Highly-Efficient and Visible Light Photocatalytical Degradation of Organic Pollutants Using TiO2-Loaded on Low-Cost Biomass Husk
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Composite
2.2. Characterization
2.3. Photocatalytic Evaluation
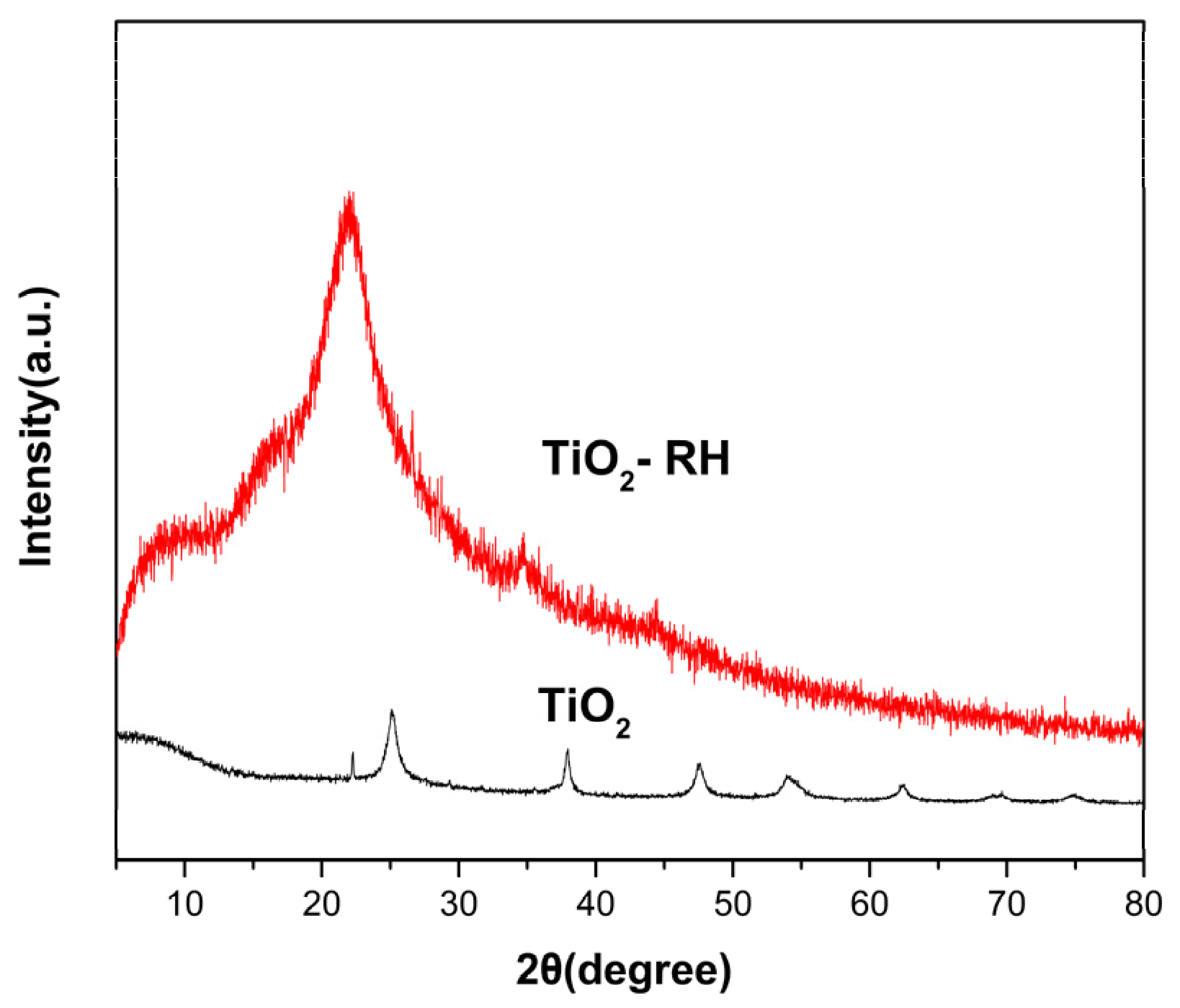
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, K.; Badru, R.; Singh, P.P.; Kaushal, S. Photodegradation of organic pollutants using heterojunctions: A review. J. Environ. Chem. Eng. 2020, 8, 103666. [Google Scholar]
- AlAbdulaal, T.H.; Ganesh, V.; AlShadidi, M.; Hussien, M.S.A.; Bouzidi, A.; Algarni, H.; Zahran, H.Y.; Abdel-wahab, M.S.; Yahia, I.S.; Nasr, S. The auto-combustion method synthesized Eu2O3-ZnO nanostructured composites for electronic and photocatalytic applications. Materials 2022, 15, 3257. [Google Scholar] [CrossRef] [PubMed]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Hussien, M.S.A.; Bouzidi, A.; Abd-Rabboh, H.S.M.; Yahia, I.S.; Zahran, H.Y.; Abdel-wahab, M.S.; Alharbi, W.; Awwad, N.S.; Ibrahim, M.A. Fabrication and characterization of highly efficient as-synthesized WO3/graphitic-C3N4 nanocomposite for photocatalytic degradation of organic compounds. Materials 2022, 15, 2482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Q.; Chen, C.; Zang, L.; Ma, W.; Zhao, J. Oxygen atom transfer in the photocatalytic oxidation of alcohols by TiO2: Oxygen isotope studies. Angew. Chem. Int. Ed. 2009, 48, 6081–6084. [Google Scholar] [CrossRef]
- Ulfa, M.; Afif, H.A.; Saraswati, T.E.; Bahruji, H. Fast removal of methylene blue via adsorption-photodegradation on TiO2/SBA-15 synthesized by slow calcination. Materials 2022, 15, 5471. [Google Scholar] [CrossRef]
- Dou, Q.Q.; Teng, C.P.; Ye, E.; Loh, X.J. Effective near-infrared photodynamic therapy assisted by upconversion nanoparticles conjugated with photosensitizers. Int. J. Nanomed. 2015, 10, 419–432. [Google Scholar]
- Liao, G.Z.; Chen, S.; Quan, X.; Chen, H.; Zhang, Y.B. Photonic crystal coupled TiO2/ polymer hybrid for efficient photocatalysis under visible light irradiation. Environ. Sci. Technol. 2010, 44, 3481–3485. [Google Scholar] [CrossRef]
- Nsib, M.F.; Hajji, F.; Mayoufifi, A.; Moussa, N.; Rayes, A.; Houas, A. In situ synthesis and characterization of TiO2/HPM cellulose hybrid material for the photocatalytic degradation of 4-NP under visible light. Comptes Rendus Chim. 2014, 17, 839–848. [Google Scholar] [CrossRef]
- Virkutyte, J.; Jegatheesan, V.; Varma, R.S. Visible light activated TiO2/microcrystalline cellulose nanocatalyst to destroy organic contaminants in water. Bioresour. Technol. 2012, 113, 288–293. [Google Scholar] [CrossRef]
- Hun, X.; Chen, Y.L.; Liu, P.; Qian, Q.R.; Luo, Y.J.; Cui, M.L.; Chen, Y.S.; Yang, D.P.; Chen, Q.H. Visible light-assisted efficient degradation of dye pollutants with biomass-supported TiO2 hybrids. Mater. Sci. Eng. C. 2018, 82, 197–203. [Google Scholar]
- Alghamdi, Y.G.; Balu, K.K.; Maqsood, A.M.; Sultan, A. Design and preparation of biomass-derived activated carbon loaded TiO2 photocatalyst for photocatalytic degradation of reactive red 120 and ofloxacin. Polymers 2022, 14, 880. [Google Scholar] [CrossRef]
- Alsaiari, M. Biomass-derived active carbon (AC) modified TiO2 photocatalyst for efficient photocatalytic reduction of chromium (VI) under visible light. Arab. J. Chem. 2021, 14, 103258. [Google Scholar] [CrossRef]
- Darwish, A.A.A.; Rashad, M.; Al-Aoh, H.A. Methyl orange adsorption comparison on nanoparticles: Isotherm, kinetics, and thermodynamic studies. Dyes Pigment. 2019, 160, 563–571. [Google Scholar] [CrossRef]
- Zou, Y.P.; Yang, T.K. Rice husk, rice husk ash and their applications. In Rice Bran and Rice Bran Oil; AOCS Press: Urbana, IL, USA, 2019; pp. 207–246. [Google Scholar]
- Priya, A.K.; Yogeshwaran, V.; Rajendran, S.; Hoang, T.K.A.; Soto-Moscoso, M.; Ghfar, A.A.; Bathula, C. Investigation of mechanism of heavy metals (Cr6+, Pb2+ & Zn2+) adsorption from aqueous medium using rice husk ash: Kinetic and thermodynamic approach. Chemosphere 2022, 286, 131796. [Google Scholar]
- Li, A.; Xie, H.; Qiu, Y.; Liu, L.; Lu, T.; Wang, W.; Qiu, G. Resource utilization of rice husk biomass: Preparation of MgO flake-modified biochar for simultaneous removal of heavy metals from aqueous solution and polluted soil. Environ. Pollut. 2022, 310, 119869. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Z.H.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef]
- Liu, X.Y.; Steven, M.Z.; Liu, G.Q.; Li, Y.Q.; Zhang, R.H. Pretreatment of wheat straw with potassium hydroxide for increasing enzymatic and microbial degradability. Bioresour. Technol. 2015, 185, 150–157. [Google Scholar] [CrossRef]
- Ye, E.; Regulacio, M.D.; Bharathi, M.S.; Pan, H.; Lin, M.; Bosman, M.; Win, K.Y.; Ramanarayan, H.; Zhang, S.Y.; Loh, X.J.; et al. An experimental and theoretical investigation of the anisotropic branching in gold nanocrosses. Nano 2016, 8, 543–552. [Google Scholar] [CrossRef]
- Huang, K.; Dou, Q.; Loh, X.J. Nanomaterial mediated optogenetics: Opportunities and challenges. RSC Adv. 2016, 6, 60896–60906. [Google Scholar] [CrossRef]
- Li, Z.; Ye, E.; Lakshminarayanan, D.R.; Loh, X.J. Recent advances of using hybrid nanocarriers in remotely controlled therapeutic delivery. Small 2016, 12, 4782–4806. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, X.; Li, J.G.; Li, X.; Xiu, Z.; Yang, H.; Xue, X. Fluorine-and iron-modified hierarchical anatase microsphere photocatalyst for water cleaning: Facile wet chemical synthesis and wavelength-sensitive photocatalytic reactivity. Langmuir 2009, 26, 4546–4553. [Google Scholar] [CrossRef] [PubMed]
- Renwick, L.C.; Brown, D.; Clouter, A.; Donaldson, K. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup. Environ. Med. 2004, 61, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Alosaimi, E.H.; Alsohaimi, I.H.; Dahan, T.E.; Chen, Q.; Younes, A.A.; El-Gammal, B.; Melhi, S. Photocatalytic degradation of methylene blue and antibacterial activity of mesoporous TiO2-SBA-15 nanocomposite based on rice husk. Adsorp. Sci. Technol. 2021, 1, 9290644. [Google Scholar] [CrossRef]
- Wang, W.; Hu, C.; Fang, J.J.; Min, L. Large-scale preparation of rice-husk-derived mesoporous SiO2@ TiO2 as efficient and promising photocatalysts for organic contaminants degradation. Appl. Surf. Sci. 2019, 467, 1187–1194. [Google Scholar] [CrossRef]
- Adrian, C.M.L.; Suzana, Y.; Man, K.L.; Bridgid, L.F.C.; Muhammad, S.; Ayaka, Y.; Menandro, N.A. The effect of industrial waste coal bottom ash as catalyst in catalytic pyrolysis of rice husk for syngas production. Energy Convers. Manag. 2018, 165, 541–554. [Google Scholar]
- Fatimah, I.; Said, A.; Hasanah, U.A. Preparation of TiO2-SiO2 using rice husk ash as silica source and the kinetics study as photocatalyst in methyl violet decolorization. Bull. Chem. React. Eng. Catal. 2015, 10, 43–49. [Google Scholar] [CrossRef]
- Faiz, M.A.; Azurahanim, C.C.; Yazid, Y.; Suriani, A.B.; Ain, M.S.N. Preparation and characterization of graphene oxide from tea waste and it’s photocatalytic application of TiO2/graphene nanocomposite. Mater. Res. Express 2020, 7, 015613. [Google Scholar] [CrossRef]


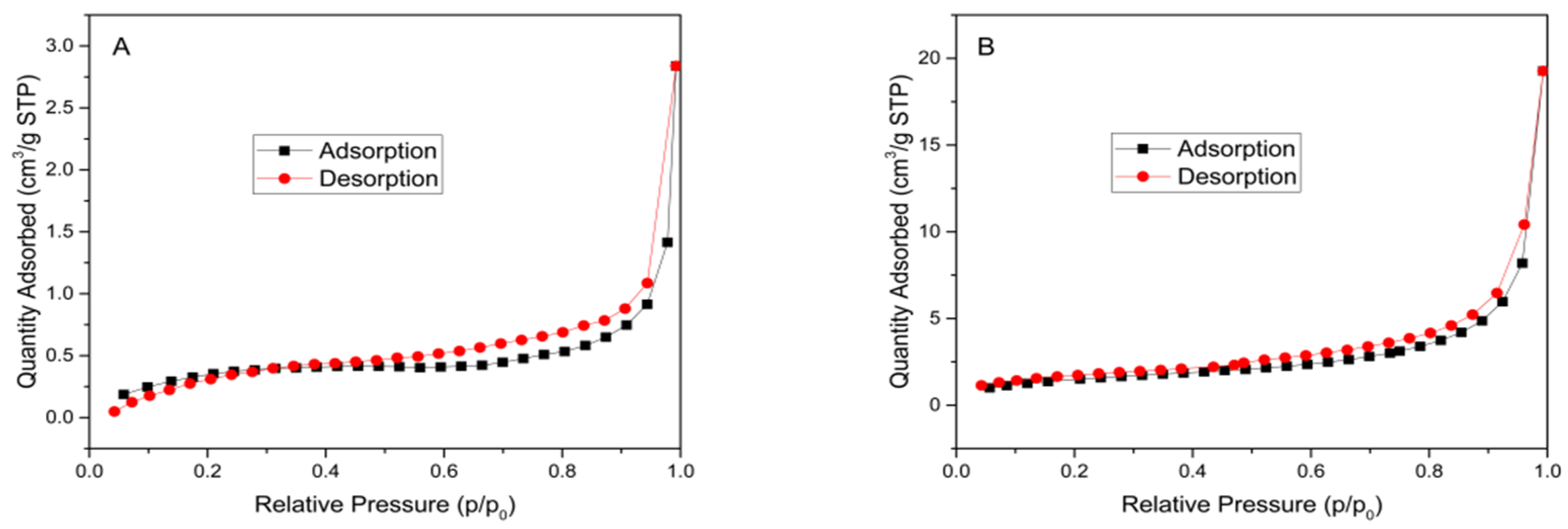



| Sample | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| RH | 1.0542 | 0.004392 | 11.2855 |
| TiO2-RH | 4.9642 | 0.029811 | 6.5425 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Lin, X.; Li, Z.; Liu, J. Highly-Efficient and Visible Light Photocatalytical Degradation of Organic Pollutants Using TiO2-Loaded on Low-Cost Biomass Husk. Materials 2022, 15, 8671. https://doi.org/10.3390/ma15238671
Li Y, Lin X, Li Z, Liu J. Highly-Efficient and Visible Light Photocatalytical Degradation of Organic Pollutants Using TiO2-Loaded on Low-Cost Biomass Husk. Materials. 2022; 15(23):8671. https://doi.org/10.3390/ma15238671
Chicago/Turabian StyleLi, Yuan, Xirong Lin, Zhanpeng Li, and Jinyun Liu. 2022. "Highly-Efficient and Visible Light Photocatalytical Degradation of Organic Pollutants Using TiO2-Loaded on Low-Cost Biomass Husk" Materials 15, no. 23: 8671. https://doi.org/10.3390/ma15238671
APA StyleLi, Y., Lin, X., Li, Z., & Liu, J. (2022). Highly-Efficient and Visible Light Photocatalytical Degradation of Organic Pollutants Using TiO2-Loaded on Low-Cost Biomass Husk. Materials, 15(23), 8671. https://doi.org/10.3390/ma15238671






