft magnetic composites with Al2O3 insulating layer by one-pot synthesis method. J. Magn. Magn. Mater. 2019, 472, 7–13. [Google Scholar] [CrossRef]
- Zhou, M.; Peng, X.L. Fundamentals of Magnetics and Magnetic Materials; Zhejiang University Press: Hangzhou, China, 2019; pp. 175–176. [Google Scholar]
- Li, W.C.; Li, W.; Ying, Y.; Yu, J.; Zheng, J.W.; Qiao, L.; Li, J.; Zhang, L.; Fan, L.; Wakiya, N.; et al. Magnetic and Mechanical Properties of Iron-Based Soft Magnetic Composites Coated with Silane Synergized by Bi2O3. J. Electron. Mater. 2021, 50, 2425–2435. [Google Scholar] [CrossRef]
- Shi, W.X.; Yang, J.; Wang, T.J.; Jin, Y. Surface organic modification of magnetic iron oxide black particles. Acta Phys.-Chim. Sin. 2001, 17, 507–510. [Google Scholar] [CrossRef]
- Berbotti, G. General properties of power losses in Soft ferromagnetic Materials. IEEE Trans. Magn. 1988, 24, 621. [Google Scholar] [CrossRef]
- Zheng, J.W.; Zheng, D.N.; Qiao, L.; Ying, Y.; Tang, Y.P.; Cai, W.; Li, W.C.; Yu, J.; Li, J.; Che, S.L. High permeability and low core loss Fe-based soft magnetic composites with Co-Ba composite ferrite insulation layer obtained by sol-gel method. J. Alloys Compd. 2022, 893, 162107. [Google Scholar] [CrossRef]
- Li, W.C.; Wang, Z.J.; Ying, Y.; Yu, J.; Zheng, J.W.; Qiao, L.; Che, S.L. In-situ formation of Fe3O4 and ZrO2 coated Fe-based soft magnetic composites by hydrothermal method. Ceram. Int. 2019, 45, 3864–3870. [Google Scholar] [CrossRef]
- Li, W.C.; Pu, Y.Y.; Ying, Y.; Kang, Y.; Yu, J.; Zheng, J.W.; Qiao, L.; Li, J.; Che, S.L. Magnetic properties and related mechanisms of iron-based soft magnetic composites with high thermal stability in situ composite-ferrite coating. J. Alloys Compd. 2020, 829, 154533. [Google Scholar] [CrossRef]
- Guziewicz, E.; Godlewski, M.; Wachnicki, L.; Krajewski, T.A.; Luka, G.; Gieraltowska, S.; Jakiela, R.; Stonert, A.; Lisowski, W.; Krawczyk, M.; et al. ALD grown zinc oxide with controllable electrical properties. Semicond. Sci. Technol. 2012, 27, 074011. [Google Scholar] [CrossRef] [Green Version]

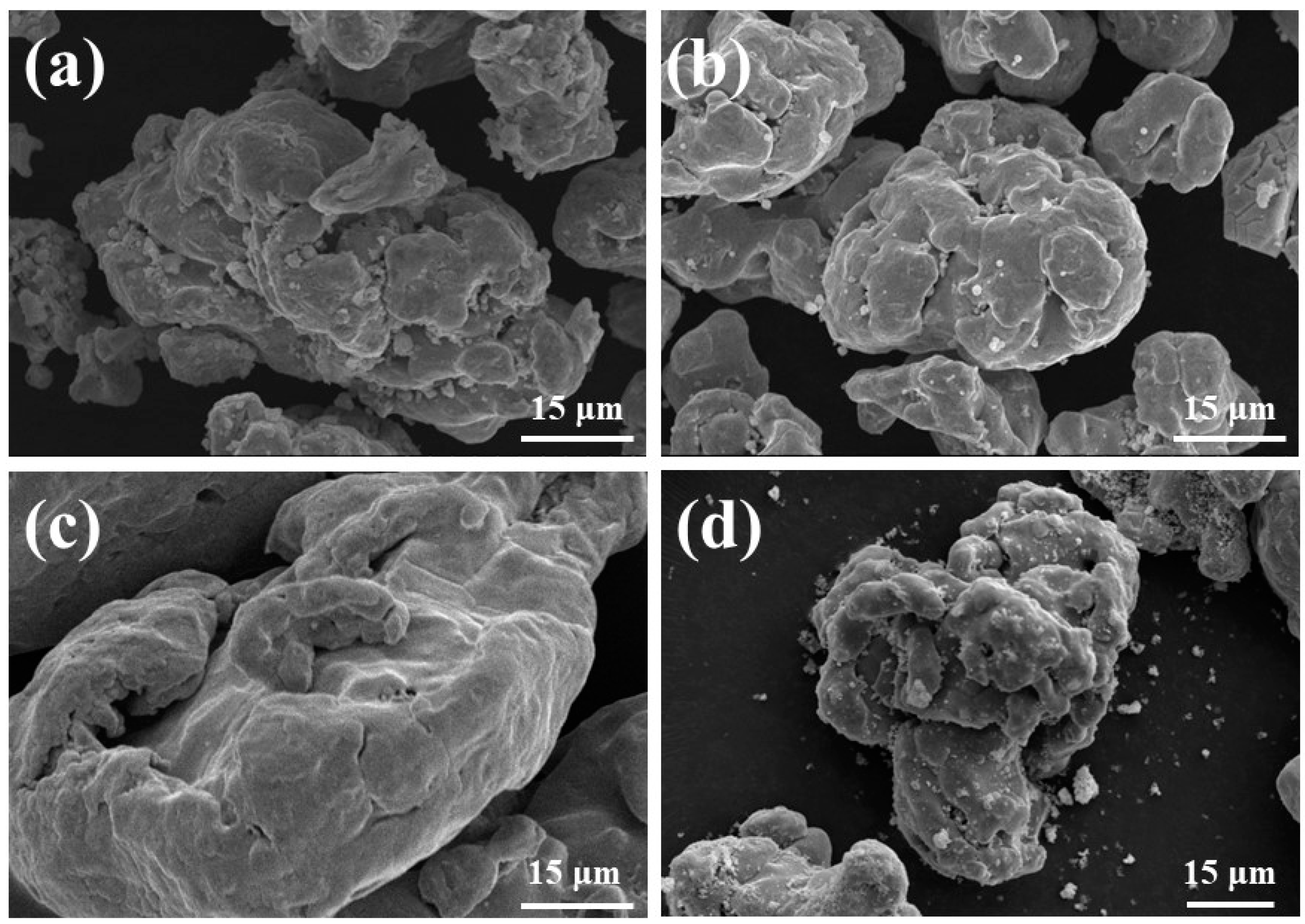
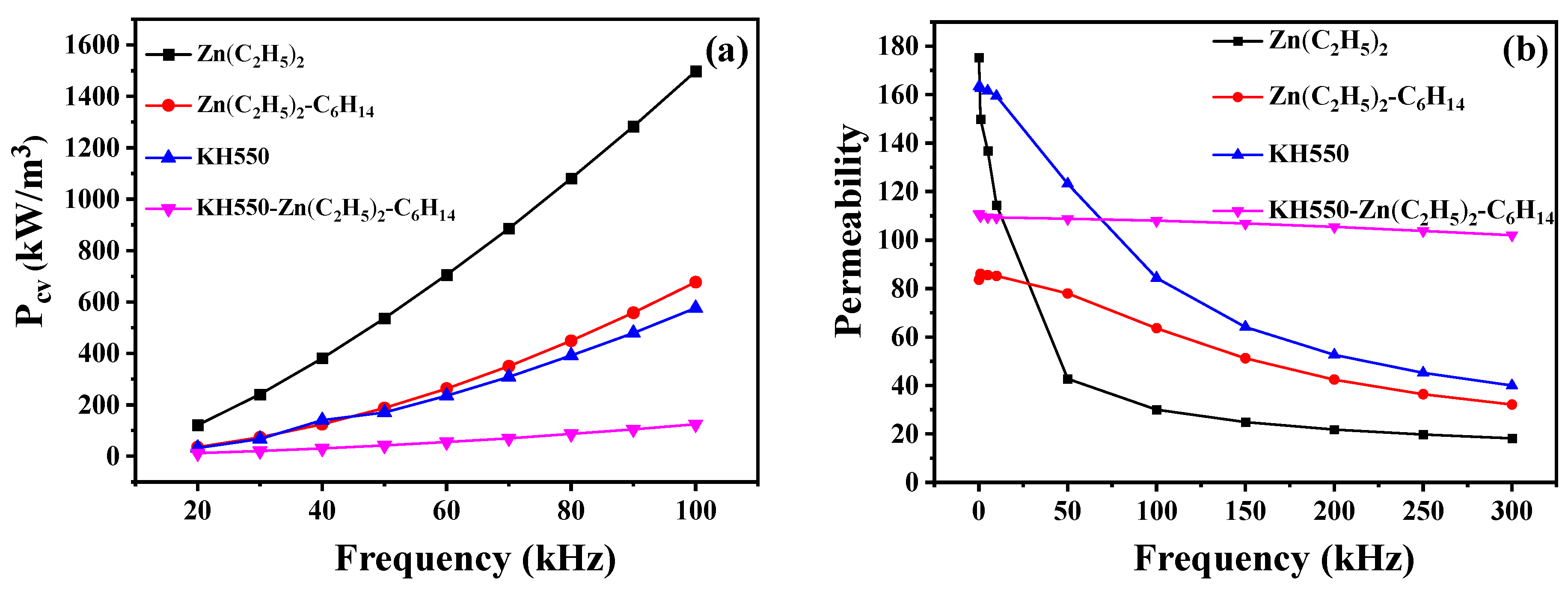

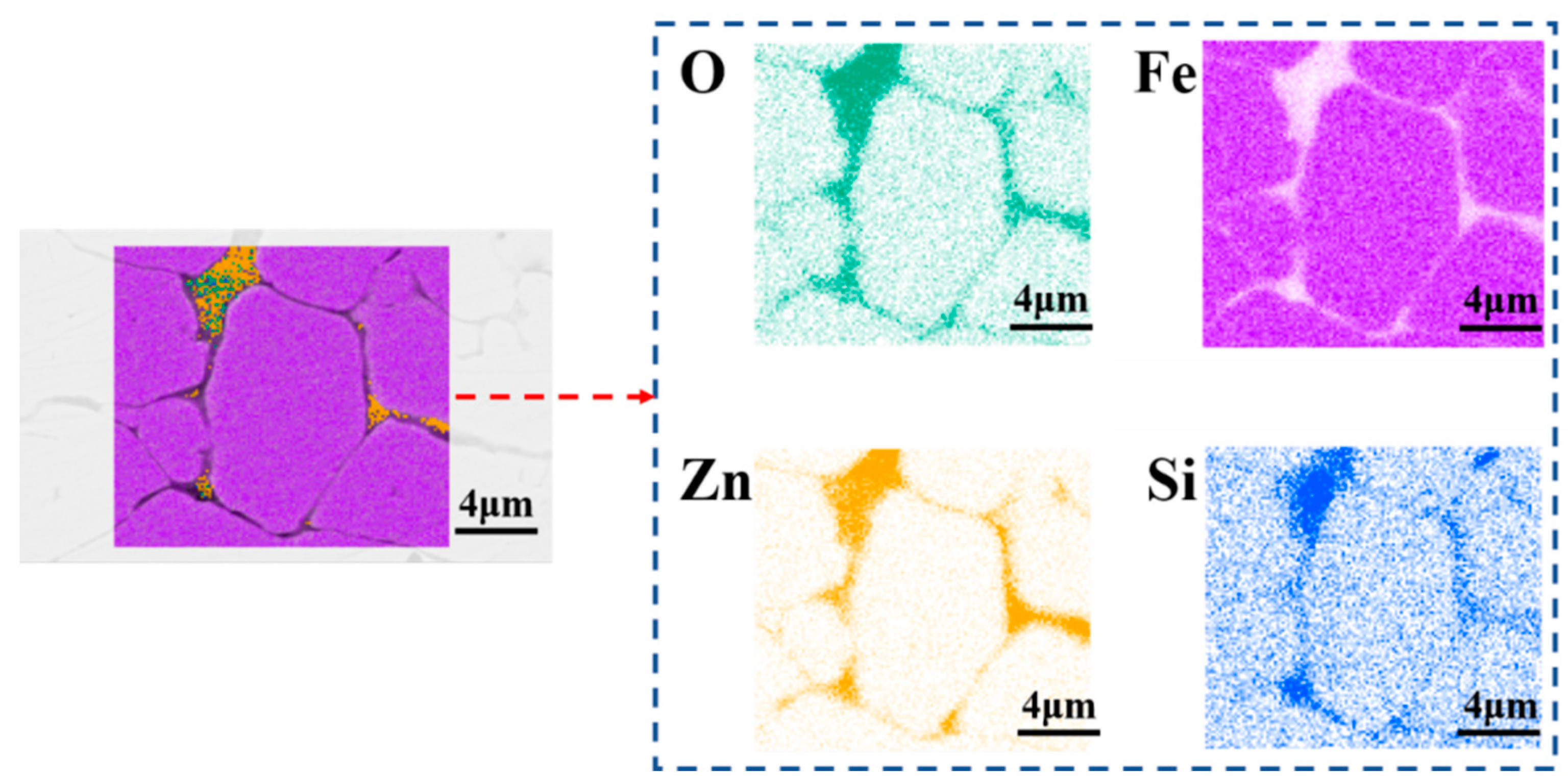

| Element (at%) | Zn(C2H5)2 | Zn(C2H5)2 + C6H14 | KH550 | KH550 and (Zn(C2H5)2 + C6H14) |
|---|---|---|---|---|
| Fe | 88.27 | 98.26 | 99.65 | 97.57 |
| Zn | 11.73 | 1.74 | 0 | 2.17 |
| Si | 0 | 0 | 0.35 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zheng, J.; Zheng, D.; Qiao, L.; Ying, Y.; Tang, Y.; Cai, W.; Li, W.; Yu, J.; Li, J.; et al. Low Core Losses of Fe-Based Soft Magnetic Composites with an Zn-O-Si Insulating Layer Obtained by Coupling Synergistic Photodecomposition. Materials 2022, 15, 8660. https://doi.org/10.3390/ma15238660
Wang S, Zheng J, Zheng D, Qiao L, Ying Y, Tang Y, Cai W, Li W, Yu J, Li J, et al. Low Core Losses of Fe-Based Soft Magnetic Composites with an Zn-O-Si Insulating Layer Obtained by Coupling Synergistic Photodecomposition. Materials. 2022; 15(23):8660. https://doi.org/10.3390/ma15238660
Chicago/Turabian StyleWang, Siyuan, Jingwu Zheng, Danni Zheng, Liang Qiao, Yao Ying, Yiping Tang, Wei Cai, Wangchang Li, Jing Yu, Juan Li, and et al. 2022. "Low Core Losses of Fe-Based Soft Magnetic Composites with an Zn-O-Si Insulating Layer Obtained by Coupling Synergistic Photodecomposition" Materials 15, no. 23: 8660. https://doi.org/10.3390/ma15238660







