Methane and Carbon Dioxide Hydrate Formation in the Presence of Metal-Based Fluid
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Apparatus
2.3. Procedure
2.3.1. Hydrate Equilibrium Temperature Measurement
2.3.2. Evaluation of Kinetics of Hydrate Formation
2.3.3. Rate Constant Modeling
3. Results and Discussion
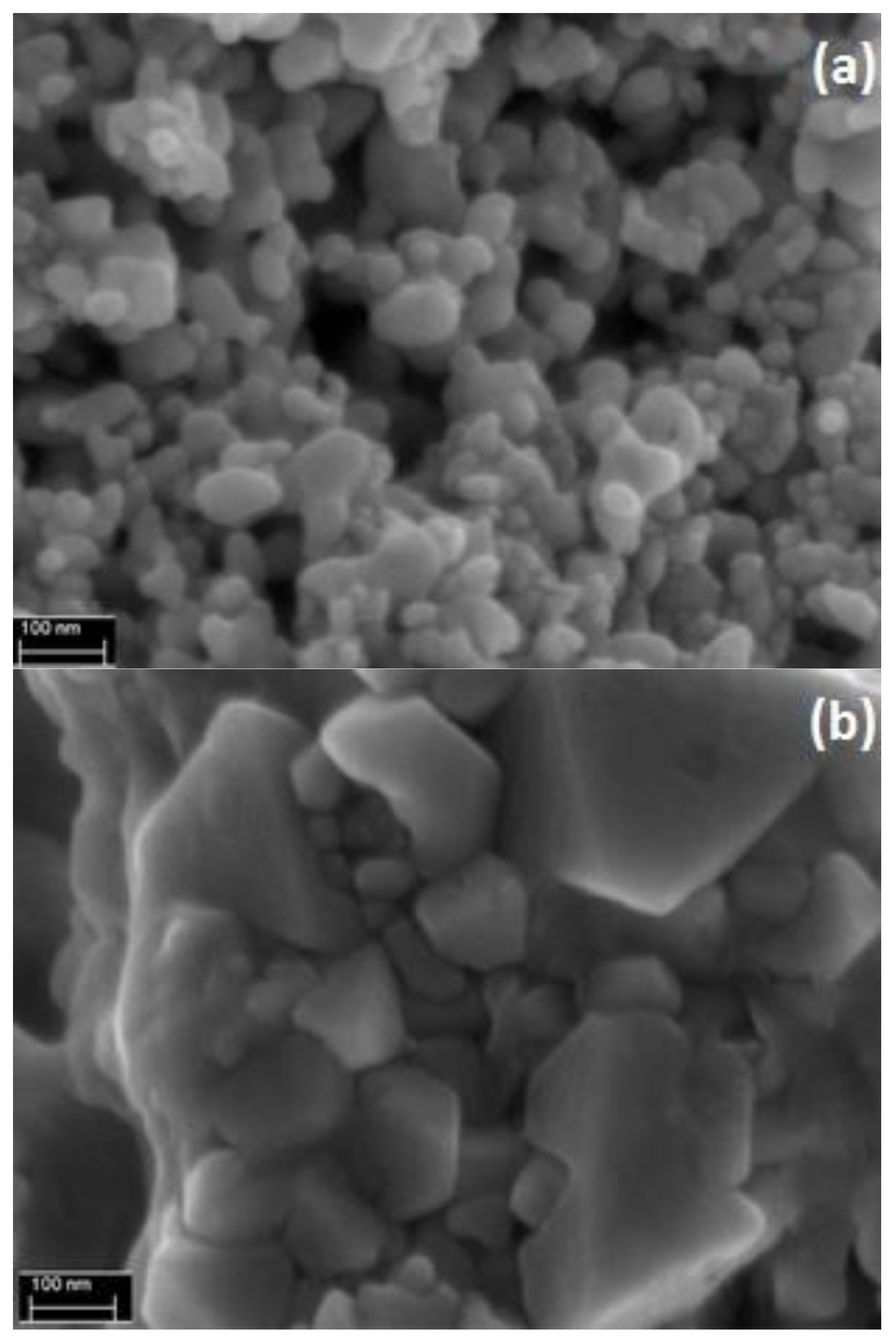
3.1. Characterization of the Silver and Copper Particles
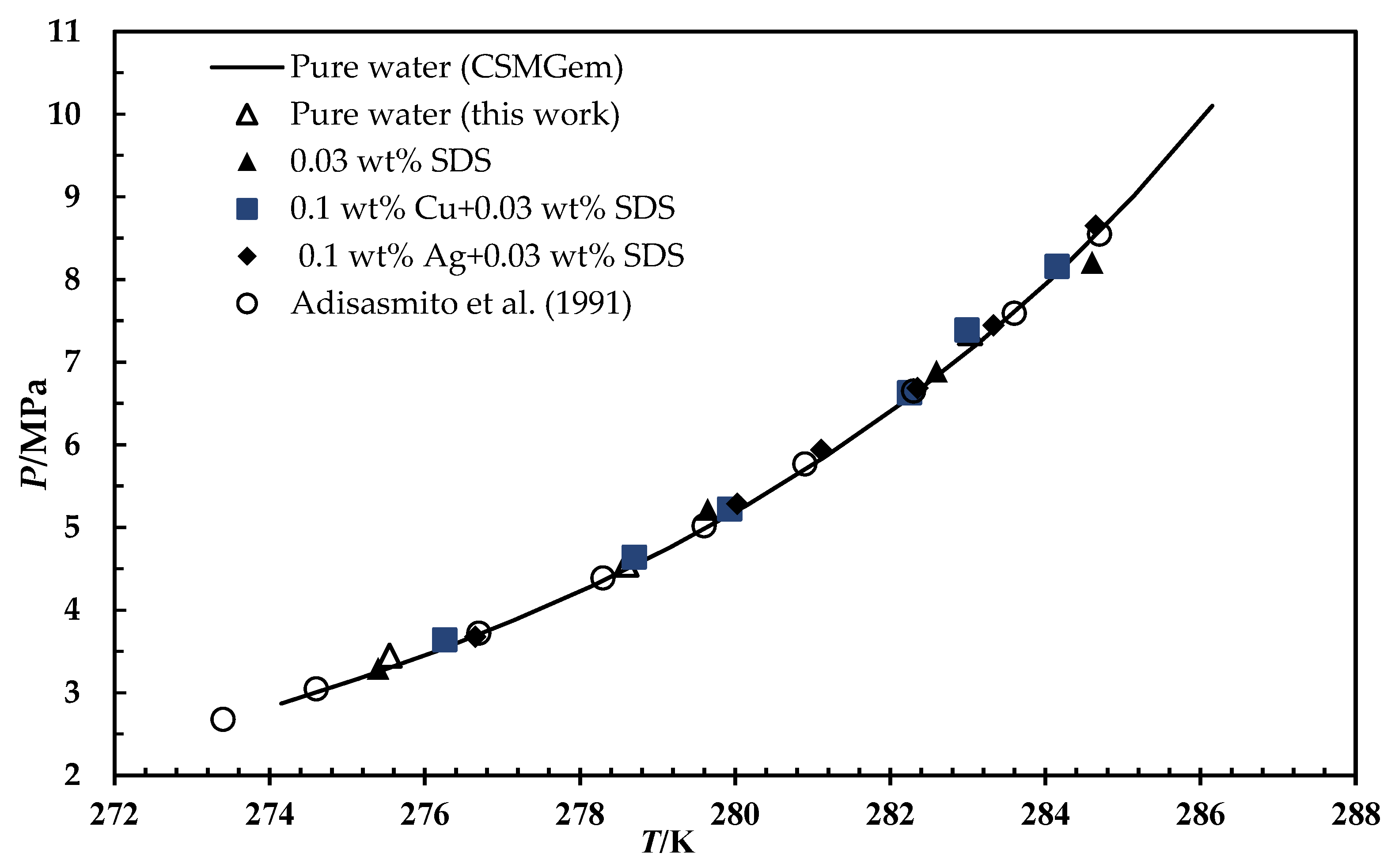
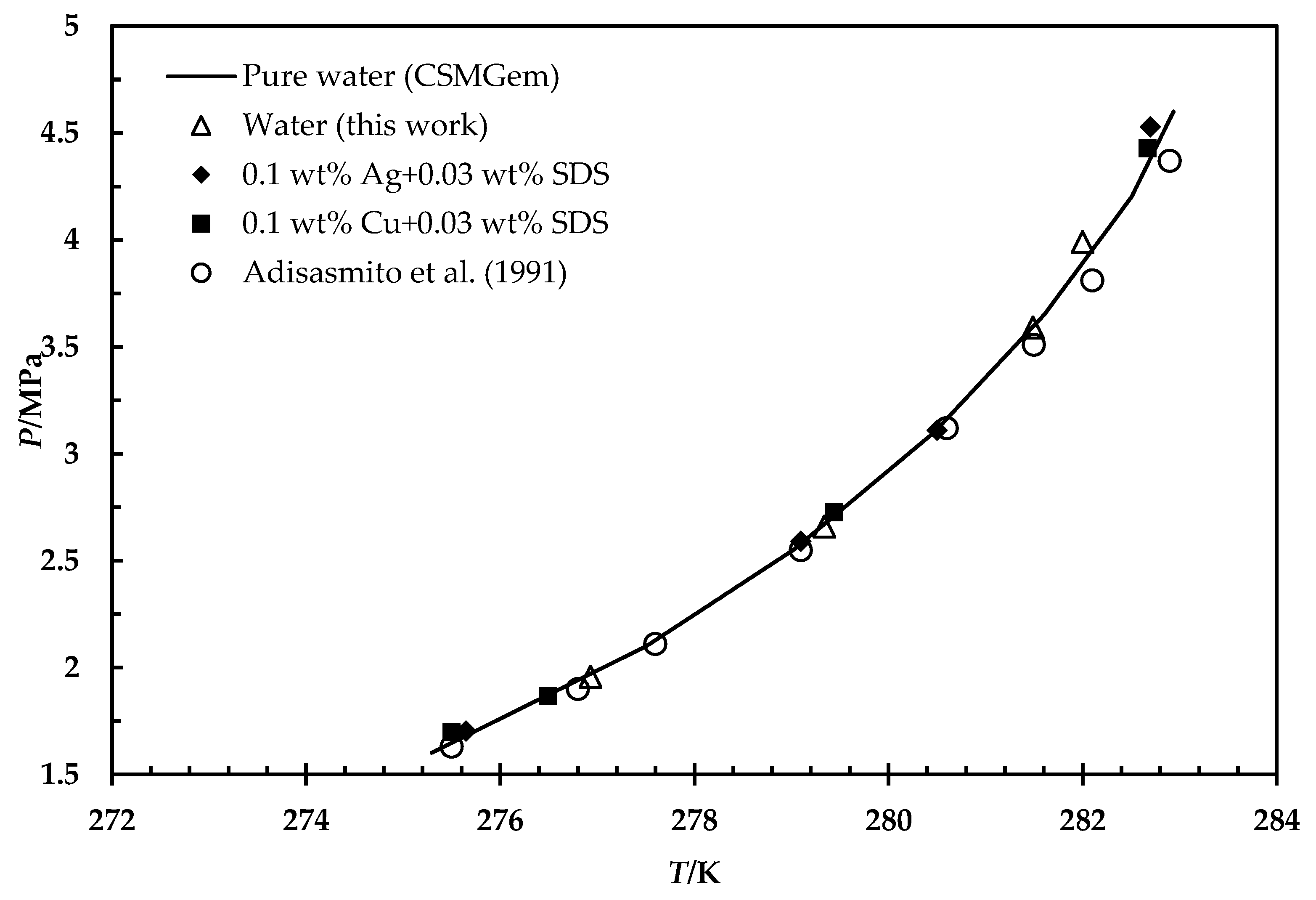
3.2. Hydrate Phase Equilibrium in the Presence of Silver and Copper Particles
3.3. Induction Time Measurement
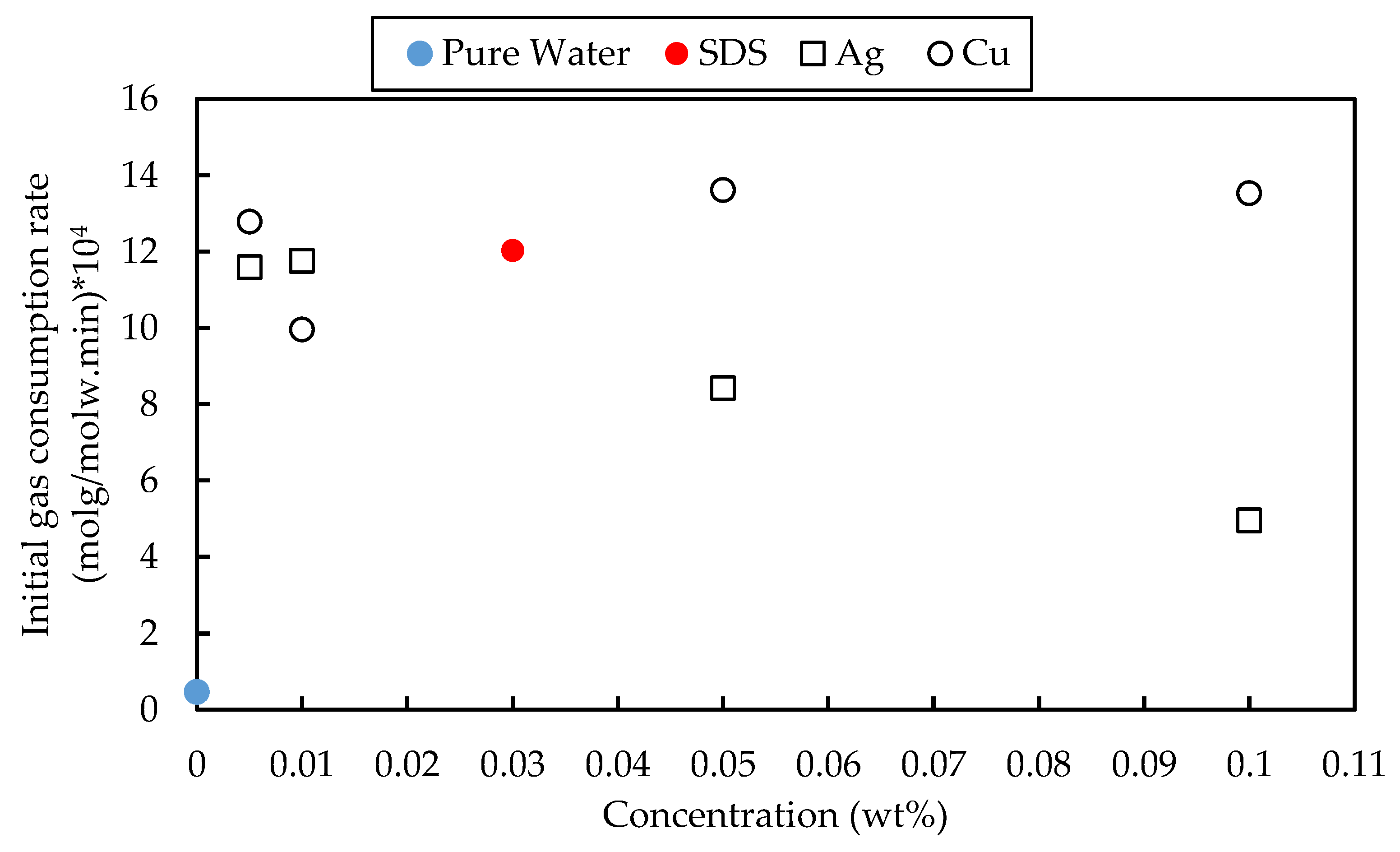
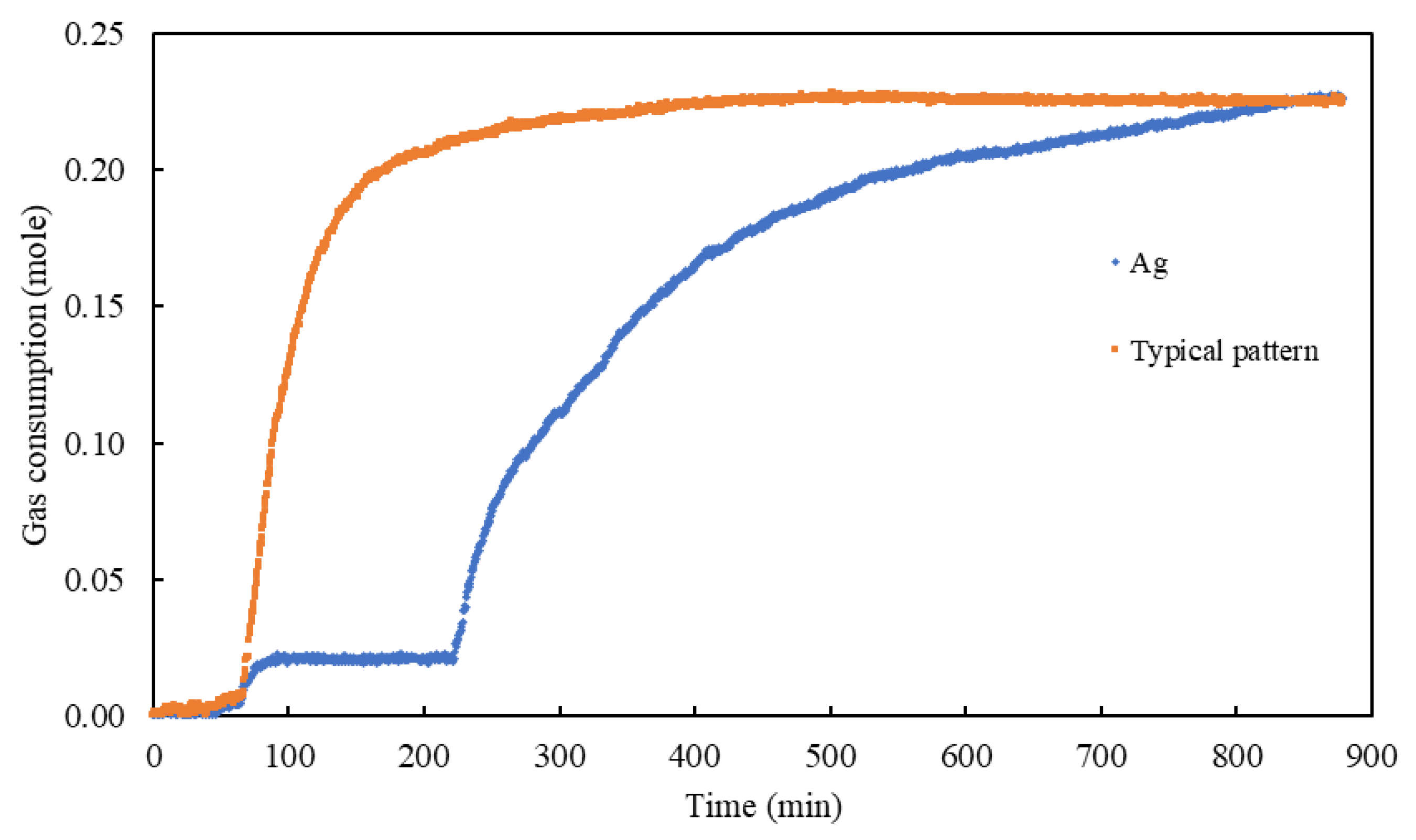
3.4. Initial Rate of the Gas Consumption
3.5. Half-Completion Time t50 and Semi-Completion Time t95 Measurements
3.6. Gas Uptake and Storage Capacity
3.7. Modelling of Apparent Rate Constant
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lal, B.; Nashed, O. Chemical Additives for Gas Hydrates; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Bharathi, A.; Nashed, O.; Lal, B.; Foo, K.S. Experimental and modeling studies on enhancing the thermodynamic hydrate inhibition performance of monoethylene glycol via synergistic green material. Sci. Rep. 2021, 11, 2396. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fan, S.; Chen, Q.; Yang, G.; Zhao, J.; Wei, N.; Wen, Y. Experimental and modeling phase equilibria of gas hydrate systems for post-combustion CO2 capture. J. Taiwan Inst. Chem. Eng. 2019, 96, 35–44. [Google Scholar] [CrossRef]
- Lin, T.-K.; Dahyar, M.; Le, M.-J.; Hsieh, B.-Z. Study of the formation mechanisms of CO2 hydrates from matching the experimental data with a porous media setting by multiphase flow-geochemical-thermal reservoir simulator. J. Taiwan Inst. Chem. Eng. 2020, 114, 115–124. [Google Scholar] [CrossRef]
- Mohamad Sabil, K.M.; Nashed, O.; Lal, B.; Foo, K.S. Formation Kinetics and Self-Preservation of CO2 Hydrates in the Presence of Carboxylated Multiwall Carbon Nanotubes. In Proceedings of the International Petroleum Technology Conference, Virtual, 16 March 2021. [Google Scholar]
- Nashed, O.; Lal, B. Gas Hydrate Promoters. In Chemical Additives for Gas Hydrates; Springer International Publishing: Cham, Switzerland, 2020; pp. 47–65. [Google Scholar]
- Nashed, O.; Lal, B.; Partoon, B.; Sabil, K.M.; Hamed, Y. Kinematic Study of Methane Hydrate Formation and Self-Preservation in the Presence of Functionalized Carbon Nanotubes. Energy Fuels 2019, 33, 7684–7695. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C. Clathrate Hydrates of Natural Gases, 3rd ed.; Speight, J.G., Ed.; Hemical Industries, A Series of Reference Books and Textbooks; CRC Press-Taylor & Francis Group: New York, NY, USA, 2008; p. 701. [Google Scholar]
- Partoon, B.; Sabil, K.M.; Lau, K.K.; Nasrifar, K.; Shariff, A.M. Selective Separation of Methane from Carbon Dioxide through sII Hydrates Formation in a Semibatch Process. Ind. Eng. Chem. Res. 2019, 58, 16834–16842. [Google Scholar] [CrossRef]
- Ganji, H.; Manteghian, M.; Zadeh, K.S.; Omidkhah, M.R.; Mofrad, H.R. Effect of different surfactants on methane hydrate formation rate; stability and storage capacity. Fuel 2007, 86, 434–441. [Google Scholar] [CrossRef]
- Wang, F.; Luo, S.-J.; Fu, S.-F.; Jia, Z.-Z.; Dai, M.; Wang, C.-S.; Guo, R.-B. Methane hydrate formation with surfactants fixed on the surface of polystyrene nanospheres. J. Mater. Chem. A 2015, 3, 8316–8323. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharjee, G.; Kulkarni, B.D.; Kumar, R. Role of Surfactants in Promoting Gas Hydrate Formation. Ind. Eng. Chem. Res. 2015, 54, 12217–12232. [Google Scholar] [CrossRef]
- Mounkachi, O.; Akrouchi, A.; Tiouitchi, G.; Lakhal, M.; Salmani, E.; Benyoussef, A.; Kara, A.; El Kenz, A.; Ez-Zahraouy, H.; El Moutaouakil, A. Stability, Electronic Structure and Thermodynamic Properties of Nanostructured MgH2 Thin Films. Energies 2021, 14, 7737. [Google Scholar] [CrossRef]
- Li, J.; Liang, D.; Guo, K.; Wang, R.; Fan, S. Formation and dissociation of HFC134a gas hydrate in nano-copper suspension. Energy Convers. Manag. 2006, 47, 201–210. [Google Scholar] [CrossRef]
- Mohammadi, A.; Manteghian, M.; Mohammadi, A.H.; Jahangiri, A. Induction time, storage capacity, and rate of methane hydrate formation in the presence of SDS and silver nanoparticles. Chem. Eng. Commun. 2017, 204, 1420–1427. [Google Scholar] [CrossRef]
- Mohammadi, A. Effect of SDS, silver nanoparticles, and SDS + silver nanoparticles on methane hydrate semicompletion time. Pet. Sci. Technol. 2017, 35, 1542–1548. [Google Scholar] [CrossRef]
- Nashed, O.; Youssouf, S.M.; Sabil, K.M.; Shariff, A.M.; Sufian, S.; Lal, B. Investigating the effect of silver nanoparticles on carbon dioxide hydrates formation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458, 012058. [Google Scholar] [CrossRef]
- Goff, G.S.; Rochelle, G.T. Oxidation Inhibitors for Copper and Iron Catalyzed Degradation of Monoethanolamine in CO2 Capture Processes. Ind. Eng. Chem. Res. 2006, 45, 2513–2521. [Google Scholar] [CrossRef]
- Pahlavanzadeh, H.; Rezaei, S.; Khanlarkhani, M.; Manteghian, M.; Mohammadi, A.H. Kinetic study of methane hydrate formation in the presence of copper nanoparticles and CTAB. J. Nat. Gas Sci. Eng. 2016, 34, 803–810. [Google Scholar] [CrossRef]
- Mohammadi, M.; Haghtalab, A.; Fakhroueian, Z. Experimental study and thermodynamic modeling of CO2 gas hydrate formation in presence of zinc oxide nanoparticles. J. Chem. Thermodyn. 2016, 96, 24–33. [Google Scholar] [CrossRef]
- Adibi, N.; Mohammadi, M.; Ehsani, M.R.; Khanmohammadian, E. Experimental investigation of using combined CH4/CO2 replacement and thermal stimulation methods for methane production from gas hydrate in the presence of SiO2 and ZnO nanoparticles. J. Nat. Gas Sci. Eng. 2020, 84, 103690. [Google Scholar] [CrossRef]
- Abdi-Khanghah, M.; Adelizadeh, M.; Naserzadeh, Z.; Barati, H. Methane hydrate formation in the presence of ZnO nanoparticle and SDS: Application to transportation and storage. J. Nat. Gas Sci. Eng. 2018, 54, 120–130. [Google Scholar] [CrossRef]
- Nashed, O.; Partoon, B.; Lal, B.; Sabil, K.M.; Shariff, A.M. Investigation of Functionalized Carbon Nanotubes’ Performance on Carbon Dioxide Hydrate Formation. Energy 2019, 174, 602–610. [Google Scholar] [CrossRef]
- Arjang, S.; Manteghian, M.; Mohammadi, A. Effect of synthesized silver nanoparticles in promoting methane hydrate formation at 4.7 MPa and 5.7 MPa. Chem. Eng. Res. Des. 2013, 91, 1050–1054. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Liu, D.; Cui, G.; Dou, B.; Wang, J.; Hao, S. Enhanced natural gas hydrates formation in the suspension with metal particles and fibers. J. Mol. Liq. 2020, 301, 112410. [Google Scholar] [CrossRef]
- Rahmati-Abkenar, M.; Manteghian, M.; Pahlavanzadeh, H. Nucleation of ethane hydrate in water containing silver nanoparticles. Mater. Des. 2017, 126, 190–196. [Google Scholar] [CrossRef]
- Rahmati-Abkenar, M.; Manteghian, M.; Pahlavanzadeh, H. Experimental and theoretical investigation of methane hydrate induction time in the presence of triangular silver nanoparticles. Chem. Eng. Res. Des. 2017, 120, 325–332. [Google Scholar] [CrossRef]
- Mohammadi, A.; Manteghian, M.; Haghtalab, A.; Mohammadi, A.H.; Rahmati-Abkenar, M. Kinetic study of carbon dioxide hydrate formation in presence of silver nanoparticles and SDS. Chem. Eng. J. 2014, 237 (Suppl. C), 387–395. [Google Scholar] [CrossRef]
- Wang, F.; Song, Y.-M.; Liu, G.-Q.; Guo, G.; Luo, S.-J.; Guo, R.-B. Rapid methane hydrate formation promoted by Ag&SDS-coated nanospheres for energy storage. Appl. Energy 2018, 213, 227–234. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, R.; Wang, F. Fast formation kinetics of methane hydrates loaded by silver nanoparticle coated activated carbon (Ag-NP@AC). Chem. Eng. J. 2021, 417, 129206. [Google Scholar] [CrossRef]
- Said, S.; Govindaraj, V.; Herri, J.-M.; Ouabbas, Y.; Khodja, M.; Belloum, M.; Sangwai, J.S.; Nagarajan, R. A study on the influence of nanofluids on gas hydrate formation kinetics and their potential: Application to the CO2 capture process. J. Nat. Gas Sci. Eng. 2016, 32, 95–108. [Google Scholar] [CrossRef]
- Torré, J.-P.; Ricaurte, M.; Dicharry, C.; Broseta, D. CO2 enclathration in the presence of water-soluble hydrate promoters: Hydrate phase equilibria and kinetic studies in quiescent conditions. Chem. Eng. Sci. 2012, 82, 1–13. [Google Scholar] [CrossRef]
- Englezos, P.; Kalogerakis, N.; Dholabhai, P.D.; Bishnoi, P.R. Kinetics of Formation of Methane and Ethane Gas Hydrates. Chem. Eng. Sci. 1987, 42, 2647–2658. [Google Scholar] [CrossRef]
- Partoon, B.; Javanmardi, J. Effect of Mixed Thermodynamic and Kinetic Hydrate Promoters on Methane Hydrate Phase Boundary and Formation Kinetics. J. Chem. Eng. Data 2013, 58, 501–509. [Google Scholar] [CrossRef]
- Adisasmito, S.; Frank, R.J.; Sloan, E.D. Hydrates of carbon dioxide and methane mixtures. J. Chem. Amp. Eng. Data 1991, 36, 68–71. [Google Scholar] [CrossRef]
- Bai, W.; Raghavendra, A.; Podila, R.; Brown, J.M. Defect density in multiwalled carbon nanotubes influences ovalbumin adsorption and promotes macrophage activation and CD4+ T-cell proliferation. Int. J. Nanomed. 2016, 11, 4357–4371. [Google Scholar] [CrossRef]
- Song, Y.; Wang, F.; Liu, G.; Luo, S.; Guo, R. Promotion Effect of Carbon Nanotubes-Doped SDS on Methane Hydrate Formation. Energy Fuels 2017, 31, 1850–1857. [Google Scholar] [CrossRef]
- Yan, K.; Li, X.; Xu, C.; Lv, Q.; Ruan, X. Molecular dynamics simulation of the intercalation behaviors of methane hydrate in montmorillonite. J. Mol. Model. 2014, 20, 2311. [Google Scholar] [CrossRef]
- Wang, R.; Liu, T.; Ning, F.; Ou, W.; Zhang, L.; Wang, Z.; Peng, L.; Sun, J.; Liu, Z.; Li, T.; et al. Effect of hydrophilic silica nanoparticles on hydrate formation: Insight from the experimental study. J. Energy Chem. 2019, 30, 90–100. [Google Scholar] [CrossRef]
- Suzuki, I.; Ishikawa, Y.; Hisamatsu, Y. The pitting corrosion of copper tubes in hot water. Corros. Sci. 1983, 23, 1095–1106. [Google Scholar] [CrossRef]
- Siangsai, A.; Inkong, K.; Kulprathipanja, S.; Kitiyanan, B.; Rangsunvigit, P. Roles of Sodium Dodecyl Sulfate on Tetrahydrofuran-Assisted Methane Hydrate Formation. J. Oleo Sci. 2018, 67, 707–717. [Google Scholar] [CrossRef]


| Chemical | Abbreviation | Diameter/nm | Purity | Supplier |
|---|---|---|---|---|
| Silver | Ag | 30–50 | >95% | Research Nanomaterials Inc. US |
| Copper | Cu | 40 | >95% | |
| Sodium dodecyl sulfate | SDS | NA | 99% | Merck |
| Methane | CH4 | NA | 99.95% | Air Product Sdn. Bhd |
| Carbon dioxide | CO2 | NA | 99.95% |
| Aqueous Phase | Solid Phase | CH4 | CO2 | ||||
|---|---|---|---|---|---|---|---|
| Mean Induction Time (min) | STD | Number of Runs | Mean Induction Time (min) | STD | Number of Runs | ||
| Pure Water | --- | 74.2 | 2.37 | 4 | 68.2 | 2.83 | 4 |
| 0.03 wt% SDS | --- | 73.9 | 5.96 | 5 | 65 | 0.05 | 3 |
| 0.03 wt% SDS | 0.005 wt% Ag | 66 | 0.26 | 3 | 66 | 0.82 | 3 |
| 0.01 wt% Ag | 62.7 | 2.76 | 4 | 66.2 | 0.56 | 3 | |
| 0.05 wt% Ag | 77.2 | 3.55 | 4 | 65.3 | 0.47 | 3 | |
| 0.1 wt% Ag | 76.1 | 4.69 | 5 | 64.6 | 0.83 | 4 | |
| 0.005 wt% Cu | 66.1 | 0.65 | 3 | 66 | 0.12 | 3 | |
| 0.01 wt% Cu | 56.8 | 5.13 | 4 | 57.8 | 6.72 | 3 | |
| 0.05 wt% Cu | 65 | 3.49 | 4 | 65 | 0.47 | 3 | |
| 0.1 wt% Cu | 65 | 2.48 | 4 | 65.1 | 0.41 | 3 | |
| Aqueous Phase | Solid Phase | Mean t50 | STD | Mean t95 | STD | Number of Runs |
|---|---|---|---|---|---|---|
| CO2 | ||||||
| Pure Water | --- | 117.0 | 3.74 | 518.0 | 24.65 | 4 |
| 0.03 wt% SDS | --- | 100.3 | 3.14 | 246.7 | 21.01 | 3 |
| 0.03 wt% SDS | 0.005 wt% Ag | 99.0 | 4.23 | 250.0 | 16.02 | 3 |
| 0.010 wt% Ag | 137.6 | 8.82 | 340.9 | 26.12 | 3 | |
| 0.050 wt% Ag | 207.7 | 38.92 | 575.4 | 129.29 | 5 | |
| 0.100 wt% Ag | 137.3 | 35.00 | 378.9 | 58.67 | 4 | |
| 0.005 wt% Cu | 103.0 | 8.93 | 240.0 | 3.50 | 3 | |
| 0.010 wt% Cu | 102.0 | 6.44 | 266.5 | 61.50 | 3 | |
| 0.050 wt% Cu | 109.5 | 1.50 | 308.5 | 8.50 | 3 | |
| 0.100 wt% Cu | 108.8 | 0.01 | 285.5 | 13.52 | 3 | |
| CH4 | ||||||
| Pure Water | --- | 656.1 | 89.26 | 1937.6 | 110.19 | 4 |
| 0.03 wt% SDS | --- | 107.0 | 10.48 | 149.4 | 30.57 | 5 |
| 0.03 wt% SDS | 0.005 wt% Ag | 92.7 | 3.55 | 135.7 | 3.32 | 3 |
| 0.010 wt% Ag | 89.4 | 20.29 | 128.4 | 17.50 | 4 | |
| 0.050 wt% Ag | 150.3 | 29.85 | 195.7 | 11.42 | 4 | |
| 0.100 wt% Ag | 152.1 | 33.71 | 200.3 | 11.25 | 5 | |
| 0.005 wt% Cu | 91.0 | 1.91 | 137.5 | 6.12 | 3 | |
| 0.010 wt% Cu | 98.6 | 3.73 | 150.6 | 10.33 | 4 | |
| 0.050 wt% Cu | 95.2 | 6.63 | 136.0 | 9.02 | 4 | |
| 0.100 wt% Cu | 114.2 | 8.87 | 157.2 | 8.55 | 4 | |
| Sample | Hydrate Former | Gas Consumption (mol) | Gas Uptake (molg/molw) | Storage Capacity v/v |
|---|---|---|---|---|
| Pure water | CH4 | 0.2087 | 0.0380 | 113.9 |
| CO2 | 0.2240 | 0.0409 | 151.8 | |
| With kinetic promoters | CH4 | 0.3320–0.3420 | 0.0606–0.0625 | 159–163.7 |
| CO2 | 0.2240–0.2300 | 0.0409–0.0420 | 151.8–155.9 |
| Aqueous Phase | Solid Phase | Apparent Rate Constant Kapp (mol/min MPa) | |
|---|---|---|---|
| CH4 | CO2 | ||
| Pure water | ---- | 0.00017 | 0.00317 |
| 0.03 wt% SDS | ---- | 0.00547 | 0.00489 |
| 0.03 wt% SDS | 0.005 wt% Ag | 0.00460 | 0.00390 |
| 0.010 wt% Ag | 0.00554 | 0.00435 | |
| 0.050 wt% Ag | 0.00400 | 0.00376 | |
| 0.100 wt% Ag | 0.00192 | 0.00341 | |
| 0.005 wt% Cu | 0.00516 | 0.00465 | |
| 0.010 wt% Cu | 0.00496 | 0.00551 | |
| 0.050 wt% Cu | 0.00589 | 0.00565 | |
| 0.100 wt% Cu | 0.00589 | 0.00426 | |
| Nanomaterial | Gas | Regression Coefficients | MAPE% | MAPE% (SDS) | ||
|---|---|---|---|---|---|---|
| k0 | k1 | k2 | ||||
| Ag | CH4 | 0.0051 | −0.0079 | −0.2416 | 7.90 | 6.75 |
| CO2 | 0.0042 | −0.0061 | −0.0147 | 5.18 | 14.14 | |
| Cu | CH4 | 0.0049 | 0.0291 | −0.1878 | 2.50 | 10.41 |
| CO2 | 0.0048 | 0.0437 | −0.4884 | 5.37 | 1.87 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nashed, O.; Partoon, B.; Lal, B.; Sabil, K.M.; Yaqub, S.; Shariff, A.M. Methane and Carbon Dioxide Hydrate Formation in the Presence of Metal-Based Fluid. Materials 2022, 15, 8670. https://doi.org/10.3390/ma15238670
Nashed O, Partoon B, Lal B, Sabil KM, Yaqub S, Shariff AM. Methane and Carbon Dioxide Hydrate Formation in the Presence of Metal-Based Fluid. Materials. 2022; 15(23):8670. https://doi.org/10.3390/ma15238670
Chicago/Turabian StyleNashed, Omar, Behzad Partoon, Bhajan Lal, Khalik Mohamad Sabil, Sana Yaqub, and Azmi Mohd Shariff. 2022. "Methane and Carbon Dioxide Hydrate Formation in the Presence of Metal-Based Fluid" Materials 15, no. 23: 8670. https://doi.org/10.3390/ma15238670
APA StyleNashed, O., Partoon, B., Lal, B., Sabil, K. M., Yaqub, S., & Shariff, A. M. (2022). Methane and Carbon Dioxide Hydrate Formation in the Presence of Metal-Based Fluid. Materials, 15(23), 8670. https://doi.org/10.3390/ma15238670







