New Pressure-Sensitive Acrylic Adhesives for Low Energy Substrates Prepared via UV-Induced Telomerization with a Fluorine-Based Telogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- -
- Taxogens/monomers: n-butyl acrylate (BA), butyl methacrylate (BMA), lauryl methacrylate (LMA) (BASF, Ludwigshafen, Germany);
- -
- Telogen: trifluoroethanol (TFEtOH, POCh, Gliwice, Poland);
- -
- Radical UV-photoinitiator: bis(2,4,6-trimethylbenzoyl)-phenylphosphineoxide (APO; Omnirad 819, IGM Resins, Waalwijk, The Netherlands).
- -
- Crosslinking monomer: aliphatic urethane acrylate (L9033; Laromer 9033, Ludwigshafen, Germany);
- -
- Radical UV-photoinitiator: hydroxycyclohexylphenyl ketone (HAP; Omnirad 184, IGM Resins, Waalwijk, The Netherlands).
2.2. Methods
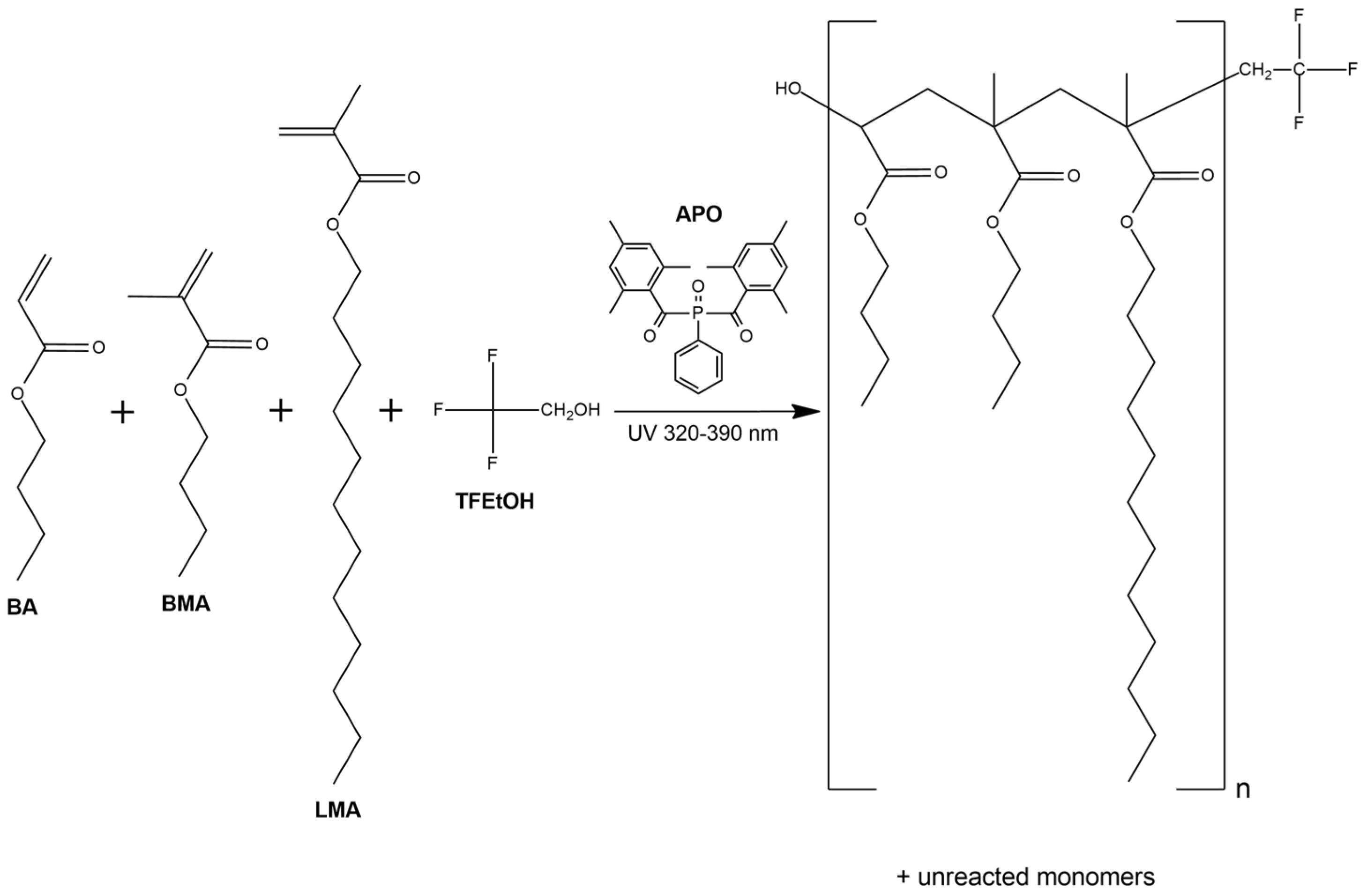
2.2.1. Fluorotelomers Syrups Preparation
2.2.2. Pressure-Sensitive Adhesives Preparation
2.2.3. Characterization of the Fluorotelomers Syrups
2.2.4. Characterization of UV-Crosslinked PSA
3. Results and Discussion
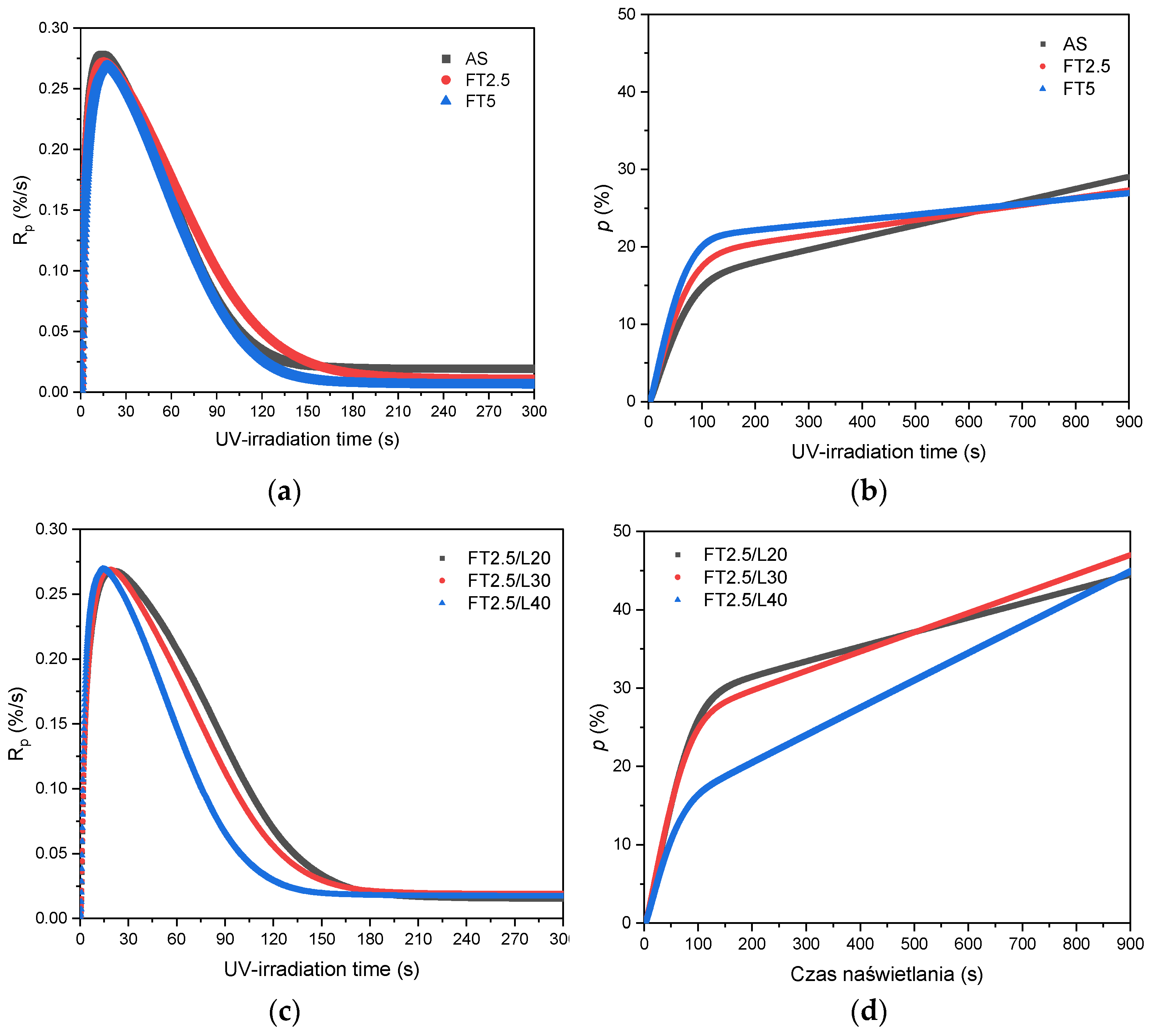
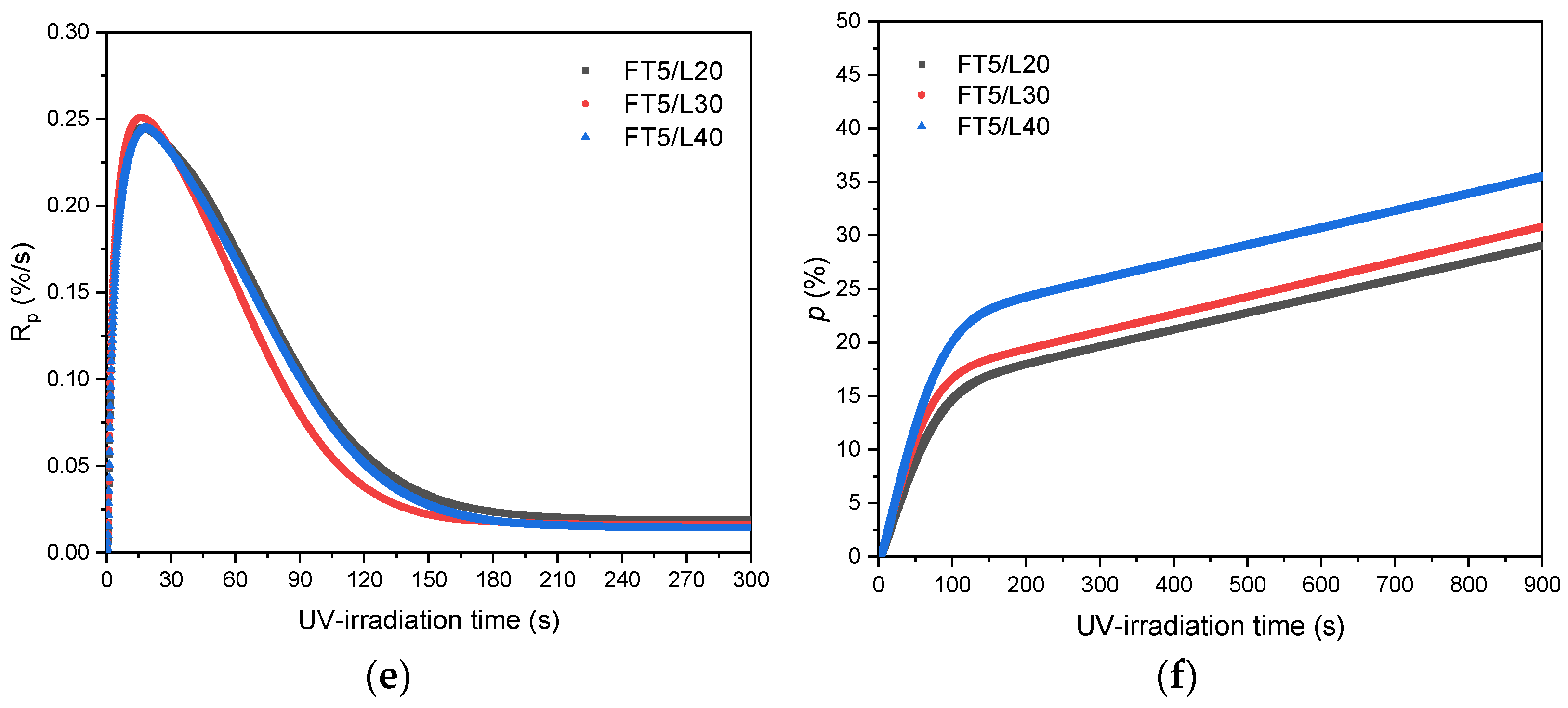
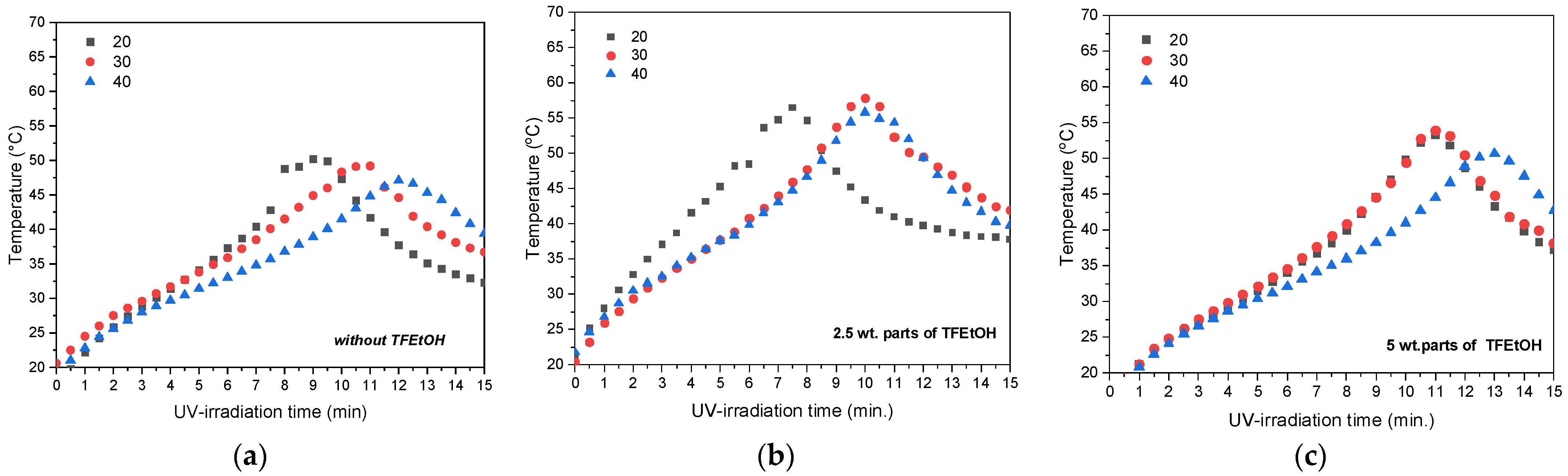
3.1. Kinetics of the UV-Phototelomerization Process
3.2. The Physicochemical Properties of the Fluorotelomers Syrups
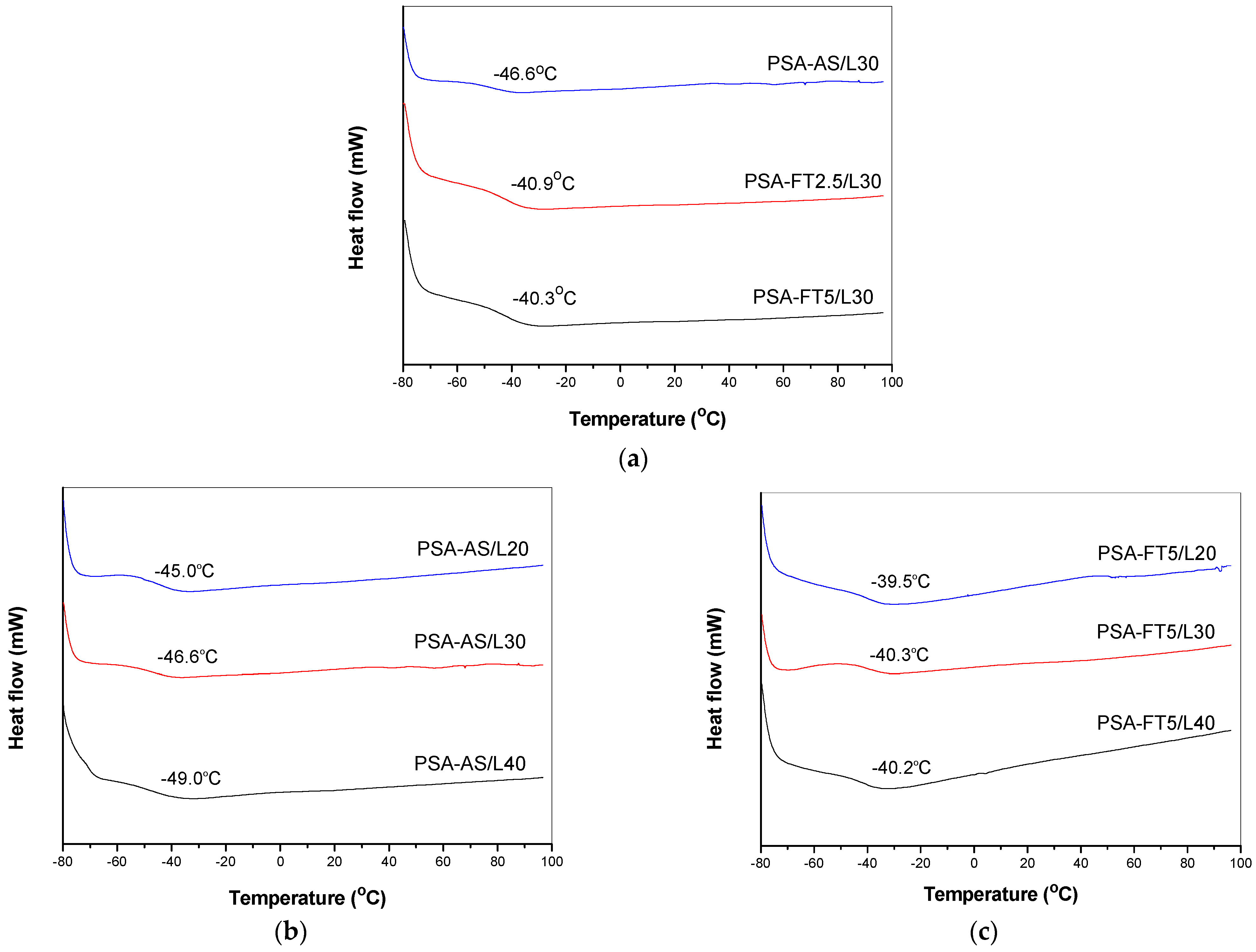
3.3. Properties of UV-Crosslinked PSA Based on Fluorotelomers
4. Conclusions
- -
- The addition of the telogen lowers the maximum reaction rate of the photopolymerization process only slightly; however, it significantly reduces molecular weights and polydispersity of the telomers. The kinetics of the UV-phototelomerization process is also influenced by the qualitative/quantitative composition of the monomer mixture.
- -
- A greater LMA concentration reduces the maximum temperature peak value during the phototelomerization process in a glass reactor with continuous mixing of the reactants, and slightly lowers the molecular weights of the fluorotelomers.
- -
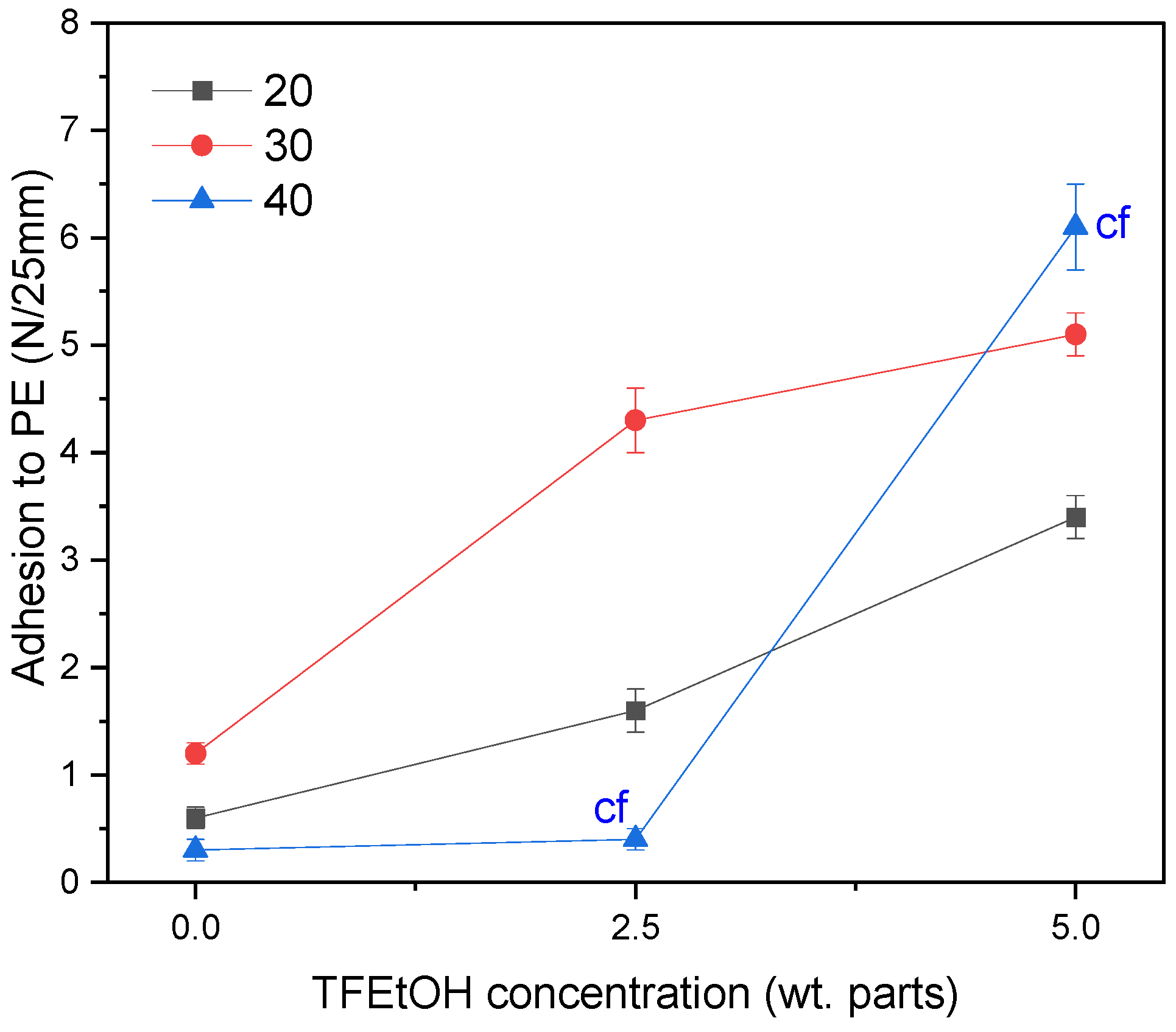
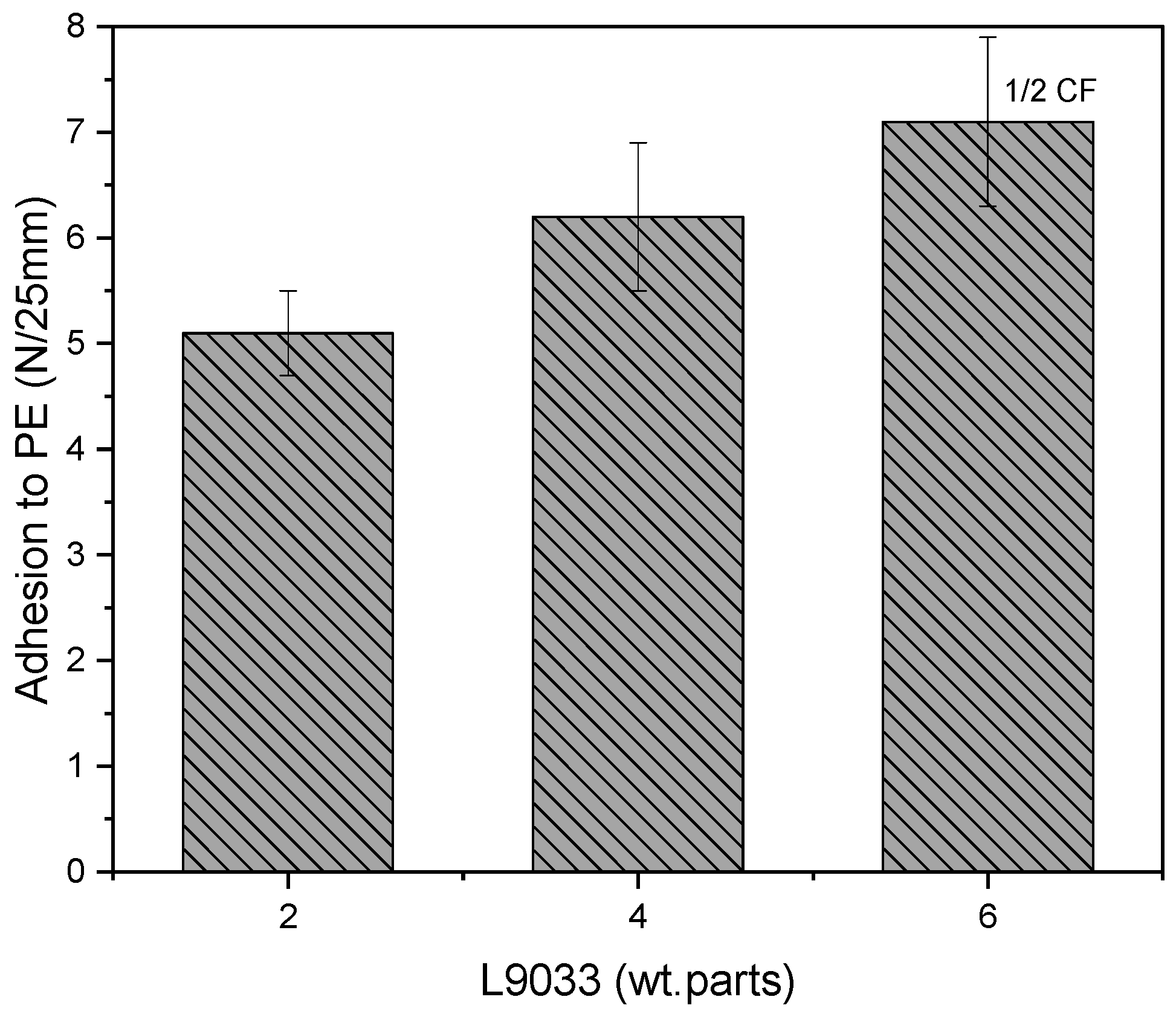
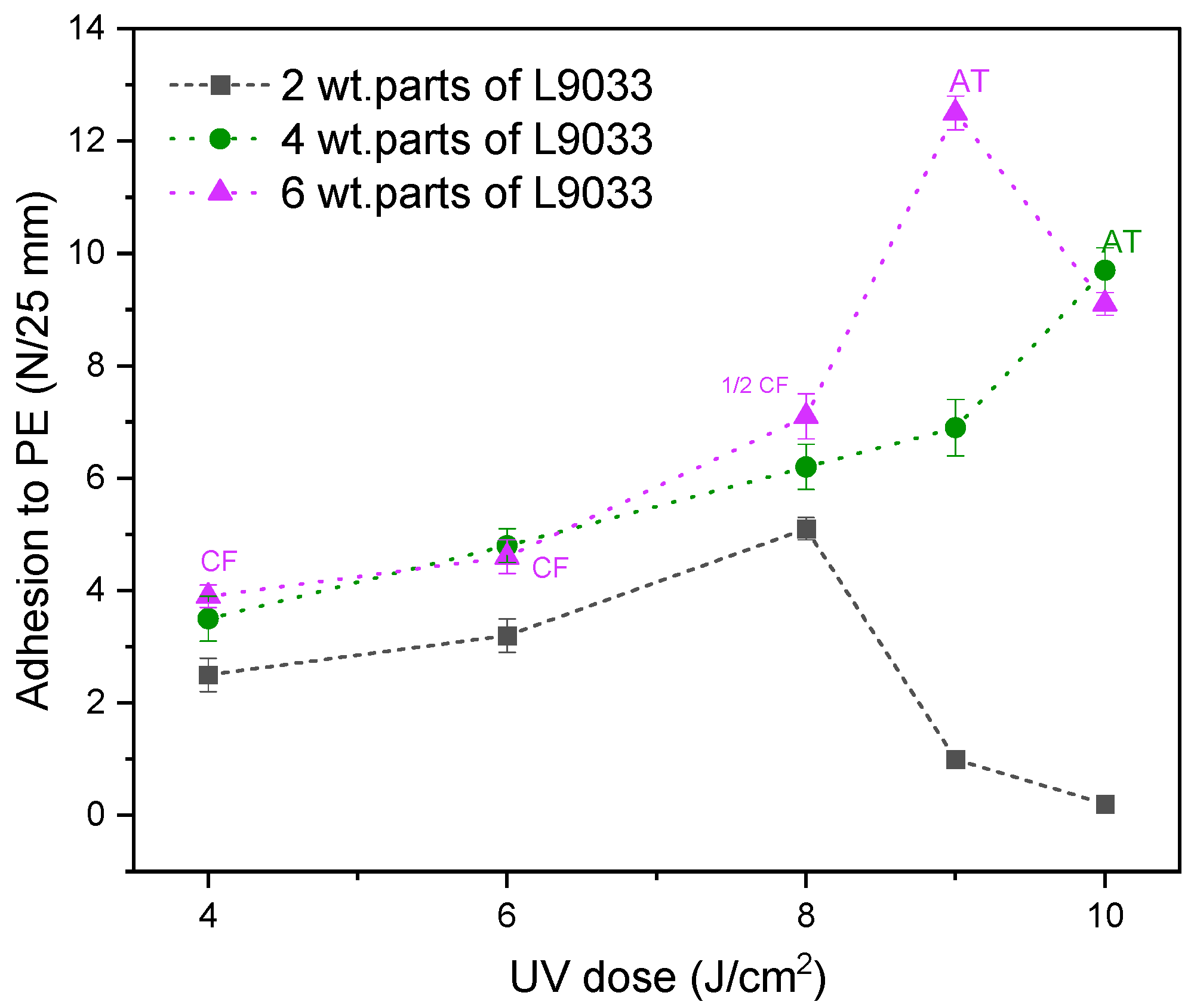
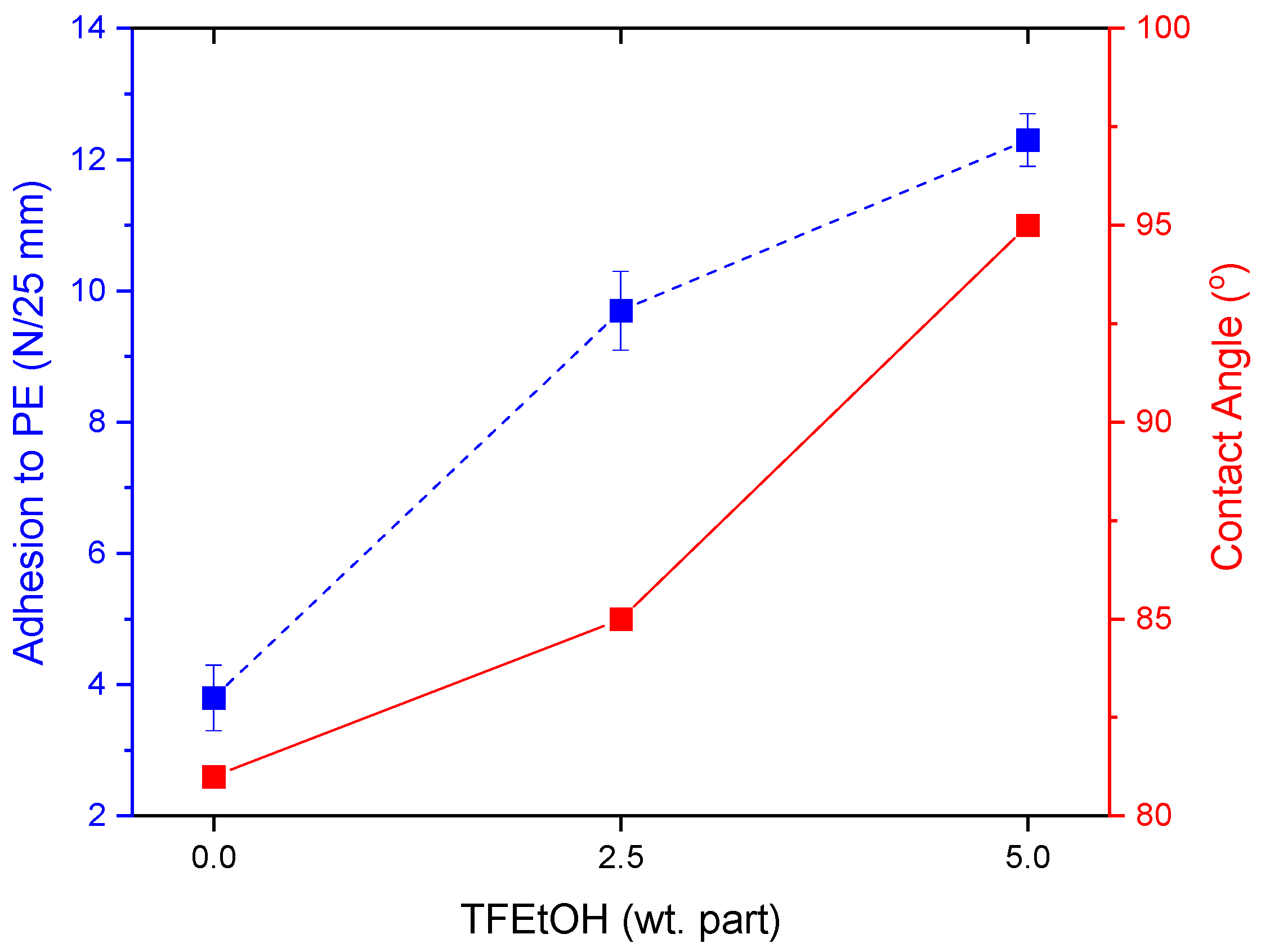
- The adhesion of the PSA tapes to the low energy surface (PE) can be significantly increased by a higher dose of the aliphatic urethane acrylate and a higher UV dose.
- -
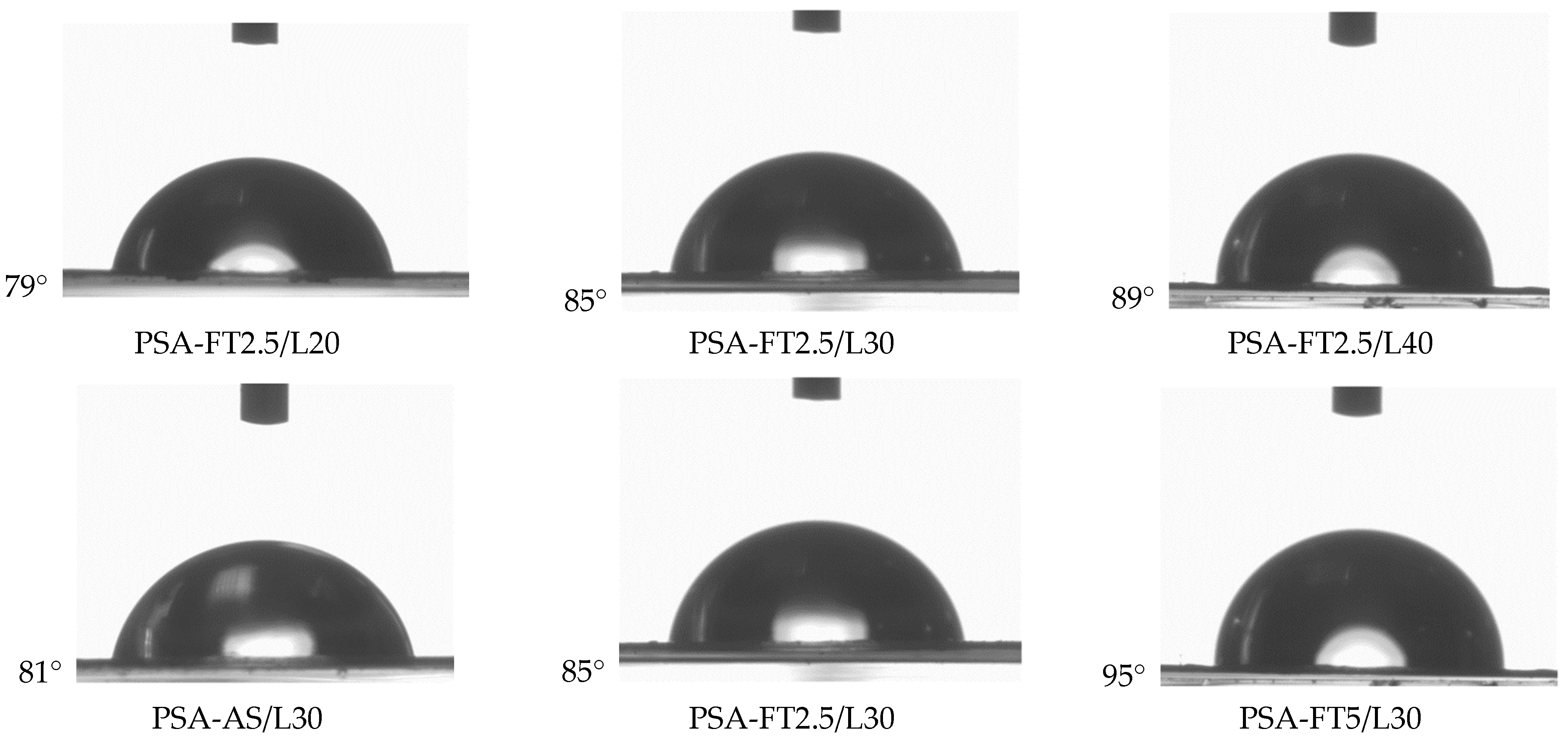
- The excellent adhesion of PSA to PE (12.3 N/25 mm), as well as a high contact angle (95° for a water/PSA system), were noted for the sample based on a telomer containing 5 wt. parts of the telogen (per 100 wt. parts of the monomers mixture), the intermediate LMA amount (30 wt.% of the monomer mixture), and 6 wt. parts of the aliphatic urethane acrylate (per 100 wt. parts of a telomer syrup).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lutz, H.; Weitzel, H.-P.; Huster, W. Polymer Science: A Comprehensive Reference. In Aqueous Emulsion Polymers; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 479–518. [Google Scholar] [CrossRef]
- Richard, P. Pressure-Sensitive Adhesives, Elastomers, and Coatings from Plant Oil. In Plastics Design Library, Handbook of Biopolymers and Biodegradable Plastics; Ebnesajjad, S., Ed.; William Andrew Publishing: Norwich, NY, USA, 2013; pp. 265–294. [Google Scholar] [CrossRef]
- Pearmain, A.J.; Haddad, A. Insulation. In Electrical Engineer’s Reference Book, 16th ed.; Laughton, M.A., Warne, D.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1–36. [Google Scholar] [CrossRef]
- Marques, A.C.; Mocanu, A.; Tomić, N.Z.; Balos, S.; Stammen, E.; Lundevall, A.; Abrahami, S.T.; Günther, R.; de Kok, J.M.M.; Teixeira de Freitas, S. Review on Adhesives and Surface Treatments for Structural Applications: Recent Developments on Sustainability and Implementation for Metal and Composite Substrates. Materials 2020, 13, 5590. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Seo, H.S.; Lee, J.H.; Shin, S. Adhesion improvement of the acrylic pressure-sensitive adhesive to low-surface-energy substrates using silicone urethane dimethacrylates. Eur. Polym. J. 2020, 137, 109949. [Google Scholar] [CrossRef]
- Elliot, A.N.A. Automotive Applications of Polymers II; Rapra Technology Limited: Shawbury, UK, 1992; pp. 3–19. [Google Scholar]
- Polášková, K.; Klíma, M.; Jeníková, Z.; Blahová, L.; Zajíčková, L. Effect of Low Molecular Weight Oxidized Materials and Nitrogen Groups on Adhesive Joints of Polypropylene Treated by a Cold Atmospheric Plasma Jet. Polymers 2021, 13, 4396. [Google Scholar] [CrossRef] [PubMed]
- Peloquin, R.; Everaerts, A.; Wilson, K.; Galic, S. Pressure Sensitive Adhesives for Use on Low Energy Surfaces. U.S. Patent 5874143, 23 February 1999. [Google Scholar]
- Caspari, D.; Kura, F.; Schutte, M.; Seeger, K. Acrylate Copolymers and Pressure-Sensitive Adhesives Obtainable Therefrom for Bonding Low-Energy Surfaces. U.S. Patent 2003235686, 25 December 2003. [Google Scholar]
- Traser, S.; Forster, J. Pressure-Sensitive Adhesives for Low Surface Energy. Substrates. Patent Application EP 2652060, 10 October 2013. [Google Scholar]
- Agirre, A.; Nase, J.; Degrandi, R.; Creton, C.; Asua, J.M. Improving adhesion of acrylic waterborne PSAs to low surface energy materials: Introduction of stearyl acrylate. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5030–5039. [Google Scholar] [CrossRef]
- Bae, K.Y.; Lim, D.H.; Park, J.W.; Kim, H.J.; Jeong, H.M.; Takemura, A. Adhesion performance and surface characteristics of low surface energy psas fluorinated by UV polymerization. Polym. Eng. Sci. 2013, 53, 1968–1978. [Google Scholar] [CrossRef]
- Wang, Y.; Long, J.; Bai, Y.; Zhang, C.; Cheng, B.; Shao, L.; Qi, S. Preparation and characterization of fluorinated acrylic pressure sensitive adhesives for low surface energy substrates. J. Fluor. Chem. 2015, 180, 103–109. [Google Scholar] [CrossRef]
- Fang, C.; Huang, X.; Ge, T.; Li, Y.; Cao, Y.; Zhu, X.; Dong, X. Effect of dodecafluoroheptyl methacrylate (DFMA) on the comprehensive properties of acrylate emulsion pressure sensitive adhesives. Int. J. Adhes. Adhes. 2020, 101, 102634. [Google Scholar] [CrossRef]
- Teng, H. Overview of the Development of the Fluoropolymer Industry. Appl. Sci. 2012, 2, 496–512. [Google Scholar] [CrossRef]
- Drobny, J.G. Technology of Fluoropolymers, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Ebnesajjad, S. Fluoroplastics; Plastic Design Library: New York, NY, USA, 2002. [Google Scholar]
- Ameduri, B.; Boutevin, B.; Guida-Pietrasanta, F.; Rousseau, A. Fluorinated oligomers, telomers and (co)polymers: Synthesis and applications. J. Fluor. Chem. 2001, 107, 397–409. [Google Scholar] [CrossRef]
- Ameduri, B.; Boutevin, B. Well-Architectured Fluoropolymers; Elsevier: Oxford, UK, 2004. [Google Scholar]
- Kabatc, J. The influence of a radical structure on the kinetics of photopolymerization. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1575–1589. [Google Scholar] [CrossRef]
- Gziut, K.; Kowalczyk, A.; Schmidt, B. Free-Radical Bulk-Photopolymerization Process as a Method of Obtaining Thermally Curable Structural Self-Adhesive Tapes and Effect of Used Type I Photoinitiators. Polymers 2020, 12, 2191. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Spanswick, J. Controlled/living radical polymerization. Mater. Today 2005, 8, 26–33. [Google Scholar] [CrossRef]
- Wakselman, C.; Lantz, A. Perfluoralkyl bromides and iodides. In Organofluorine Chemistry: Principles and Applications; Plenum Press: New York, NY, USA, 1994; Volume 15, pp. 177–194. [Google Scholar]
- Salamone, J.C. Polymeric Materials Encyclopedia; CRC Press: Boca Raton, FL, USA, 1996; Volume 4. [Google Scholar]
- Park, K.H.; Lee, D.Y.; Yoon, S.H.; Kim, S.H.; Han, M.S.; Jeon, S.; Kim, Y.; Lim, Y.K.; Hwang, D.-H.; Jung, S.-H.; et al. Adhesion Improvement of Solvent-Free Pressure-Sensitive Adhesives by Semi-IPN Using Polyurethanes and Acrylic Polymers. Polymers 2022, 14, 3963. [Google Scholar] [CrossRef] [PubMed]
- Benedek, I. Pressure-Sensitive Adhesives and Applications; Marcel Deker Inc.: New York, NY, USA, 2004; pp. 92–102. [Google Scholar]


| Fluorotelomers or Acrylic Copolymers Components (wt. parts) | |||||
|---|---|---|---|---|---|
| BA | BMA | LMA | TFEtOH * | APO * | Syrup Acronym |
| 65 | 15 | 20 | 0 | 0.5 | AS/L20 |
| 55 | 30 | 0 | 0.5 | AS/L30 | |
| 45 | 40 | 0 | 0.6 | AS/L40 | |
| 65 | 20 | 2.5 | 0.45 | FT2.5/L20 | |
| 55 | 30 | 2.5 | 0.5 | FT2.5/L30 | |
| 45 | 40 | 2.5 | 0.6 | FT2.5/L40 | |
| 65 | 20 | 5.0 | 0.45 | FT5/L20 | |
| 55 | 30 | 5.0 | 0.6 | FT5/L30 | |
| 45 | 40 | 5.0 | 0.65 | FT5/L40 | |
| Syrup Acronym | η (Pa·s) | SC (wt.%) | Mw (g/mol) | Mn (g/mol) | Mw/Mn |
|---|---|---|---|---|---|
| AS/L20 | >>60 | no data | 120,000 | 24,000 | 5.9 |
| AS/L30 | 6.5 | 74 | 107,000 | 22,000 | 4.9 |
| AS/L40 | 6.2 | 73 | 92,000 | 19,000 | 4.8 |
| FT2.5/L20 | 6.6 | 64 | 29,000 | 1500 | 1.9 |
| FT2.5/L30 | 11.4 | 68 | 26,000 | 1400 | 1.9 |
| FT2.5/L40 | 7.0 | 75 | 23,000 | 1200 | 1.9 |
| FT5/L20 | 8.2 | 76 | 24,000 | 1300 | 1.8 |
| FT5/L30 | 5.8 | 76 | 22,500 | 1300 | 1.7 |
| FT5/L40 | 6.5 | 74 | 21,000 | 1200 | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, A.; Kraśkiewicz, A.; Kowalczyk, K. New Pressure-Sensitive Acrylic Adhesives for Low Energy Substrates Prepared via UV-Induced Telomerization with a Fluorine-Based Telogen. Materials 2022, 15, 8667. https://doi.org/10.3390/ma15238667
Kowalczyk A, Kraśkiewicz A, Kowalczyk K. New Pressure-Sensitive Acrylic Adhesives for Low Energy Substrates Prepared via UV-Induced Telomerization with a Fluorine-Based Telogen. Materials. 2022; 15(23):8667. https://doi.org/10.3390/ma15238667
Chicago/Turabian StyleKowalczyk, Agnieszka, Agata Kraśkiewicz, and Krzysztof Kowalczyk. 2022. "New Pressure-Sensitive Acrylic Adhesives for Low Energy Substrates Prepared via UV-Induced Telomerization with a Fluorine-Based Telogen" Materials 15, no. 23: 8667. https://doi.org/10.3390/ma15238667
APA StyleKowalczyk, A., Kraśkiewicz, A., & Kowalczyk, K. (2022). New Pressure-Sensitive Acrylic Adhesives for Low Energy Substrates Prepared via UV-Induced Telomerization with a Fluorine-Based Telogen. Materials, 15(23), 8667. https://doi.org/10.3390/ma15238667









