Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: PART I—Pre-Treatment and Conversion Coating
Abstract
:1. Foreword
2. Surface Cleaning and Pre-Treatment
2.1. Introduction
- Improvement in the corrosion resistance of the metallic substrate.
- Preparation of an adequate surface for the subsequent layers in the coating system.
2.2. Mechanical Pre-Treatment
2.3. Chemical Pre-Treatment
2.3.1. Alkaline Degreasing
2.3.2. Acid Pickling
2.3.3. Case Study: AZ91 Magnesium Alloy
| Pre-Treatment | Concentration/ Other Parameters | Substrate | Subsequent Coating | Effects on Properties of the Substrate or the Coating | Reference/Year |
|---|---|---|---|---|---|
| -acid pickling: 1-H3PO4 2-HF 3-HNO3 | Duration: 60–600 s 85% 50% 70% | AM50 and AZX310 | - | Among the tested acid solution, HNO3 exhibited the most effective result to reduce the corrosion rate in 3.5 wt.% NaCl. | [85] 2017 |
| -acid pickling: 1-CH3COOH + Ca(NO3)2 2-C2H2O4.2H2O 3-C6H8O7 | Duration: 15–120 s 100–200 g/L + 50 g/L 20 g/L 40–120 g/L | AZ31 | - | 4 µm etching is claimed to be sufficient to ensure the reduction of Fe impurity level close to that of bulk. Acetic acid-based solution showed the best result in terms of impurity removal. | [62] 2010 |
| -acid pickling: 1-H2SO4 2-HNO3 3-H3PO4 | Duration: 15–120 s 10–50 g/L 20–80 g/L 40–80 g/L | AZ31 | - | 5 µm etching is claimed to be sufficient to ensure the reduction of Fe impurity level below 100 ppm. Nitric acid had the best performance to reduce the corrosion rate of bare AZ31. | [61] 2010 |
| -alkaline cleaning: NaOH + Na3PO4.12H2O + NaSiO3.10H2O + OP-10 -acid pickling: H3PO4 (85% V/V)+ Na2MoO4.2H2O -activation: NH4HF2 | Duration: 8–10 min 40 g/L 20 g/L 20 g/L 3 mL/L Duration: 5–10 s 200 mL/L 1–20 g/L Duration: 6–10 min 200 g/L | AZ91D | Electroless Ni–P plating | Reduction in etching rate with increase in the concentration of Na2MoO4.2H2O. Increase in Ni–P plating rate with increase in concentration of Na2MoO4 from 0.5 g/L to 7 g/L. | [84] 2011 |
| -acid pickling: 1-HF 2-HCl 3-HNO3 | Duration: 20 s 0.5 and 11 wt.% 0.5 wt.% 0.5 wt.% | AZ91D | Stannate conversion coating | Best corrosion protection performance in 0.05 M NaCl in the case of HF compared to other acid pickling solutions. | [86] 2011 |
| -acid pickling: 1-H3PO4 85% 2-HCl 37% 3-HNO3 68% 4-C6H8O7 -conditioning: NaOH | 200 mL/L, 30 s 5 mL/L, 30 s 30 mL/L, 30 s 20 g/L, 45 s 200 g/L, 65 °C, 30 min | AZ91D | Zn immersion coating | H3PO4 and HNO3 pickling solutions preferentially attacked the β phase/matrix interface. Combination of acid pickling + conditioning treatment can provide an electrochemically uniform substrate, which results in a uniform subsequent Zn immersion coating. | [52] 2012 |
| -acid pickling: H3PO4 -activation: 1-HF 2-NH4HF2 | Duration: 1 min 50~700 mL/L Duration: 8 min 10–300 mL/L 5–150 mL/L | AZ31 | Electroless Ni–P plating | The highest etching rate of H3PO4 was achieved at 400 mL/L concentration. Pickling with H3PO4 improve the corrosion resistance of the subsequent electroless Ni–P plating when the concentration is less than 400 mL/L. The best corrosion resistance performance obtained when pickling with H3PO4 and subsequent NH4HF2 activation were performed. | [83] 2014 |
| -acid pickling: 1-hydrofluoric acid 2-acetic acid 3-N3PO4 + NaOH | 10% v/v, 10min 0.05 M, 30 s 10 g/L + 50 g/L, 40 min | AZ91 | Sol–gel (TEOS/MTMS) | Na3PO4 + NaOH pre-treatment offers a better surface condition for the subsequent sol–gel deposition as compared to the acid pickling pre-treatments, which, in turn, leads to a more corrosion protective sol–gel coating. | [54] 2019 |
| 1-sand blasting 2-grinding 3-polishing | -corundum particles (180 µm) -emery paper #150, 400 and 1000 −2.5 µm alumina slurry | AZ91 | Phosphate conversion coating | Lower surface roughness resulted in a more uniform and denser coating. Different coating composition was observed at the valleys and peaks of the rough surface treated by grinding. | [13] 2019 |
| -acid pickling: 1-HNO3 | Duration: 90 s 1 M | AZ31 | Polycaprolactone (PCL) electrospinning | Pre-treatment with HNO3 significantly reduce the corrosion rate of the bare AZ31 and PCL-coated samples in SBF solution. | [117] 2016 |
| -acid Pickling: 1-HNO3 2-H3PO4 | Duration: 30–180 s 1 M 1 M | Mg0.6Ca | CaP conversion coating (as the result of immersion in SBF) | Higher deposition rate of CaP phase in SBF after acid pickling. Lower corrosion rate in SBF after acid pickling. Slightly higher corrosion resistance of the substrate treated by HNO3 compared to that treated by H3PO4 | [118] 2021 |
| -pre-treatment NO−3 + EDTA | Duration: 30 min Temperature: 60 °C 0.1 M PH: ~13.5 | AZ91 | Phosphate conversion coting | The successful dissolution of Alx(Mn,Fe)y impurity phase and formation of electrochemically uniform surface. Formation of more uniform phosphate conversion coating with superior corrosion protection properties. | [110] 2022 |
3. Conversion Coatings
3.1. Introduction
- Phosphate with permanganate;
- Metal phosphates (Ca2+, Zn2+, Mn2+, Sr2+);
- Rare earth (Ce3+/4+), La3+, Y3+);
- Permanganate with vanadate/molybdate/wolframate/zirconate;
- Permanganate with HF;
- Fluorides;
- Hexafluorozirconate, hexafluorotitanate, and other fluorometallates;
- Stannates;
- Phytates and other organic polymers;
- Al–Mg layered double hydroxides;
- Cr(III)-less favorable option owing to generation of Cr(VI) during exploitation.
| H+ dissociation | H2SiF6 ⇄ 2H+ + SiF62− | (1) |
| SiF62− dissociation | SiF62− + 4H2O ⇄ Si(OH)4(s) + 4H+ + 6F− | (2) |
| TiCl4 dissociation | TiCl4 + 4H2O ⇄ Ti(OH)4(s) + 4HCl | (3) |
| Reaction of TiO2 and F− | TiF62− + 2H2O ⇄ Ti(OH)4(s) + 4H+ + 6F− | (4) |
| Magnesium dissolution | Mg → Mg2+ + 2e− | (5) |
| Aluminum dissolution | Al → Al3+ + 3e− | (6) |
| Proton reduction | 2H+ + 2e− → H2(g) | (7) |
| Hydroxide formation | Mg2+ + 2OH− → Mg(OH)2(s) | (8) |
| Reaction of Mg2+ and F− | Mg2+ + 2F− → MgF2(s) | (9) |
| Hydroxide formation | Al3+ + 3OH− → Al(OH)3(s) | (10) |
| Reaction of Al3+ and F− | Al3+ + 3F− → AlF3(s) | (11) |
| Reaction with more F− | Al3+ + 6F− → AlF63−(aq) or/and AlF3(s) + 3F− → AlF63−(aq) | (12) |
| Mg(OH)2 dehydration | Mg(OH)2(s) → MgO(s) + H2O | (13) |
| Al(OH)3 dehydration | Al(OH)3(s) → Al2O3(s) + H2O | (14) |
| Ti(OH)4 dehydration | Ti(OH)4(s) → TiO2 + 2H2O | (15) |
| Si(OH)4 dehydration | Si(H)3(s) → SiI2(s) + 2H2O | (16) |
3.2. Phosphate-Based Conversion Coatings (PCC)
3.3. Rare Earth-Based Conversion Coatings
3.4. Vanadate-Based Conversion Coating
3.5. Molybdate-Based Conversion Coating
3.6. Stannate-Based Conversion Coating
3.7. Selenite-Based Conversion Coating
3.8. Magnesium Fluoride Conversion Coating
3.9. Hexafluoro–Zirconate/Titanate/Hafnate/Niobate-Based Conversion Coating
3.10. Organic-Based Conversion Coating
3.11. Layered Double Hydroxide (LDH)
3.12. Post-Treatment on Conversion Coatings
| Treatment | Pre-Treatment | Bath Component/ Concentration/ Mechanism | Initial Bulk pH | Duration, Bath T | Thickness, Surface Composition/ Morphology | Performance | Testing Medium | Alloy | Reference/ Year | Advantage/ Disadvantage |
|---|---|---|---|---|---|---|---|---|---|---|
| Cr(VI) | Alkaline decreasing Nitric acid pickling Chromic acid pickling HF activation | K2Cr2O7 40 g/L K2SO4 20 g/L | n/a | 1–14 min 75 °C with air bubbling | 11 µm Cr2O3, Cr(OH)3, K2CrO4, MgO | Ecorr shift −1.61 to –1.3 V icorr decreased from 0.079 to 0.02 A/m2 for bare to CCC coated substrate | 1% NaCl | EV31A | [80] 2014 | Highly effective, with self-healing effect, abrasion resistance, commercially available, one step process, long bath life / carcinogenic, toxic, banned by EU regulations, urgent need for replacement |
| Cr(III) | Grinding to 1000, ultrasonication in acetone | (0.3 M CrCl3 0.05 M NH4H2PO2) in choline chloride:ethylene glycol (1:2) | n/a | 30–60 min 30 ± 5 °C under ultrasonic treatment followed by methanol rinsing | 3 µm Cr2O3 Microcracks | Ecorr shift −1.51 to –1.45 V icorr decreased from 609 µA/cm2 to 1.25 µA/cm2 for bare to Cr(III)CC coated substrate | 3.5% NaCl | AZ31 | [135] 2016 | Commercially available, robust and easy bath maintenance and process control, one step process / moderate corrosion protection, weak self-healing properties, contains minor amount of carcinogenic Cr(VI) |
| Pickling/activation processes according to SAE-AMS-M-3171 | e.g., 1–5 g/L Cr2(SO4)3, 1–5 g/L K2ZrF6, 0–5 g/L MeBF4 0–5 g/L ZnSO4 0.5–1.5 g/L soluble cellulose 0–10 g/L surfactant | 3.7–4.0 | 5–15 min Ambient temp up to 50 °C | Adhesion 2–2.5 times higher than for DOW 7 chromate treated process | n/a | n/a | AZ91C-T6 ZE41-T5 | [136] 2010 | ||
| Phosphate | Alkali washing in 60 wt.% NaOH, grinding to 1200 grit, cleaning in pure water and ethanol | 35 g/L Mn(H2PO4)2, 0.5 g/L of NaF or C6H5Na3O7 or C6H8O7 | 2.5 | 1 s–20 min 95 °C | Lamellar structure with block particles. Intermediate layer: Mg3(PO4)2, AlPO4, and Mg(OH)2. Outer layer: MnHPO4. | Ecorr shift from −1.5 to −0.34, −0.468, and −1.37 V for the bare to phosphated substrate containing citric acid, NaF and Na-citrate, respectively. Icorr reduction from 460 µA/cm2 to 5 nA/cm2, 32 nA/cm2 and 5 µA/cm2 for the bare to phosphated substrate containing citric acid, NaF and Na-citrate, respectively. | 3.5 wt.% NaCl | AZ31 | [205] (2013) | Commercially available, eco-benign, good adhesion with paint, / moderate corrosion protection effect, requires elevated temperatures, multi-step process, requires accurate operation, bath maintenance and control due to low stability of bath’s pH |
| Grinding to 1200 grits, cleaned with industrial alcohol in ultrasonic bath, degreased in NaOH, acid pickling in mixture of HF and C2H6O2 | 4–36 mL/L H3PO4, 40–90 g/L Ba(H2PO4)2 1–3 g/L NaF | n/a | 10–30 min, 60–100 °C | Mg, MgO, and some amorphous phases | Corrosion spots appear after 20 h of SST for the phosphate conversion coated sample, while white massive corrosion blocks after 8 h of SST covered the untreated sample | SST, damp heat test | AZ31 | [400] (2009) | ||
| Grinding to 4000 grits in ethanol, rinsed in ethanol | 0.1 M Mg(OH)2, 0.24 M H3PO4 | 3.2 | 20 min, 45 °C | 2.5 µm thick coating after 20 min of phosphating. Micro-cracks structure. Coating composed of MgO/Mg(OH)2 and Mg-PO4 compounds | Ecorr shift from −1.61 V to −1.41 V for the bare to phosphated substrate. Icorr reduced from 223 µA/cm2 to 6.9 µA/cm2 for the bare to treated substrate. Pit initiation time was delayed from 10 min to 24 h for the bare to the phosphated substrate. | 0.1 M and 0.05 M NaCl | AZ31 | [401] (2017) | ||
| Grinding to 2000 grits in ethanol, rinsed in ethanol | Step 1: H3PO4, step 2: 0.05 M (NH4)2HPO4 | Step1: 5 Step2: n/a | Step 1: 30 min, 40 °C Step 2: 30–60 min, 80 °C | Inner layer: MgHPO4.3H2O Outer layer: MgNH4PO4.6H2O | Corrosion resistance of phosphated substrates is about 20 times better than untreated samples. Ecorr shift from −1.6 V to −1.53 V for the bare to 2-step treated substrate. Icorr reduction from 63 µA/cm2 to 3.7 µA/cm2for the bare to 2-step treated substrate. | SBF | AZ31 | [402] (2015) | ||
| grinding to 2500 grits, degreased in absolute ethanol, acid pickling in HNO3 and then HF solution. Rinsed by distilled water between each step | 10 g/L Y(NO3)3 then in NH4H2PO4 bath with concentration of 1–2.5% | n/a | 30–180 s, 75–90 °C | Y2O3, YOx/y, Mg3(PO4)2, AlPO4 and YPO4 | Ecorr shift positively about 180mV compared to the uncoated one at, Icorr reduced from 70.2 µA/cm2 to 7.7 µA/cm2 for bare to conversion coated sample | 3.5% NaCl | AZ91 | [403] (2016) | ||
| Phosphate-permanganate | Grinding to 1500 grit, polishing with 0.3 µm Al2O3 paste, pure water cleaning, alkaline degreasing with NaOH + Na3PO4, pure water cleaning, acid pickling with H3PO4, surface activation with HF | 20 g/L KMnO4, 60 g/L MnHPO4 | n/a | 10 min, 50 °C | Network-like cracks in coating containing metal oxides (Mg, Mn and Al), Hydroxide, phosphates and spinel for AZ series alloy | Equivalent or slightly better passive capability than the conventional Cr6+-based conversion treatment of AZ series alloys, but an inferior capability for the pure Mg | 5 wt.% NaCl | AZ61,AZ80, AZ91, and pure Mg | [187] (2002) | |
| Grinding to 2000 grit, rinsed with DI water, cleaned in acetone, dried in a stream of hot air | 0.87 M NH4H2PO4, 0.063–0.51 M KMnO4 | n/a | 10 min, 60 °C | Three layer: 1- porous layer on substrate 2- compact intermediate layer 3: cellular overlay. Thickness in the range of 8–1 µm | Less than 10% corroded fraction after 24 h SST for the phosphate solution containing 0.51 M KMnO4, while more than 50% of bare AZ31 was corroded after 24 h | Solution of 0.05 M NaCl and 0.10 M Na2SO4. SST (ASTM B117) | AZ31 | [189] (2013) | ||
| Degreasing with ethanol, acid pickling with H3PO4, tap water rinsing, NaOH activating, tap water rinsing | 100 g/L NH4H2PO4, 30 g/L KMnO4 | 3.5 | 40 °C | First layer: homogenous but with many cracks/ Second layer: nodules of Mn-rich oxides | Reduction in corrosion rate from SST by phosphate-permanganate conversion coating. +200 mV shift to Ecorr and two orders of magnitude reduction in icorr, comparing untreated and phosphate-permanganate coated sample | Salt spray ASTM B117, Electrochemical tests in solution containing Na2SO4, NaHCO3 and NaCl (pH 8.2) | AZ91 and AM50 | [404] (2010) | ||
| Grinding to 1200 grits, DI water, air stream drying | 0.1 M KMnO4 0.025 M Mn(NO3)2 0.02 M KH2PO4 | 1.7 | 90 s 25 °C | 230 nm nearly crack-free | Ecorr shift –1.56 V to –1.41 V icorr decreased from 20 µA/cm2 to 1.6 µA/cm2 for bare to PCC coated substrate compared to 0.4 µA/cm2 for DOW1 CCC Sufficient electrical conductivity, Poor crystallinity | 0.05 M NaCl + 0.1 M Na2SO4 | AZ31 | [81] (2015) | ||
| Blasting (alumina F220-500), degreasing (e.g. NaOH), pickling (H3PO4), activating (or HF) | 0.2 M KMnO4 0.1 M Na3PO4 2 g/L Ca(NO3)2 2 g/L Y(NO3)3 | 2.5–5 H3PO4 | n/a | n/a | CC treated uncoated samples withstood 168 h of SSF and 500 h of humidity test, CC treated samples coated with primer and resin withstood 2000 h of SSF, Good adhesion of organic coats | SSF (ASTM B117 Sec. 8.1 and 10.1); Humidity tests; Cross-cut adhesion tests | EV31A, AZ91, AM60 | [405] 2015, 2017 | ||
| Zinc -phosphate | Grinding to 1000 grits, degreased in absolute acetone, rinsed by DI water | Primary bath: 2 g/L ZnO, 12 g/L H3PO4, 1g/L NaF, 4 g/L C4H4O6Na2, 6 g/L NaNO3, 0.5 g/L Na4P2O7 + 2 g/L nano-CeO2 or 2 g/L nano-ZnO or 2 g/L nano-ZrO2 | n/a | 60 min, 60 °C | n/a | Icorr reduction from 1.24 mA/cm2 to 0.06 mA/cm2 for original phosphate coated to the nano-CeO2 modified coating. Ecorr shifted from −1.42 V to −1.30 V for original phosphate coated to the nano-CeO2 modified coating. Significant reduction in crack ratio and size on the nano-CeO2 modified coating compared to the original phosphate coating | 3.5% NaCl | AZ91D | [406] (2017) | |
| Grinding to 2000 grit, degreased in KOH, rinsed in distilled water | 1 M H3PO4, 0.004–0.068 M Zn(NO3)2.6H2O, 0.042 M NaNO2, 0.021 M NaNO3, 0.024 M NaF, 0.034 M Na2HPO4.12H2O | 2.1–4 | 50 °C | Outer porous hopeite crystal and inner dense amorphous compound | Icorr reduction to 50 time lower value from bare to the treated sample at pH of 3.07 | 0.5 M NaCl | AZ31 | [175] (2013) | ||
| Grinding to 3000 grits, alkaline degreasing, acid pickling | 50 g/L Zn(H2PO4)2, 20 g/L NaH2PO4, 30 g/L 50% Mn(NO3)2, 5 g/L C6H8O7, 0.2 g/L C18H29NaO3S | 1.8–2.6 | 15 min, 45 °C | Homogeneous and ordered crystals containing Zn3(PO4)2 and MnHPO4. Some cracks | Ecorr shift from −1.571 V to −0.370 V for bare to coated substrate in phosphate solution of pH 2. Icorr reduction from 129 µA/cm2 to 5 µA/cm2 for bare to coated substrate in phosphate solution of pH 2. | 3.5% NaCl | Mg-8.5Li | [204] (2014) | ||
| Heat treatment of samples for 0–24 h at 400 °C, grinding to 2000 grits, cleaned with distilled water, degreased in KOH, rinsed in distilled water | 12.4 g/L H3PO4 (85 wt.%), 5 g/L Zn(NO3)2.6.H2O, 20 g/L NaH2PO4.12H2O, 3 g/L NaNO2, 1.84 g/L NaNO3, 1 g/L NaF | 3–3.2 | 50 °C | Inner layer of MgZn2(PO4)2 and Mg3(PO4)2. Outer layer of hopeite (Zn3(PO4)2·4H2O) | the sample with 24 h heat treatment withstood 24 h in immersion test, While the bare sample withstood only 3 h in immersion test | 0.5 M NaCl | AZ91 | [407] (2013) | ||
| Grinding to 1000 grits, rinsed with DI water, degrease in alcohol | 2 g/L ZnO, 12 g/L H3PO4, 1 g/L NaF, 4 g/L C4H6O6Na2, 6 g/L NaNO3, + 0.5 g/L of Basic bath TSPP or ATMP or EDTA | n/a | 20 min, 45 °C | n/a | Ecorr shift from −1.45 V to −1.40 V, −1.43 V and −1.45 V for basic phosphate solution to solution containing TSPP, ATMP and EDTA, respectively. Icorr reduction from 30 µA/cm2 to 8.5 µA/cm2, 10 µA/cm2and 28 µA/cm2 in the presence of TSPP, ATMP and EDTA, respectively. | Salt-water test (SWI), 3.5% NaCl | AZ91 | [203] (2014) | ||
| Calcium-phosphate | Grinding to 2000 grits, degreased in absolute acetone, rinsed by DI water, dried under atmospheric condition | 40 g/L Ca(NO3)2, 40 mL/L H3PO4 then in the 5 g/L NaF solution, then surface modification in a 0.05 M ethanol of stearic acid solution | Phosphating 2.8; Fluoride 12 | Phosphating: 20 min, 37 °C ± 2 °C fluoride bath: 2h, 80 °C, 15 h stearic acid | Micro-protrusions, submicro-lumps and nano-grains with diameter of about 1–2 µm. Ca3(PO4)2, Ca(H2PO4), Ca10(PO4)6F2, and MgF2 | Icorr reduction from 129 µA/cm2 to 1.3 µA/cm2 for bare to the substrate coated with phosphate, fluoride and stearic acid. Ecorr shift from −1.54 V to −1.36 V for bare to the substrate coated with phosphate, fluoride and stearic acid. | 3.5% NaCl | Mg-5Zn-1.5Ca | [199] (2017) | |
| Grinding to 2000 grit, cleaning in acetone. No pre-treatment such as alkaline degreasing or acid pickling. | 12 g/L Ca(NO3)2.4H2O, 1.2 g/L CaO, 8 mL/L H3PO4 (85% v/v) | 2.4–3.2 | 5 s–40 min, 15 °C, 37 °C, 60 °C | Ca9Mg(HPO4)(PO4)6, MgHPO4.3H2O. Thickest coating at bath pH of 3.2 | Lowest icorr 2.9 µA/cm2 obtained at pH 3.0 | SBF solution | AZ60 | [145] (2016) | ||
| Grinding to 2000 grits, cleaned in DI water and ethanol and then dried in open air | 0.05 M Ca(NO3)2.4H2O, 0.03 M NaH2PO4.2H2O | n/a | 48 h, room T | CaHPO4.2H2O, Ca2P2O7 (after heat treatment) with thickness of 30 µm | Ecorr shifted from −1.666 V to −1.566 V and −1.515 V fore bare, Ca-P coated and Ca-P coated followed with heat treatment. Icorr reduced from 35 µA/cm2 to 3.5 µA/cm2 and 1 µA/cm2 for bare, Ca-P coated and Ca-P coated followed with heat treatment. | Hank solution | ZK60 | [146] (2012) | ||
| Grinding to 300 grits, alkaline cleaning (NaOH, Na3PO4), acid pickling (CH3COOH+NaNO3) etching, HF activation, DI water | 2 g/L Ce(NO3)3 2 g/L La(NO3)3 2 g/L KMnO4 | 4.0 | 5 min 40 °C | 15 µm La2O3, CeO2, Mn2O3, and MnO2 homogeneous with microcracks | Ecorr and icorr decreased from 1.58 V/0.13 mA/cm2 to 1.44V/0.031 mA/cm2 for bare to RE coated substrate, compared to 1.11 V/0.056 mA/cm2 for Cr(VI) CC Excellent adhesion to substrate | 3.5% NaCl | Mg–Li | [246] (2009) | ||
Rare-earth element Rare-earth element | Grinding to 600 grits, polishing by 1-µm diamond paste, acetone, distilled water, degreasing with NaOH and Na3PO4 at 80 °C, DI water | 0.02 M Ce(NO3)3 5 g/L H2O2 (30 wt.%) | 4 | 15 min 25–55 °C | MgO, Mg(OH)2, CeO2, and Ce2O3, The highest uniformity and compactness of coating observed at 35 °C | Ecorr and icorr decreased from 1.543 V/0.25 mA/cm2 to 1.504 V/3 µA/cm2 for bare to RE coated substrate | 3.5% NaCl | AZ91 | [241] (2015) | Commercially available, high corrosion resistance / expensive, unsatisfactory long term stability |
| Grinding to 2500 grits, polishing by 3.5-µm diamond paste, degreased in ethanol, acid pickling by HNO3 (0.8%) and then 40% HF, rinsed with distilled water and subsequent drying before each step | 10 g/L Y(NO3)3 | n/a | 30 °C | Y2O3, YOx/y, Al2O3, and MgO | Improvement in corrosion resistance was not so significant, however, the post-treatment with the silica sol coating reduced the corrosion current density by two orders of magnitude, Ecorr shifted positively about 140 mV, The corrosion current density decreased about two orders of magnitude | 3.5% NaCl | AZ91 | [219] (2017) | ||
| Grinding to 180 grits, cleaned with isopropyl, rinsed with DI water, dried in room temperature, etched in HNO3, alkaline cleaning in Na2SiO3.5H2O | 4 wt.% CeCl3.7H2O, 6.7 wt.% H2O2, 0.25 wt.% organic gelatin | n/a | 5–180 s in CeCC solution, followed by 5min at 85 °C immersion in 2.5wt.% NaH2PO4 | Three-layer coating: nanocrystalline MgO, nanocrystalline CeCC and outer amorphous CeCC layer | Best corrosion behavior for the thinner CeCC (100 nm) | NSST | AZ31 | [236] (2016) | ||
| Grinding to 1200 grits, rinsed with DI water, degreases with acetone, acid pickling in 0.15M HCl or 0.46 M HF, rinsed in DI water, dried in stream of air | 0.05 M Ce(NO3)3.6H2O, 0.254 M H2O2 | 2.9 | 180 s, room T | 200 nm thickness on HCl pickled samples with chemical composition of Mg(OH)2, Al(OH)3. 300 nm thickness HF-pickled samples contained MgF2, as well. CC contained Mg/Al hydroxide and CeO2 | Adhesion grade was 1B, 3B, and 5B for the cerium coating on the as-polished AZ31, the HCl-pickled AZ31, and the HF-pickled AZ31, respectively. Corroded area after 24 h of SST was >80% for the cerium-coated as-polished AZ31, 20~25% for the cerium-coated HCL-pickled AZ31, and <1% for the cerium-coated HF-pickled AZ31. | SST, 3.5% NaCl for electrochemical tests, adhesion test according to ASTM D3359-02 | AZ31 | [408] (2012) | ||
| Grinding to 800 grits, Rinsed with DI water, dried in a stream of hot air, | 0.05 M Al (NO3)3, 0.001–0.05 M Ce(NO3)3 | n/a | 2 min, 15–20 °C | 6 µm compact coating with some observed micro-cracks, Al(OH)3, Al2O3, Mg(OH)2, MgO, Ce2O and Ce2O3 | The most positive Ecorr at Ce(NO3)3 concentration of 0.005 M, which also exhibited the lowest icorr of value 0.022 mA/cm2 | 5 wt.% NaCl | AZ91 | [409] (2013) | ||
| Grinding to 1500 grits, cleaning in acetone, degreased with NaOH+Na3PO4 | 6 g/L La(NO3)3, 3 g/L Na2MoO4 | 4 | 25 °C | 5–6 µm, Cracked layer with “dry-mud” morphology | Ecorr shifted 500 mV to more positive values with respect to bare substrate. two orders of magnitude in icorr | 3.5 wt.% NaCl | AZ31 | [229] (2010) | ||
| Grinding to 1000 grit, degreased in acetone, washed with triply distilled water | 5–50 mM Ce(NO3−)3·6H2O in purified N2 gas saturated atmosphere. 1–20 mM H2O2, 1–10 mM ascorbic acid | n/a | 50 °C | CeO, CeO2, Ce2O3, MgO, Mg(OH)2 | Small positive shift of Ecorr and 4-time reduction of icorr by addition of ascorbic acid to the bath. | Ringer solution | AZ91 | [221] (2016) | ||
| Vanadate | Grinding to 2400 grits, ultrasonication in acetone, stream air drying | NaVO3 30 g/L Vanadium oxide precipitation | 8 | 10 min/ 80 °C | 0.1–1.6 µm Vanadium oxides microcracks | Ecorr and icorr decreased from −1.63 V /0.1 mA/cm2 to −1.37 V/0.56 µA/cm2 for bare to vanadate coated alloy | 0.1% NaCl | AZ61 | [254] 2007 | High corrosion protection ability for a number of mg alloys / toxic if swallowed, suspected of damaging fertility, toxic to aquatic life with long lasting effects [258]. not industrially feasible |
| Grinding to 800 grits, acetone, air drying | NaVO3 50 g/L Vanadium oxide precipitation | 10 min/ RT | 1.5–2.5 µm Vanadium oxides microcracks | n/a | 3.5% NaCl | AZ31 | [253] 2011 | |||
| Molybdate | Grinding to 300 grits, alkaline decreasing 40 g/L NaOH, 10 g/L Na3PO4·12H2O Acid pickling (200 mL/L CH3COOH 50g/L NaNO3) | 25 g/L Na2MoO4·12H2O 4 g/L NaF (optional SiO2 nanoparticles) | 3 | 10 min/ 66 °C | 12 µm Multiple microcracks SiO2 addition decreases the number of microcracks | For MoO4− −1.04 V/16.1 µA/cm2 For MoO4−+SiO2 −0.81 V/3.6 µA/cm2 | 3.5% NaCl | AZ31 | [260] 2013 | Moderate corrosion protection / limited commercial availability |
| Stannate | Grinding to 1500 grits, air drying, acid pickling and activation 0.25%HF + 0.25%HCl | 0.25 M Na2SnO3, 0.073 M CH3COONa, 0.13 M Na3PO4, 0.05 M NaOH. | alkaline | 1 h/ 40 °C potentiostatic conditions | 0.6–1.8 µm deposit composed of MgSnO3·3H2O | Ecorr decreased from −1.77 V to −1.55 V for bare to stannate treated alloy | Borate buffer (0.15 M H3BO3 and 0.05 M Na2B4O7, pH 8.5) | AZ91 | [266] 2007 | Commercially available, environmentally acceptable / moderate corrosion protection, long time treatment, typically requires elevated temperature |
| Grinding to 2400 grits, polishing with 3 and 1um diamond paste, ultrasonication in acetone, stream hot air drying, activated in 11.25% HF | 30–60 g/L K2SnO3·3H2O, 10 g/L CH3COONa·3H2O, 50 g/L Na2P2O7 2.5–15 g/L NaOH Nucleation and growth of round particles | 12.6–13.2 | 2–60 min/ 60–90 °C | Few microns thick round agglomerates of submicron particles with remaining discontinuity in surface coverage | SST with rating numbers varying from 8 (bare AZ61) to 4 (stannate treated AZ61) | 5% NaCl SST | AZ61 | [410] 2006 | ||
| Grinding to 1000 grits, cold air stream drying, acid pickling and activation HF, HCl, HNO3 | 50 g/L K2SnO3, 10 g/L CH3COONa, 50 g/L Na2P2O7 5 g/L KOH | 12.4 | 1–10 min/ 82 °C | Round agglomerated submicron particles of MgSnO3·3H2O | Ecorr and icorr decreased from -1.60 V /12 µA/cm2 to –1.44 V/0.67 µA/cm2 for bare to stannate treated alloy | 0.05 M NaCl + 0.1 M Na2SO4 | AZ91 | [86] 2011 | ||
| Grinding to 800 grits, acetone, air drying | 25 g/L K2SnO3·3H2O + NaOH | 12.9 | 30 min/ RT | Corrosion rate decreased by 1/3–1/2 | 3.5% NaCl | AZ91 | [411] 2013 | |||
| Fluoride | Untreated | 7–28 M HF | Highly acidic | 1–24 h/ RT | Up to 2 µm Mg(OH)xF2−x | Ecorr and icorr decreased from −1.473 V/0.11 mA/cm2 to −1.468 V/0.017 mA/cm2 for bare to HF treated alloy | 3.5% NaCl | AZ31 | [99] 2010 | Commercially available / moderate corrosion protection HF is highly toxic, fatal if swallowed, in contact with skin or if inhaled [293] |
| Fluoro-metallates Zr, Ti or Zr/Ti fluorides | Grinding to 1200 grits | 0.01 M TiCl4, 0.01 M H2SiF6 5 mL/L HNO3 | 4 by NaOH | 0.5–10 min/ 40 °C | 0.2–0.5 µm micro-cracks Mg(OH)2, MgF2, Si(OH)4, Ti(OH)4 | Ecorr and icorr decreased from −1.55 V/9.9 µA/cm2 to −1.48 V/0.48 µA/cm2 for bare to treated alloy | 0.05 M NaCl 0.1 M Na2SO4; SST | AZ31 | [125] 2012 | Commercially available, excellent paint adhesion, good corrosion resistance, Single step process, operable at room temperature, Well-compatible with pre- and post-treatment / requires accurate process operation, active R&D topic |
| Grinding to 2000 grits, ultrasonication in acetone, hot air drying, 20% HF 20 h/RT | 0.2 M Zr(NO3)4: methanol:AcAc (molar 1:4:8) aged for 48 h, concluded by alloy dipping | Withdrawal speed: 6 m/h | Micron and submicron pores and cracks | Ecorr and icorr decreased from −1.614 V/12.9 µA/cm2 to −1.516 V/0.53 µA/cm2 for bare to treated alloy | 3.5% NaCl | AZ91 | [289] 2008 | |||
| Grinding to 2000 grits, degreasing in NaOH (40 g/L) +Na2SiO3 (40 g/L) | (a) H2TiF6 0.5 g/L and H2ZrF6 1.5 g/L (b) H2ZrF6 1.5 g/L + tannic acid 1.5 g/L (c) H2TiF6 0.5 g/L + tannic acid 1.5 g/L | 2.5 by NaOH | 3 min/ 25–30 °C | 5–6 μm Micro-cracks MgF2, Mg(OH)2, MgO, TiO2, ZrO2, Ti, and Zr metal–organic complex | icorr decreased from 93.72 μA/cm2 to 1.047 μA/cm2 | 3.5 wt.% NaCl | AZ91 | [306] 2015 | ||
| Grinding for SST: alkaline (NaOH, Na2CO3, Na3PO4, soap) and acidic (NH4F, H3PO4) treatment followed by air drying | 0.03–0.1 M Ce(NO3)3 0.03–0.1 M ZrO(NO3)2 0.02–0.05 M NbxOyFz | 4 by NH4F | 24 h /RT | CeO2, Ce2O3, ZrO2, Nb2O5, MgO, MgF2, composition did not change after anodic/cathodic polarization | Ecorr and icorr decreased from –2.07 V/626 µA/cm2 to –1.76 V/13 A/cm2 for bare to 24 h treated alloy | 0.5 M Na2SO4, or SST | AZ91 | [296] 2008 | ||
| Phytic acid (phytate) | Grinding to 1400 grits, ultrasonication in acetone for 10 min | Phytic acid/0.5 g/L | 5 | 20 min/RT | 4–5 µm, Mg/Al phytate microcrackes | Ecorr shifted from –1.906 V to –1.735 V icorr decreased from 429.4 to 373.0 mA/cm2 for bare to PA coated substrate excellent adhesion to substrate and epoxy coating | 5% NaCl | AZ61 | [318] | Excellent adhesion to substrate and epoxy coating, environmentally benign / limited corrosion protection, relatively expensive, commercially unavailable |
| Grinding to 1200 grits, DI water | Phytic acid/0.5%/chemisorption | 5 | 10–30 min/ 29 °C | 14–20 µm magnesium phytate (Mg12−x HxPhy) | icorr 37 µA/cm2 excellent adhesion | Phosphate buffer solution. pH 7.4 37 °C | AZ31 | [324] | ||
| Grinding to 1500 grits, alkaline degreasing and acid pickling | Phytic acid/ 20 g/L/ deposition | 6 | 10 min/ 35 °C | 7 µm Mg/Al phytate, macroscopic: smooth gray microscopic: flower-like cracked deposits | Ecorr shifted from −1.645 V to −0.905 V icorr decreased from 1.1 mA/cm2 to 2.3 µA/cm2 for bare to PA coated substrate excellent adhesion | 3.5% NaCl | Mg-Li alloy Mg - 11 wt.% Li, 3 wt.% Al, 0.5 wt.% RE | [322] | ||
| Grinding to 2000 grits, alkaline degreasing and acid pickling | Phytic acid / 20 g/L/ deposition | 9–10 | 0.5–3 min 25 ± 5 °C, Then hot air drying | transparent, microcracks | Ecorr shifted from −1.46 V to –1.31 V | 0.05 M NaCl | AZ31 | [412] | ||
| Grinding to 2000 grits, washing in acetone and DI water, hot air drying | Phytic acid/ 5 g/L/ deposition | 8 | 20 min RT | 0.34 µm Integrated and uniform | icorr 6 orders of magnitude lower than bare alloy | RT 3.5% NaCl | AZ91 | [323] | ||
| Grinding to 1200 grits, washing in acetone | Phytic acid/ 50% heat post-treatment improves corrosion resistance | n/a | 3 h RT | 2.2 µm | Ecorr shifted from −1.64 V to –1.50 V icorr decreased from 24 µA/cm2 to 1.2 µA/cm2 for bare to PA coated substrate excellent adhesion | Phosphate buffer solution | Pure Mg | [413] | ||
| Grinding to 4000 grits, washing in acetone, ethanol and DI water, followed by alkaline degreasing and acid pickling, followed by acetone, alcohol and DI water and hot air drying. Then 3M NaOH for 12 h at 60 °C, washed in DI water and dried in vacuum oven | Phytic acid/ 5 g/L covalent immobilization | 5 | 20 min 60 °C | n/a | Ecorr shifted from −1.44 V to –1.45 V icorr decreased from 0.27 mA/cm2 to 0.14 mA/cm2 for bare to PA coated substrate excellent adhesion | Phosphate buffer solution 37 °C | Pure Mg | [60] | ||
| Grinding to 2000 grits, ultrasonic treatment in ethanol, dried by warm air | Slurry prepared at 55 °C for 48 h 0.06 M Mg(NO3)2·6H2O, 0.03 M Al(NO3)3·9H2O +0.06 M Na2MoO4 and 0.2 M NaOH | alkaline | AZ31 sample was kept in slurry for 36 h 100 °C in autoclave | 17 µm Typical LDH flakes, MgAl-LDH, (Mg6Al2(OH) 16MoO4·4H2O) | Ecorr shifted from −1.54 V to –1.21 V icorr decreased from 31.7 µA/cm2 to 0.16 µA/cm2 for bare to LDH coated alloy | 3.5% NaCl | AZ31 | [384] 2014 | ||
| LDH | Grinding to 2000 grits, ultrasonic treatment in ethanol, dried in air stream | Slurry prepared at 40 °C for 48 h + 12 h [Mg(NO3)2 Al(NO3)3 at molar ratio 3:1 + Na2CO3/NaOH | alkaline | AZ31 sample was kept in slurry for 24–48 h 100 °C in autoclave | 7 µm typical LDH flakes, MgAl-LDH, (Mg6Al2(OH)16CO3·4H2O) | Ecorr shifted from –1.56 V to –1.18 V icorr decreased from 30.4 µA/cm2 to 0.07 µA/cm2 for bare to LDH coated alloy | 3.5% NaCl | AZ31 | [377] 2014 | High corrosion protective ability, environmentally benign, can be loaded with corrosion inhibitors for active corrosion protection, can be grown at RT and ambient pressure / at the early development stage, relatively expensive, active R&D topic |
| Grinding to 5000 grits, PEO treatment, Ultrasonic treatment in ethanol, dried in air stream | 0.1 M NaNO3 | 8 by NaOH | 12 h 100 °C in autoclave | 8 µm typical LDH flakes, LDH-MgAl-NO3 or LDH-MgAl-VO3 | Ecorr shifted from –0.74 V to –0.47 V icorr decreased from 3.9 µA/cm2 to 0.95 µA/cm2 for PEO treated to PEO-LDH-NO3 coated alloy | 3.5% NaCl | AZ31 | [369] 2017 | ||
| Grinding to 5000 grits, PEO treatment, Ultrasonic treatment in ethanol, dried in air stream | 0.05 M Al(NO3)3, 0.3 M NH4NO3 | 8.72–12.04 | 12 h 100 °C in autoclave | Typical LDH flakes, MgAl-LDH, Mg(OH)2 | Ecorr shifted from −1.51 V to −1.34 V, icorr shifted from 32.68 to 0.118 μA/cm2 for bath pH 8.72 to 11.72 | 3.5% NaCl | AZ31 | [370] 2017 | ||
| Grinding to 1200 grits, DI water, dried in air | Al(NO3)3 EDTA, NTA | 8–12 | 15 min to 6 h at 95 °C and 48 h at 25 °C ambient pressure | 20–60 nm typical LDH flakes, MgAl-LDH | n/a | n/a | AZ91 | [385] 2018 | ||
| PEO-treated AZ91 PEO electrolyte 1 g/L KOH, 8 g/L Na3PO4 and 12 g/L NaAlO2 | 0.05 M Al(NO3)3, 0.5 M NaNO3, 0.5 g of AZ91 flakes 0.05 M DTPA 0.003 M salicylate-Na | 10.0 | 0.5 to 8 h at 70 or 95 °C, ambient pressure | Typical LDH flakes were grown on top of PEO and inside PEO pores | n/a | n/a | AZ91 | [373] 2020 |
4. Summary and Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchheit, R.G.; Hughes, A.E. Chromate and Chromate-Free Conversion Coatings; ASM International: Almere, The Netherlands, 2003. [Google Scholar]
- Costa, M.; Klein, C.B. Toxicity and carcinogenicity of chromium compounds in humans. Crit. Rev. Toxicol. 2006, 36, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Park, R.M.; Bena, J.F.; Stayner, L.T.; Smith, R.J.; Gibb, H.J.; Lees, P.S. Hexavalent chromium and lung cancer in the chromate industry: A quantitative risk assessment. Risk Anal. Int. J. 2004, 24, 1099–1108. [Google Scholar] [CrossRef]
- ECHA. Substances Restricted under REACH. 2022. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.239.176 (accessed on 1 February 2022).
- Wierzbicka, E.; Vaghefinazari, B.; Mohedano, M.; Visser, P.; Posner, R.; Blawert, C.; Zheludkevich, M.; Lamaka, S.; Matykina, E.; Arrabal, R. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part II—PEO and Anodizing. Materials 2022, 15, 8515. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Wierzbicka, E.; Visser, P.; Posner, R.; Arrabal, R.; Matykina, E.; Mohedano, M.; Blawert, C.; Zheludkevich, M.L.; Lamaka, S.V. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part III—Corrosion Inhibitors and Combining Them with Other Protection Strategies. Materials 2022, 15, 8489. [Google Scholar] [CrossRef]
- Zvonkina, I.J. Adhesion of polymer coatings: Principles and evaluation. In Industrial Applications for Intelligent Polymers and Coatings; Springer: Cham, Switzerland, 2016; pp. 605–617. [Google Scholar]
- Sathyanarayana, M.N.; Yaseen, M. Role of promoters in improving adhesions of organic coatings to a substrate. Prog. Org. Coat. 1995, 26, 275–313. [Google Scholar] [CrossRef]
- Walter, R.; Kannan, M.B. Influence of surface roughness on the corrosion behaviour of magnesium alloy. Mater. Des. 2011, 32, 2350–2354. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Blanquet, A.; Staiger, M.P.; Dias, G.J.; Woodfield, T.B.F. On the role of surface roughness in the corrosion of pure magnesium in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1310–1318. [Google Scholar] [CrossRef]
- Gawlik, M.M.; Wiese, B.; Desharnais, V.; Ebel, T.; Willumeit-Römer, R. The effect of surface treatments on the degradation of biomedical Mg alloys-a review paper. Materials 2018, 11, 2561. [Google Scholar] [CrossRef] [Green Version]
- Höche, D.; Nowak, A.; John-Schillings, T. Surface cleaning and pre-conditioning surface treatments to improve the corrosion resistance of magnesium (Mg) alloys. In Corrosion Prevention of Magnesium Alloys: A Volume in Woodhead Publishing Series in Metals and Surface Engineering; Woodhead Publishing: Sawston, UK, 2013; pp. 87–109. [Google Scholar]
- Zhang, C.; Liu, B.; Yu, B.; Lu, X.; Wei, Y.; Zhang, T.; Mol, J.M.C.; Wang, F. Influence of surface pretreatment on phosphate conversion coating on AZ91 Mg alloy. Surf. Coat. Technol. 2019, 359, 414–425. [Google Scholar] [CrossRef]
- Song, G.-L.; Xu, Z. The surface, microstructure and corrosion of magnesium alloy AZ31 sheet. Electrochim. Acta 2010, 55, 4148–4161. [Google Scholar] [CrossRef]
- Song, G.; Xu, Z. Effect of Pre-treatment on corrosion performance of AZ31B magnesium alloy Panel. SAE Tech. Pap. 2010. [Google Scholar] [CrossRef]
- Phani, A.R.; Gammel, F.J.; Hack, T.; Haefke, H. Enhanced corrosioon resistance by sol-gel-based ZrO2-CeO2 coatings on magnesium alloys. Mater. Corros. 2005, 56, 77–82. [Google Scholar] [CrossRef]
- Seli, H.; Silikas, N.; Chen, X.; Thomas, A. Preliminary study of hydroxyapatite particles air abrasive blasting on Mg-4Zn-0.3Ca surface. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019. [Google Scholar]
- Zuleta, A.A.; Correa, E.; Castaño, J.G.; Echeverría, F.; Baron-Wiecheć, A.; Skeldon, P.; Thompson, G.E. Study of the formation of alkaline electroless Ni-P coating on magnesium and AZ31B magnesium alloy. Surf. Coat. Technol. 2017, 321, 309–320. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, W. Electroless nickel plating on AZ91 Mg alloy substrate. Surf. Coat. Technol. 2006, 200, 5087–5093. [Google Scholar] [CrossRef]
- Wang, B.J.; Xu, D.K.; Dong, J.H.; Ke, W. Effect of the crystallographic orientation and twinning on the corrosion resistance of an as-extruded Mg–3Al–1Zn (wt.%) bar. Scr. Mater. 2014, 88, 5–8. [Google Scholar] [CrossRef]
- Bagherifard, S.; Hickey, D.J.; Fintová, S.; Pastorek, F.; Fernandez-Pariente, I.; Bandini, M.; Webster, T.J.; Guagliano, M. Effects of nanofeatures induced by severe shot peening (SSP) on mechanical, corrosion and cytocompatibility properties of magnesium alloy AZ31. Acta Biomater. 2018, 66, 93–108. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Li, Y.; Chen, J.; Zou, Z. Effects of tensile and compressive deformation on corrosion behaviour of a Mg-Zn alloy. Corros. Sci. 2015, 90, 445–450. [Google Scholar] [CrossRef]
- Tekumalla, S.; Gupta, M. An insight into ignition factors and mechanisms of magnesium based materials: A review. Mater. Des. 2017, 113, 84–98. [Google Scholar] [CrossRef]
- Czerwinski, F. Controlling the ignition and flammability of magnesium for aerospace applications. Corros. Sci. 2014, 86, 1–16. [Google Scholar] [CrossRef]
- Shokrani, A.; Dhokia, V.; Newman, S.T. Environmentally conscious machining of difficult-to-machine materials with regard to cutting fluids. Int. J. Mach. Tools Manuf. 2012, 57, 83–101. [Google Scholar] [CrossRef]
- Song, G.-L.; Liu, M. The effect of surface pretreatment on the corrosion performance of Electroless E-coating coated AZ31. Corros. Sci. 2012, 62, 61–72. [Google Scholar] [CrossRef]
- Bagherifard, S. Enhancing the structural performance of lightweight metals by shot peening. Adv. Eng. Mater. 2019, 21, 1801140. [Google Scholar] [CrossRef]
- Nilawar, S.; Uddin, M.; Chatterjee, K. Surface engineering of biodegradable implants: Emerging trends in bioactive ceramic coatings and mechanical treatments. Mater. Adv. 2021, 2, 7820–7841. [Google Scholar] [CrossRef]
- Li, X.; Lu, L.; Li, J.; Zhang, X.; Gao, H. Mechanical properties and deformation mechanisms of gradient nanostructured metals and alloys. Nat. Rev. Mater. 2020, 5, 706–723. [Google Scholar] [CrossRef]
- Lin, B.; Zhang, J.; Sun, Q.; Han, J.; Li, H.; Wang, S. Microstructure, corrosion behavior and hydrogen evolution of USSP processed AZ31 magnesium alloy with a surface layer containing amorphous Fe-rich composite. Int. J. Hydrog. Energy 2021, 46, 10172–10182. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, P.; She, J.; Jiang, B.; Tang, A.; Pan, F.; Han, Q. A study of the corrosion behavior of AZ31 Mg alloy in depth direction after surface nanocrystallization. Surf. Coat. Technol. 2020, 396, 125968. [Google Scholar] [CrossRef]
- Mhaede, M.; Pastorek, F.; Hadzima, B. Influence of shot peening on corrosion properties of biocompatible magnesium alloy AZ31 coated by dicalcium phosphate dihydrate (DCPD). Mater. Sci. Eng. C 2014, 39, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Eidivandi, S.; Boroujeny, B.S.; Dustmohammadi, A.; Akbari, E. The effect of surface mechanical attrition treatment (SMAT) time on the crystal structure and electrochemical behavior of phosphate coatings. J. Alloys Compd. 2020, 821, 153252. [Google Scholar] [CrossRef]
- Uddin, M.; Hall, C.; Santos, V.; Visalakshan, R.; Qian, G.; Vasilev, K. Synergistic effect of deep ball burnishing and HA coating on surface integrity, corrosion and immune response of biodegradable AZ31B Mg alloys. Mater. Sci. Eng. C 2021, 118, 111459. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Song, G.-L.; Wu, P.-P.; Zheng, D.-J.; Wang, Z.-M. A burnished and Al-alloyed magnesium surface with improved mechanical and corrosion properties. Corros. Sci. 2021, 184, 109395. [Google Scholar] [CrossRef]
- Cao, C.; Zhu, J.; Tanaka, T.; Pham, D.N. Investigation of Corrosion Resistance Enhancement for Biodegradable Magnesium Alloy by Ball Burnishing Process. Int. J. Autom. Technol. 2020, 14, 175–183. [Google Scholar] [CrossRef]
- Pu, Z.; Yang, S.; Song, G.-L.; Dillon, O., Jr.; Puleo, D.; Jawahir, I. Ultrafine-grained surface layer on Mg–Al–Zn alloy produced by cryogenic burnishing for enhanced corrosion resistance. Scr. Mater. 2011, 65, 520–523. [Google Scholar] [CrossRef]
- Pu, Z.; Song, G.-L.; Yang, S.; Outeiro, J.; Dillon, O., Jr.; Puleo, D.; Jawahir, I. Grain refined and basal textured surface produced by burnishing for improved corrosion performance of AZ31B Mg alloy. Corros. Sci. 2012, 57, 192–201. [Google Scholar] [CrossRef]
- Liu, H.; Tong, Z.; Zhou, W.; Yang, Y.; Jiao, J.; Ren, X. Improving electrochemical corrosion properties of AZ31 magnesium alloy via phosphate conversion with laser shock peening pretreatment. J. Alloys Compd. 2020, 846, 155837. [Google Scholar] [CrossRef]
- Pu, Z.; Dillon, O., Jr.; Puelo, D.; Jawahir, I. Cryogenic machining and burnishing of magnesium alloys to improve In Vivo corrosion resistance. In Surface Modification of Magnesium and Its Alloys for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 103–133. [Google Scholar]
- Salahshoor, M.; Guo, Y. Biodegradation control of magnesium-calcium biomaterial via adjusting surface integrity by synergistic cutting-burnishing. Procedia CIRP 2014, 13, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Xin, Y.; Chen, X.-B.; Xu, D.; Chu, P.K.; Liu, C.; Guan, B.; Huang, X.; Liu, Q. Evading strength-corrosion tradeoff in Mg alloys via dense ultrafine twins. Nat. Commun. 2021, 12, 4616. [Google Scholar] [CrossRef]
- Bland, L.G.; Gusieva, K.; Scully, J.R. Effect of Crystallographic Orientation on the Corrosion of Magnesium: Comparison of Film Forming and Bare Crystal Facets using Electrochemical Impedance and Raman Spectroscopy. Electrochim. Acta 2017, 227, 136–151. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Li, Y.D.; Li, Y.X.; Wu, Y.H.; Zheng, Y.F.; Han, Y. Effect of surface mechanical attrition treatment on biodegradable Mg–1Ca alloy. Mater. Sci. Eng. C 2014, 35, 314–321. [Google Scholar] [CrossRef]
- Op’t Hoog, C.; Birbilis, N.; Estrin, Y. Corrosion of pure Mg as a function of grain size and processing route. Adv. Eng. Mater. 2008, 10, 579–582. [Google Scholar] [CrossRef]
- Chen, G.; Fu, Y.; Cui, Y.; Gao, J.; Guo, X.; Gao, H.; Wu, S.; Lu, J.; Lin, Q.; Shi, S. Effect of surface mechanical attrition treatment on corrosion fatigue behavior of AZ31B magnesium alloy. Int. J. Fatigue 2019, 127, 461–469. [Google Scholar] [CrossRef]
- Singh, D.; Basha, D.A.; Wadsö, L.; Orlov, D.; Matsushita, Y.; Singh, A.; Hosmani, S.S. Evolution of gradient structured layer on AZ91D magnesium alloy and its corrosion behaviour. J. Alloys Compd. 2021, 882, 160659. [Google Scholar] [CrossRef]
- Fabijanic, D.; Taylor, A.; Ralston, K.D.; Zhang, M.-X.; Birbilis, N. Influence of surface mechanical attrition treatment attrition media on the surface contamination and corrosion of magnesium. Corrosion 2013, 69, 527–535. [Google Scholar] [CrossRef]
- Johnston, S.; Shi, Z.; Dargusch, M.S.; Atrens, A. Influence of surface condition on the corrosion of ultra-high-purity Mg alloy wire. Corros. Sci. 2016, 108, 66–75. [Google Scholar] [CrossRef]
- Zhang, X.; Cano, Z.P.; Wilson, B.; McDermid, J.R.; Kish, J.R. Effect of surface preparation on the corrosion resistance of friction stir linear lap welded AZ31B-H24. Can. Metall. Q. 2017, 56, 308–321. [Google Scholar] [CrossRef]
- NPCS Board of Consultants & Engineers. Electroplating, Anodizing & Metal Treatment Hand Book; NIIR Project Consultancy Services: Delhi, India, 2003. [Google Scholar]
- Yang, H.Y.; Chen, X.B.; Guo, X.W.; Wu, G.H.; Ding, W.J.; Birbilis, N. Coating pretreatment for Mg alloy AZ91D. Appl. Surf. Sci. 2012, 258, 5472–5481. [Google Scholar] [CrossRef]
- Kappes, M.; Iannuzzi, M.; Carranza, R.M. Hydrogen embrittlement of Magnesium and Magnesium Alloys: A review. J. Electrochem. Soc. 2013, 160, C168–C178. [Google Scholar] [CrossRef]
- Dalmoro, V.; Azambuja, D.S.; Alemán, C.; Armelin, E. Hybrid organophosphonic-silane coating for corrosion protection of magnesium alloy AZ91: The influence of acid and alkali pre-treatments. Surf. Coat. Technol. 2019, 357, 728–739. [Google Scholar] [CrossRef]
- Jiang, S.; Cai, S.; Lin, Y.; Bao, X.; Ling, R.; Xie, D.; Sun, J.; Wei, J.; Xu, G. Effect of alkali/acid pretreatment on the topography and corrosion resistance of as-deposited CaP coating on magnesium alloys. J. Alloys Compd. 2019, 793, 202–211. [Google Scholar] [CrossRef]
- Fockaert, L.I.; Taheri, P.; Abrahami, S.T.; Boelen, B.; Terryn, H.; Mol, J.M.C. Zirconium-based conversion film formation on zinc, aluminium and magnesium oxides and their interactions with functionalized molecules. Appl. Surf. Sci. 2017, 423, 817–828. [Google Scholar] [CrossRef]
- Riaz, U.; Rahman, Z.U.; Asgar, H.; Shah, U.; Shabib, I.; Haider, W. An insight into the effect of buffer layer on the electrochemical performance of MgF2 coated magnesium alloy ZK60. Surf. Coat. Technol. 2018, 344, 514–521. [Google Scholar] [CrossRef]
- Li, L.-Y.; Cui, L.-Y.; Liu, B.; Zeng, R.-C.; Chen, X.-B.; Li, S.-Q.; Wang, Z.-L.; Han, E.-H. Corrosion resistance of glucose-induced hydrothermal calcium phosphate coating on pure magnesium. Appl. Surf. Sci. 2019, 465, 1066–1077. [Google Scholar] [CrossRef]
- Chakraborty Banerjee, P.; Singh Raman, R.K. Electrochemical impedance spectroscopic investigation of the role of alkaline pre-treatment in corrosion resistance of a silane coating on magnesium alloy, ZE41. Electrochim. Acta 2011, 56, 3790–3798. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, G.; Wang, J.; Zhao, S.; Zhao, Y.; Huang, N. Covalent immobilization of phytic acid on Mg by alkaline pre-treatment: Corrosion and degradation behavior in phosphate buffered saline. Corros. Sci. 2013, 75, 280–286. [Google Scholar] [CrossRef]
- Nwaogu, U.C.; Blawert, C.; Scharnagl, N.; Dietzel, W.; Kainer, K.U. Influence of inorganic acid pickling on the corrosion resistance of magnesium alloy AZ31 sheet. Corros. Sci. 2009, 51, 2544–2556. [Google Scholar] [CrossRef] [Green Version]
- Nwaogu, U.C.; Blawert, C.; Scharnagl, N.; Dietzel, W.; Kainer, K.U. Effects of organic acid pickling on the corrosion resistance of magnesium alloy AZ31 sheet. Corros. Sci. 2010, 52, 2143–2154. [Google Scholar] [CrossRef] [Green Version]
- Song, G.L.; Xu, Z. Surface processing and alloying to improve the corrosion resistance of magnesium (Mg) alloys. In Corrosion Prevention of Magnesium Alloys: A Volume in Woodhead Publishing Series in Metals and Surface Engineering; Woodhead Publishing: Sawston, UK, 2013; pp. 110–134. [Google Scholar]
- ASM International. ASM Handbook, Corrosion–Fundamentals, Testing and Protection; Volume 13A; ASM International, The Materials Information Society: Materials Park, OH, USA, 2003. [Google Scholar]
- Song, G.; Atrens, A. Understanding magnesium corrosion. A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Birbilis, N.; Williams, G.; Gusieva, K.; Samaniego, A.; Gibson, M.A.; McMurray, H.N. Poisoning the corrosion of magnesium. Electrochem. Commun. 2013, 34, 295–298. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, X.; Curioni, M.; Pawar, S.; Liu, H.; Fan, Z.; Scamans, G.; Thompson, G. Corrosion behavior of pure magnesium with low iron content in 3.5 wt.% NACL solution. J. Electrochem. Soc. 2015, 162, C362–C368. [Google Scholar] [CrossRef]
- Supplit, R.; Koch, T.; Schubert, U. Evaluation of the anti-corrosive effect of acid pickling and sol-gel coating on magnesium AZ31 alloy. Corros. Sci. 2007, 49, 3015–3023. [Google Scholar] [CrossRef]
- Gawlik, M.M.; Wiese, B.; Welle, A.; González, J.; Desharnais, V.; Harmuth, J.; Ebel, T.; Willumeit-Römer, R. Acetic acid etching of Mg-xGd alloys. Metals 2019, 9, 117. [Google Scholar] [CrossRef]
- ASF Metals; ASM International; Handbook Committee. ASM Handbook: Volume 5, Surface Engineering; ASM International: Almere, The Netherlands, 1994. [Google Scholar]
- Wang, Z.C.; Jia, F.; Yu, L.; Qi, Z.B.; Tang, Y.; Song, G.L. Direct electroless nickel–boron plating on AZ91D magnesium alloy. Surf. Coat. Technol. 2012, 206, 3676–3685. [Google Scholar] [CrossRef]
- Korrapati, V.K.; Scharnagl, N.; Letzig, D.; Zheludkevich, M.L. Self-assembled layers for the temporary corrosion protection of magnesium-AZ31 alloy. Corros. Sci. 2020, 169, 108619. [Google Scholar] [CrossRef]
- Korrapati, V.K.; Scharnagl, N.; Letzig, D.; Zheludkevich, M.L. Bilayer coatings for temporary and long–term corrosion protection of magnesium–AZ31 alloy. Prog. Org. Coat. 2022, 163, 106608. [Google Scholar] [CrossRef]
- Singh, C.; Meena, L.; Tiwari, S.; Singh, R. Establishing environment friendly surface treatment for AZ91 magnesium alloy for subsequent electroless nickel plating. J. Electrochem. Soc. 2018, 165, C71. [Google Scholar] [CrossRef]
- Seifzadeh, D.; Mohsenabadi, H.K.; Rajabalizadeh, Z. Electroless Ni–P plating on magnesium alloy by innovative, simple and non-toxic oxalate pretreatment and its corrosion protection. RSC Adv. 2016, 6, 97241–97252. [Google Scholar] [CrossRef]
- Chen, X.B.; Yang, H.Y.; Abbott, T.B.; Easton, M.A.; Birbilis, N. Corrosion-resistant electrochemical platings on magnesium alloys: A state-of-the-art review. Corrosion 2012, 68, 518–535. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, G.; Hu, B.; Lei, X.; Yi, H.; Zhang, J. Electrochemical behaviors of the magnesium alloy substrates in various pretreatment solutions. Appl. Surf. Sci. 2010, 256, 2988–2994. [Google Scholar] [CrossRef]
- Xiang, Y.; Hu, W.; Liu, X.; Zhao, C.; Ding, W. A study on surface state during the pretreatment of electroless nickel plating on magnesium alloys. Trans. Inst. Met. Finish. 2001, 79, 27–29. [Google Scholar] [CrossRef]
- Xu, C.; Chen, L.; Yu, L.; Zhang, J.; Zhang, Z.; Wang, J. Effect of pickling processes on the microstructure and properties of electroless Ni-P coating on Mg-7.5Li-2Zn-1Y alloy. Prog. Nat. Sci. Mater. Int. 2014, 24, 655–662. [Google Scholar] [CrossRef]
- Pommiers-Belin, S.; Frayret, J.; Uhart, A.; Ledeuil, J.; Dupin, J.-C.; Castetbon, A.; Potin-Gautier, M. Determination of the chemical mechanism of chromate conversion coating on magnesium alloys EV31A. Appl. Surf. Sci. 2014, 298, 199–207. [Google Scholar] [CrossRef]
- Jian, S.Y.; Chu, Y.R.; Lin, C.S. Permanganate conversion coating on AZ31 magnesium alloys with enhanced corrosion resistance. Corros. Sci. 2015, 93, 301–309. [Google Scholar] [CrossRef]
- Chai, Z.; Zhu, C.J.K.; Zhao, Y.; Wang, C.; Cai, F.; Chen, M.; Wang, L. Pretreatment behaviors and improved corrosion resistance for Cu/Co-Ni-Cu coating electrodeposition on Magnesium alloy. J. Electrochem. Soc. 2016, 163, D493–D499. [Google Scholar] [CrossRef]
- Xie, Z.H.; Chen, F.; Xiang, S.R.; Zhou, J.L.; Song, Z.W.; Yu, G. Studies of several pickling and activation processes for electroless Ni-P plating on AZ31 magnesium alloy. J. Electrochem. Soc. 2015, 162, D115–D123. [Google Scholar] [CrossRef]
- Lei, X.; Yu, G.; Gao, X.; Ye, L.; Zhang, J.; Hu, B. A study of chromium-free pickling process before electroless Ni–P plating on magnesium alloys. Surf. Coat. Technol. 2011, 205, 4058–4063. [Google Scholar] [CrossRef]
- Arthanari, S.; Nallaiyan, R.; Kwang Seon, S. Electrochemical corrosion behavior of acid treated strip cast AM50 and AZX310 magnesium alloys in 3.5 wt.% NaCl solution. J. Magnes. Alloy. 2017, 5, 277–285. [Google Scholar] [CrossRef]
- Huang, Y.H.; Lee, Y.L.; Lin, C.S. Acid pickling pretreatment and stannate conversion coating treatment of AZ91D magnesium alloy. J. Electrochem. Soc. 2011, 158, C310–C317. [Google Scholar] [CrossRef]
- Li, J.z.; Huang, J.g.; Tian, Y.w.; Liu, C.s. Corrosion action and passivation mechanism of magnesium alloy in fluoride solution. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2009, 19, 50–54. [Google Scholar] [CrossRef]
- Okulov, I.V.; Lamaka, S.V.; Wada, T.; Yubuta, K.; Zheludkevich, M.L.; Weissmüller, J.; Markmann, J.; Kato, H. Nanoporous magnesium. Nano Res. 2018, 11, 6428–6435. [Google Scholar] [CrossRef]
- Casanova, P.; Jaimes, K.; Parada, N.; Hernández-Barrios, C.; Aparicio, M.; Viejo, F.; Coy, A. Synthesis and evaluation of MgF2 coatings by chemical conversion on magnesium alloys for producing biodegradable orthopedic implants of temporary use. In Journal of Physics Conference Series; IOP Publishing: Bristol, UK, 2013; p. 012003. [Google Scholar]
- Lee, Y.; Chen, F.; Lin, C. Corrosion resistance studies of cerium conversion coating with a fluoride-free pretreatment on AZ91D magnesium alloy. J. Electrochem. Soc. 2012, 160, C28. [Google Scholar] [CrossRef]
- Durán, K.; Hernández-Barrios, C.; Coy, A.; Viejo, F. Effect of fluoride conversion pretreatment time and the microstructure on the corrosion performance of TEOS-GPTMS sol–gel coatings deposited on the WE54 magnesium alloy. J. Mater. Res. Technol. 2021, 15, 4220–4242. [Google Scholar] [CrossRef]
- Barajas, J.; Joya, J.; Durán, K.; Hernández-Barrios, C.; Coy, A.; Viejo, F. Relationship between microstructure and formation-biodegradation mechanism of fluoride conversion coatings synthesised on the AZ31 magnesium alloy. Surf. Coat. Technol. 2019, 374, 424–436. [Google Scholar] [CrossRef]
- Yuan, J.; Li, P.; Yuan, R.; Mao, D.; Zhao, S.; Feng, T. Influence of pickling time on electroless Ni–P coating on magnesium alloy. Mater. Corros. 2021, 72, 642–651. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, W. The effect of substrate on the electroless nickel plating of Mg and Mg alloys. Surf. Coat. Technol. 2006, 200, 3553–3560. [Google Scholar] [CrossRef]
- Shao, Z.; Cai, Z.; Hu, R.; Wei, S. The study of electroless nickel plating directly on magnesium alloy. Surf. Coat. Technol. 2014, 249, 42–47. [Google Scholar] [CrossRef]
- Liu, X.-K.; Liu, Z.-L.; Ping, L.; Xiang, Y.-H.; Hu, W.-B.; Ding, W.-J. Properties of fluoride film and its effect on electroless nickel deposition on magnesium alloys. Trans. Nonferrous Met. Soc. China 2010, 20, 2185–2191. [Google Scholar] [CrossRef]
- Zuleta, A.A.; Correa, E.; Sepúlveda, M.; Guerra, L.; Castaño, J.G.; Echeverría, F.; Skeldon, P.; Thompson, G.E. Effect of NH4HF2 on deposition of alkaline electroless Ni–P coatings as a chromium-free pre-treatment for magnesium. Corros. Sci. 2012, 55, 194–200. [Google Scholar] [CrossRef]
- Chen, X.B.; Easton, M.A.; Birbilis, N.; Yang, H.Y.; Abbott, T.B. Corrosion-resistant electrochemical plating of magnesium (Mg) alloys. In Corrosion Prevention of Magnesium Alloys; Song, G.-L., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 315–346. [Google Scholar] [CrossRef]
- da Conceicao, T.F.; Scharnagl, N.; Blawert, C.; Dietzel, W.; Kainer, K.U. Surface modification of magnesium alloy AZ31 by hydrofluoric acid treatment and its effect on the corrosion behaviour. Thin Solid Film. 2010, 518, 5209–5218. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Shizhao, S.; Chen, M.; Fahlman, B.D.; Liu, D.; Bi, H. In vitro and in vivo corrosion, mechanical properties and biocompatibility evaluation of MgF2-coated Mg-Zn-Zr alloy as cancellous screws. Mater. Sci. Eng. C 2017, 75, 1268–1280. [Google Scholar] [CrossRef]
- Mao, L.; Shen, L.; Chen, J.; Wu, Y.; Kwak, M.; Lu, Y.; Xue, Q.; Pei, J.; Zhang, L.; Yuan, G. Enhanced bioactivity of Mg–Nd–Zn–Zr alloy achieved with nanoscale MgF2 surface for vascular stent application. ACS Appl. Mater. Interfaces 2015, 7, 5320–5330. [Google Scholar] [CrossRef]
- Snihirova, D.; Wang, L.; Lamaka, S.V.; Wang, C.; Deng, M.; Vaghefinazari, B.; Hoche, D.; Zheludkevich, M.L. Synergistic Mixture of Electrolyte Additives: A Route to a High-Efficiency Mg-Air Battery. J. Phys. Chem. Lett. 2020, 11, 8790–8798. [Google Scholar] [CrossRef]
- Mouls, B.; Arurault, L.; Taberna, P.L.; Bonningue, C. Macroscopic, thermodynamic, kinetic and microscopic study of nitric acid pickling of Elektron 21 (EV31A) magnesium alloy. J. Magnes. Alloy. 2014, 2, 363–376. [Google Scholar] [CrossRef]
- Wang, L.; Snihirova, D.; Deng, M.; Vaghefinazari, B.; Höche, D.; Lamaka, S.V.; Zheludkevich, M.L. Revealing physical interpretation of time constants in electrochemical impedance spectra of Mg via Tribo-EIS measurements. Electrochim. Acta 2022, 404, 139582. [Google Scholar] [CrossRef]
- Rahim, S.A.; Joseph, M.A.; Hanas, T. Tailoring biomineralization and biodegradation of Mg-Ca alloy by acetic acid pickling. Mater. Res. Express 2020, 7, 054002. [Google Scholar] [CrossRef]
- da Conceicao, T.F.; Scharnagl, N.; Dietzel, W.; Hoeche, D.; Kainer, K.U. Study on the interface of PVDF coatings and HF-treated AZ31 magnesium alloy: Determination of interfacial interactions and reactions with self-healing properties. Corros. Sci. 2011, 53, 712–719. [Google Scholar] [CrossRef]
- Vander Voort, G.F.; AIH Committee. ASM Handbook; ASM International: Almere, The Netherlands, 2004. [Google Scholar]
- ASTM D1732-03 Standard Practices for Preparation of Magnesium Alloy Surfaces for Painting. 2018. Available online: https://www.astm.org/Standards/D1732.htm (accessed on 1 February 2019).
- Lamaka, S.V.; Höche, D.; Petrauskas, R.P.; Blawert, C.; Zheludkevich, M.L. A new concept for corrosion inhibition of magnesium: Suppression of iron re-deposition. Electrochem. Commun. 2016, 62, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.; Yu, B.; Zhang, B.; Zhou, P.; Zhang, T.; Wang, F. Chemically depleting the noble impurities from AZ91-T4 magnesium alloy: A new and efficient pretreatment method to improve the corrosion resistance of phosphate conversion coatings. Corros. Sci. 2021, 191, 109725. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Vaghefinazari, B.; Mei, D.; Petrauskas, R.P.; Hoche, D.; Zheludkevich, M.L. Comprehensive screening of Mg corrosion inhibitors. Corros. Sci. 2017, 128, 224–240. [Google Scholar] [CrossRef]
- Raman, R.K.S.; Birbilis, N.; Efthimiadis, J. Corrosion of Mg alloy AZ91—The role of microstructure. Corros. Eng. Sci. Technol. 2004, 39, 346–350. [Google Scholar] [CrossRef]
- Zhao, M.C.; Liu, M.; Song, G.; Atrens, A. Influence of the β-phase morphology on the corrosion of the Mg alloy AZ91. Corros. Sci. 2008, 50, 1939–1953. [Google Scholar] [CrossRef]
- Maddela, S.; O’Keefe, M.J.; Wang, V.C.M.; Kuo, H.H. Influence of surface pretreatment on coating morphology and corrosion performance of ceriumbased conversion coatings on AZ91D alloy. Corrosion 2010, 66. [Google Scholar] [CrossRef]
- Yang, H.; Guo, X.; Chen, X.; Birbilis, N. A homogenisation pre-treatment for adherent and corrosion-resistant Ni electroplated coatings on Mg-alloy AZ91D. Corros. Sci. 2014, 79, 41–49. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Snihirova, D.; Wang, C.; Wang, L.; Deng, M.; Höche, D.; Lamaka, S.V.; Zheludkevich, M.L. Exploring the effect of sodium salt of Ethylenediaminetetraacetic acid as an electrolyte additive on electrochemical behavior of a commercially pure Mg in primary Mg-air batteries. J. Power Sources 2022, 527, 231176. [Google Scholar] [CrossRef]
- Hanas, T.; Sampath Kumar, T.S.; Perumal, G.; Doble, M. Tailoring degradation of AZ31 alloy by surface pre-treatment and electrospun PCL fibrous coating. Mater. Sci. Eng. C 2016, 65, 43–50. [Google Scholar] [CrossRef]
- Rahim, S.A.; Muhammad Rabeeh, V.P.; Joseph, M.A.; Hanas, T. Does acid pickling of Mg-Ca alloy enhance biomineralization? J. Magnes. Alloys 2021, 9, 1028–1038. [Google Scholar] [CrossRef]
- Chen, X.-B.; Easton, M.A.; Birbilis, N.; Yang, H.-Y.; Abbott, T.B. Corrosion-resistant coatings for magnesium (Mg) alloys. In Corrosion Prevention of Magnesium Alloys; Song, G.-L., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 282–312. [Google Scholar]
- Saji, V.S.; Narayanan, T.S.; Chen, X. Conversion Coatings for Magnesium and Its Alloys; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Friedrich, H.E.; Mordike, B.L. Magnesium Technology; Springer: Berlin/Heidelberg, Germany, 2006; Volume 212. [Google Scholar]
- Saji, V.S. Organic conversion coatings for magnesium and its alloys. J. Ind. Eng. Chem. 2019, 75, 20–37. [Google Scholar] [CrossRef]
- Pommiers, S.; Frayret, J.; Castetbon, A.; Potin-Gautier, M. Alternative conversion coatings to chromate for the protection of magnesium alloys. Corros. Sci. 2014, 84, 135–146. [Google Scholar] [CrossRef]
- Chen, X.; Birbilis, N.; Abbott, T. Review of corrosion-resistant conversion coatings for magnesium and its alloys. Corrosion 2011, 67, 035005-1–035005-16. [Google Scholar] [CrossRef]
- Yang, Y.C.; Tsai, C.Y.; Huang, Y.H.; Lin, C.S. Formation mechanism and properties of titanate conversion coating on AZ31 magnesium alloy. J. Electrochem. Soc. 2012, 159, C226–C232. [Google Scholar] [CrossRef]
- Zhang, X.; van den Bos, C.; Sloof, W.G.; Hovestad, A.; Terryn, H.; de Wit, J.H.W. Comparison of the morphology and corrosion performance of Cr(VI)- and Cr(III)-based conversion coatings on zinc. Surf. Coat. Technol. 2005, 199, 92–104. [Google Scholar] [CrossRef]
- Wen, N.-T.; Lin, C.-S.; Bai, C.-Y.; Ger, M.-D. Structures and characteristics of Cr(III)-based conversion coatings on electrogalvanized steels. Surf. Coat. Technol. 2008, 203, 317–323. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Wen, N.-T.; Chen, W.-K.; Ger, M.-D.; Pan, G.-T.; Yang, T.C.-K. The effects of immersion time on morphology and electrochemical properties of the Cr(III)-based conversion coatings on zinc coated steel surface. Corros. Sci. 2008, 50, 3494–3499. [Google Scholar] [CrossRef]
- Qi, J.T.; Hashimoto, T.; Walton, J.R.; Zhou, X.; Skeldon, P.; Thompson, G.E. Trivalent chromium conversion coating formation on aluminium. Surf. Coat. Technol. 2015, 280, 317–329. [Google Scholar] [CrossRef]
- Chen, W.-K.; Bai, C.-Y.; Liu, C.-M.; Lin, C.-S.; Ger, M.-D. The effect of chromic sulfate concentration and immersion time on the structures and anticorrosive performance of the Cr(III) conversion coatings on aluminum alloys. Appl. Surf. Sci. 2010, 256, 4924–4929. [Google Scholar] [CrossRef]
- Kramer, K.; Salet, L.K. Trivalent Chromium-Containing Composition for Use in Corrosion Resistant Coatings on Metal Surfaces. U.S. Patent 9487866B2, 8 November 2016. [Google Scholar]
- Bhatia, P. Corrosion Resistant Trivalent Chromium Phosphated Chemical Conversion Coatings. European Patent EP1378585A1, 7 January 2004. [Google Scholar]
- Qi, J.; Ye, Z.; Gong, N.; Qu, X.; Mercier, D.; Światowska, J.; Skeldon, P.; Marcus, P. Formation of a trivalent chromium conversion coating on AZ91D magnesium alloy. Corros. Sci. 2021, 186, 109459. [Google Scholar] [CrossRef]
- Brady, M.P.; Leonard, D.N.; Meyer, H.M.; Thomson, J.K.; Unocic, K.A.; Elsentriecy, H.H.; Song, G.L.; Kitchen, K.; Davis, B. Advanced characterization study of commercial conversion and electrocoating structures on magnesium alloys AZ31B and ZE10A. Surf. Coat. Technol. 2016, 294, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Gu, C.; Tong, Y.; Yan, W.; Tu, J. A Smart Superhydrophobic Coating on AZ31B Magnesium Alloy with Self-Healing Effect. Adv. Mater. Interfaces 2016, 3, 1500694. [Google Scholar] [CrossRef]
- Matzdorf, C.A.; Nickerson, W.C.; Green, J.L. Process for Preparing Chromium Conversion Coatings for Magnesium Alloys. U.S. Patent 2010/0032060A1, 11 February 2010. [Google Scholar]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Van Phuong, N.; Lee, K.; Chang, D.; Kim, M.; Lee, S.; Moon, S. Zinc phosphate conversion coatings on magnesium alloys: A review. Met. Mater. Int. 2013, 19, 273–281. [Google Scholar] [CrossRef]
- Song, G.L. Corrosion Prevention of Magnesium Alloys; Elsevier Science: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Narayanan, T.S.N.S. Surface pretreatment by phosphate conversion coatings—A review. Rev. Adv. Mater. Sci. 2005, 9, 130–177. [Google Scholar]
- Díaz, B.; Freire, L.; Mojío, M.; Nóvoa, X.R. Optimization of conversion coatings based on zinc phosphate on high strength steels, with enhanced barrier properties. J. Electroanal. Chem. 2015, 737, 174–183. [Google Scholar] [CrossRef]
- Hafeez, M.A.; Farooq, A.; Zang, A.; Saleem, A.; Deen, K.M. Phosphate chemical conversion coatings for magnesium alloys: A review. J. Coat. Technol. Res. 2020, 17, 827–849. [Google Scholar] [CrossRef]
- ECHA. Information on Chemicals. Available online: https://echa.europa.eu/information-on-chemicals (accessed on 1 November 2018).
- ECHA. EP Supports Ban of Phosphates in Consumer Detergents. Available online: http://europa.eu/rapid/press-release_IP-11-1542_en.htm (accessed on 1 November 2018).
- Su, Y.; Guo, Y.; Huang, Z.; Zhang, Z.; Li, G.; Lian, J.; Ren, L. Preparation and corrosion behaviors of calcium phosphate conversion coating on magnesium alloy. Surf. Coat. Technol. 2016, 307, 99–108. [Google Scholar] [CrossRef]
- Li, K.; Wang, B.; Yan, B.; Lu, W. Microstructure in vitro corrosion and cytotoxicity of Ca-P coatings on ZK60 magnesium alloy prepared by simple chemical conversion and heat treatment. J. Biomater. Appl. 2013, 28, 375–384. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Xiao, G.Y.; Lu, Y.P. Phosphate chemical conversion coatings on metallic substrates for biomedical application: A review. Mater. Sci. Eng. C 2015, 47, 97–104. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Effect of [Ca2+] and [PO43−] levels on the formation of calcium phosphate conversion coatings on die-cast magnesium alloy AZ91D. Corros. Sci. 2012, 55, 226–232. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Magnesium in the murine artery: Probing the products of corrosion. Acta Biomater. 2014, 10, 1475–1483. [Google Scholar] [CrossRef]
- Zai, W.; Zhang, X.; Su, Y.; Man, H.C.; Li, G.; Lian, J. Comparison of corrosion resistance and biocompatibility of magnesium phosphate (MgP), zinc phosphate (ZnP) and calcium phosphate (CaP) conversion coatings on Mg alloy. Surf. Coat. Technol. 2020, 397, 125919. [Google Scholar] [CrossRef]
- Gao, J.; Su, Y.; Qin, Y.-X. Calcium phosphate coatings enhance biocompatibility and degradation resistance of magnesium alloy: Correlating in vitro and in vivo studies. Bioact. Mater. 2021, 6, 1223–1229. [Google Scholar] [CrossRef]
- Mao, L.; Zhu, H.; Chen, L.; Zhou, H.; Yuan, G.; Song, C. Enhancement of corrosion resistance and biocompatibility of Mg-Nd-Zn-Zr alloy achieved with phosphate coating for vascular stent application. J. Mater. Res. Technol. 2020, 9, 6409–6419. [Google Scholar] [CrossRef]
- Tomozawa, M.; Hiromoto, S. Microstructure of hydroxyapatite-and octacalcium phosphate-coatings formed on magnesium by a hydrothermal treatment at various pH values. Acta Mater. 2011, 59, 355–363. [Google Scholar] [CrossRef]
- Hiromoto, S.; Tomozawa, M. Hydroxyapatite coating of AZ31 magnesium alloy by a solution treatment and its corrosion behavior in NaCl solution. Surf. Coat. Technol. 2011, 205, 4711–4719. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Hu, Y.; Zhang, F.; Huang, Y.-D.; Wang, Z.-L.; Li, S.-Q.; Han, E.-H. Corrosion resistance of cerium-doped zinc calcium phosphate chemical conversion coatings on AZ31 magnesium alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 472–483. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.; Lan, Z.; Kong, L.; Huang, Y.; Cui, H. Characterization of calcium-modified zinc phosphate conversion coatings and their influences on corrosion resistance of AZ31 alloy. Surf. Coat. Technol. 2011, 205, 3347–3355. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Su, Y.; Zai, W.; Li, G.; Wen, C. In Vitro Degradation Behaviors of Manganese-Calcium Phosphate Coatings on an Mg-Ca-Zn Alloy. Scanning 2018, 2018, 6268579. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Li, G.; Lian, J. A chemical conversion hydroxyapatite coating on AZ60 magnesium alloy and its electrochemical corrosion behaviour. Int. J. Electrochem. Sci. 2012, 7, 11497–11511. [Google Scholar]
- Su, Y.; Lu, Y.; Su, Y.; Hu, J.; Lian, J.; Li, G. Enhancing the corrosion resistance and surface bioactivity of a calcium-phosphate coating on a biodegradable AZ60 magnesium alloy via a simple fluorine post-treatment method. Rsc Adv. 2015, 5, 56001–56010. [Google Scholar] [CrossRef]
- Cheng, Z.; Lian, J.; Hu, X.; Yang, X.; Li, G. Ca-P conversion coating on AZ60 magnesium alloy for biomedical application. Chem. Res. Chin. Univ. 2014, 30, 543–548. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. A simple route towards a hydroxyapatite–Mg(OH)2 conversion coating for magnesium. Corros. Sci. 2011, 53, 2263–2268. [Google Scholar] [CrossRef]
- You, M.; Echeverry-Rendón, M.; Zhang, L.; Niu, J.; Zhang, J.; Pei, J.; Yuan, G. Effects of composition and hierarchical structures of calcium phosphate coating on the corrosion resistance and osteoblast compatibility of Mg alloys. Mater. Sci. Eng. C 2021, 120, 111734. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, D.; Su, Y.; Lu, C.; Niu, L.; Lian, J.; Li, G. Improvement of the biodegradation property and biomineralization ability of magnesium–hydroxyapatite composites with dicalcium phosphate dihydrate and hydroxyapatite coatings. ACS Biomater. Sci. Eng. 2016, 2, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cai, S.; Shen, S.; Xu, G.; Li, Y.; Ling, R.; Wu, X. In-situ defect repairing in hydroxyapatite/phytic acid hybrid coatings on AZ31 magnesium alloy by hydrothermal treatment. J. Alloys Compd. 2016, 658, 649–656. [Google Scholar] [CrossRef]
- Dong, Q.; Zhou, X.; Feng, Y.; Qian, K.; Liu, H.; Lu, M.; Chu, C.; Xue, F.; Bai, J. Insights into self-healing behavior and mechanism of dicalcium phosphate dihydrate coating on biomedical Mg. Bioact. Mater. 2021, 6, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Luthringer, B.; Tang, S.; Hu, J.; Blawert, C.; Zheludkevich, M.L. Evolution and performance of a MgO/HA/DCPD gradient coating on pure magnesium. J. Alloys Compd. 2021, 883, 160793. [Google Scholar] [CrossRef]
- Yang, X.; Xie, B.; Wang, L.; Qin, Y.; Henneman, Z.J.; Nancollas, G.H. Influence of magnesium ions and amino acids on the nucleation and growth of hydroxyapatite. CrystEngComm 2011, 13, 1153–1158. [Google Scholar] [CrossRef]
- Ding, H.; Pan, H.; Xu, X.; Tang, R. Toward a detailed understanding of magnesium ions on hydroxyapatite crystallization inhibition. Cryst. Growth Des. 2014, 14, 763–769. [Google Scholar] [CrossRef]
- Hiromoto, S. Self-healing property of hydroxyapatite and octacalcium phosphate coatings on pure magnesium and magnesium alloy. Corros. Sci. 2015, 100, 284–294. [Google Scholar] [CrossRef]
- Dong, Q.; Dai, J.; Qian, K.; Liu, H.; Zhou, X.; Yao, Q.; Lu, M.; Chu, C.; Xue, F.; Bai, J. Dual self-healing inorganic-organic hybrid coating on biomedical Mg. Corros. Sci. 2022, 200, 110230. [Google Scholar] [CrossRef]
- Mei, D.; Lamaka, S.V.; Gonzalez, J.; Feyerabend, F.; Willumeit-Römer, R.; Zheludkevich, M.L. The role of individual components of simulated body fluid on the corrosion behavior of commercially pure Mg. Corros. Sci. 2019, 147, 81–93. [Google Scholar] [CrossRef]
- Mei, D.; Lamaka, S.V.; Lu, X.; Zheludkevich, M.L. Selecting medium for corrosion testing of bioabsorbable magnesium and other metals–a critical review. Corros. Sci. 2020, 171, 108722. [Google Scholar] [CrossRef]
- Kouisni, L.; Azzi, M.; Zertoubi, M.; Dalard, F.; Maximovitch, S. Phosphate coatings on magnesium alloy AM60 part 1: Study of the formation and the growth of zinc phosphate films. Surf. Coat. Technol. 2004, 185, 58–67. [Google Scholar] [CrossRef]
- Li, G.; Lian, J.; Niu, L.; Jiang, Z.; Jiang, Q. Growth of zinc phosphate coatings on AZ91D magnesium alloy. Surf. Coat. Technol. 2006, 201, 1814–1820. [Google Scholar] [CrossRef]
- Phuong, N.V.; Lee, K.H.; Chang, D.; Moon, S. Effects of Zn2+ concentration and pH on the zinc phosphate conversion coatings on AZ31 magnesium alloy. Corros. Sci. 2013, 74, 314–322. [Google Scholar] [CrossRef]
- Van Phuong, N.; Moon, S. Comparative corrosion study of zinc phosphate and magnesium phosphate conversion coatings on AZ31 Mg alloy. Mater. Lett. 2014, 122, 341–344. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Sun, X.-X.; Song, Y.-W.; Zhang, F.; Li, S.-Q.; Cui, H.-Z.; Han, E.-H. Influence of solution temperature on corrosion resistance of Zn-Ca phosphate conversion coating on biomedical Mg-Li-Ca alloys. Trans. Nonferrous Met. Soc. China 2013, 23, 3293–3299. [Google Scholar] [CrossRef]
- Yuan, J.; Yuan, R.; Wang, J.; Li, Q.; Xing, X.; Liu, X.; Hu, W. Fabrication and corrosion resistance of phosphate/ZnO multilayer protective coating on magnesium alloy. Surf. Coat. Technol. 2018, 352, 74–83. [Google Scholar] [CrossRef]
- Sun, X.; Susac, D.; Li, R.; Wong, K.C.; Foster, T.; Mitchell, K.A.R. Some observations for effects of copper on zinc phosphate conversion coatings on aluminum surfaces. Surf. Coat. Technol. 2002, 155, 46–50. [Google Scholar] [CrossRef]
- Niu, L.Y.; Jiang, Z.H.; Li, G.Y.; Gu, C.D.; Lian, J.S. A study and application of zinc phosphate coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2006, 200, 3021–3026. [Google Scholar] [CrossRef]
- Toong, D.W.Y.; Ng, J.C.K.; Huang, Y.; Wong, P.E.H.; Leo, H.L.; Venkatraman, S.S.; Ang, H.Y. Bioresorbable metals in cardiovascular stents: Material insights and progress. Materialia 2020, 12, 100727. [Google Scholar] [CrossRef]
- Chen, X.B.; Nisbet, D.R.; Li, R.W.; Smith, P.; Abbott, T.B.; Easton, M.A.; Zhang, D.-H.; Birbilis, N. Controlling initial biodegradation of magnesium by a biocompatible strontium phosphate conversion coating. Acta Biomater. 2014, 10, 1463–1474. [Google Scholar] [CrossRef]
- Wang, W.; Nune, K.C.; Tan, L.; Zhang, N.; Dong, J.; Yan, J.; Misra, R.D.K.; Yang, K. Bone regeneration of hollow tubular magnesium-strontium scaffolds in critical-size segmental defects: Effect of surface coatings. Mater. Sci. Eng. C 2019, 100, 297–307. [Google Scholar] [CrossRef]
- Chen, X.B.; Yang, H.Y.; Abbott, T.B.; Easton, M.A.; Birbilis, N. Corrosion protection of magnesium and its alloys by metal phosphate conversion coatings. Surf. Eng. 2014, 30, 871–879. [Google Scholar] [CrossRef]
- Amaravathy, P.; Kumar, T.S. Bioactivity enhancement by Sr doped Zn-Ca-P coatings on biomedical magnesium alloy. J. Magnes. Alloy. 2019, 7, 584–596. [Google Scholar] [CrossRef]
- Wang, J.; Jin, C.; Mei, D.; Ding, Y.; Chang, L.; Zhu, S.; Wang, L.; Feng, Y.; Guan, S. Synthesis and degradation behaviour of Zn-modified coating on Mg alloy. Surf. Eng. 2021, 37, 963–971. [Google Scholar] [CrossRef]
- Chong, K.Z.; Shih, T.S. Conversion-coating treatment for magnesium alloys by a permanganate-phosphate solution. Mater. Chem. Phys. 2003, 80, 191–200. [Google Scholar] [CrossRef]
- Hawke, D.; Albright, D.L. A phosphate-permanganate conversion coating for magnesium. Met. Finish. 1995, 93, 34, 36–38. [Google Scholar] [CrossRef]
- Lee, Y.L.; Chu, Y.R.; Li, W.C.; Lin, C.S. Effect of permanganate concentration on the formation and properties of phosphate/permanganate conversion coating on AZ31 magnesium alloy. Corros. Sci. 2013, 70, 74–81. [Google Scholar] [CrossRef]
- Lin, C.S.; Lee, C.Y.; Li, W.C.; Chen, Y.S.; Fang, G.N. Formation of phosphate/permanganate conversion coating on AZ31 magnesium alloy. J. Electrochem. Soc. 2006, 153, B90–B96. [Google Scholar] [CrossRef]
- Zucchi, F.; Frignani, A.; Grassi, V.; Trabanelli, G.; Monticelli, C. Stannate and permanganate conversion coatings on AZ31 magnesium alloy. Corros. Sci. 2007, 49, 4542–4552. [Google Scholar] [CrossRef]
- Amini, R.; Sarabi, A.A. The corrosion properties of phosphate coating on AZ31 magnesium alloy: The effect of sodium dodecyl sulfate (SDS) as an eco-friendly accelerating agent. Appl. Surf. Sci. 2011, 257, 7134–7139. [Google Scholar] [CrossRef]
- Maurya, R.; Siddiqui, A.R.; Balani, K. An environment-friendly phosphate chemical conversion coating on novel Mg-9Li-7Al-1Sn and Mg-9Li-5Al-3Sn-1Zn alloys with remarkable corrosion protection. Appl. Surf. Sci. 2018, 443, 429–440. [Google Scholar] [CrossRef]
- Mosiałek, M.; Mordarski, G.; Nowak, P.; Simka, W.; Nawrat, G.; Hanke, M.; Socha, R.P.; Michalska, J. Phosphate-permanganate conversion coatings on the AZ81 magnesium alloy: SEM, EIS and XPS studies. Surf. Coat. Technol. 2011, 206, 51–62. [Google Scholar] [CrossRef]
- Hamid, Z.A.; El-khair, M.T.A.; Hassan, H.B. Synthesis and protection of AM50 magnesium alloy and its composites using environmentally pretreatment electrolyte. Surf. Coat. Technol. 2011, 206, 1041–1050. [Google Scholar] [CrossRef]
- Song, Y.; Xu, Z.; Dong, K.; Shan, D.; Han, E.H. Investigation of microcracks on conversion film of AZ80 Mg alloy. Surf. Eng. 2018, 35, 527–535. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, B.; Hou, Y.; Duan, G.; Yang, L.; Zhang, B.; Zhang, T.; Wang, F. Revisiting the cracking of chemical conversion coating on magnesium alloys. Corros. Sci. 2021, 178, 109069. [Google Scholar] [CrossRef]
- Liao, S.; Yu, B.; Zhang, X.; Lu, X.; Zhou, P.; Zhang, C.; Chen, X.; Zhang, T.; Wang, F. New design principles for the bath towards chromate- and crack-free conversion coatings on magnesium alloys. J. Magnes. Alloy. 2021, 9, 505–519. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Zai, W.; Li, G.; Liu, S.; Lian, J.; Jiang, Z. Fabrication of Superhydrophobic Calcium Phosphate Coating on Mg-Zn-Ca alloy and Its Corrosion Resistance. J. Mater. Eng. Perform. 2017, 26, 6117–6129. [Google Scholar] [CrossRef]
- Sun, R.X.; Wang, P.F.; Zhao, D.D.; Sun, Z.Z.; Li, C.Q.; Chen, K.Z. An environment-friendly calcium phosphate conversion coating on AZ91D alloy and its corrosion resistance. Mater. Corros. 2015, 66, 383–386. [Google Scholar] [CrossRef]
- Zai, W.; Su, Y.; Man, H.C.; Lian, J.; Li, G. Effect of pH value and preparation temperature on the formation of magnesium phosphate conversion coatings on AZ31 magnesium alloy. Appl. Surf. Sci. 2019, 492, 314–327. [Google Scholar] [CrossRef]
- Li, G.Y.; Lian, J.S.; Niu, L.Y.; Jiang, Z.H. Influence of pH of phosphating bath on the zinc phosphate coating on AZ91D magnesium alloy. Adv. Eng. Mater. 2006, 8, 123–127. [Google Scholar] [CrossRef]
- Zhou, Y.; Xion, Q.Y.; Xiong, J.P.; He, J.Y. Effects of chelating agents on electrochemical behavior and microstructure of phosphate conversion coatings on magnesium alloy AZ91D. Int. J. Electrochem. Sci. 2014, 9, 5258–5271. [Google Scholar]
- Wang, G.; Cao, N.; Wang, Y. Characteristics and corrosion studies of zinc-manganese phosphate coatings on magnesium-lithium alloy. RSC Adv. 2014, 4, 59772–59778. [Google Scholar] [CrossRef]
- Cui, X.J.; Liu, C.H.; Yang, R.S.; Fu, Q.S.; Lin, X.Z.; Gong, M. Duplex-layered manganese phosphate conversion coating on AZ31 Mg alloy and its initial formation mechanism. Corros. Sci. 2013, 76, 474–485. [Google Scholar] [CrossRef]
- Li, Q.; Xu, S.; Hu, J.; Zhang, S.; Zhong, X.; Yang, X. The effects to the structure and electrochemical behavior of zinc phosphate conversion coatings with ethanolamine on magnesium alloy AZ91D. Electrochim. Acta 2010, 55, 887–894. [Google Scholar] [CrossRef]
- Shang, W.; He, C.; Wen, Y.; Wang, Y.; Zhang, Z. Performance evaluation of triethanolamine as corrosion inhibitor for magnesium alloy in 3.5 wt.% NaCl solution. RSC Adv. 2016, 6, 113967–113980. [Google Scholar] [CrossRef]
- Niu, L.Y.; Li, G.Y.; Jiang, Z.H.; Sun, L.P.; Han, D.; Lian, J.S. Influence of sodium metanitrobenzene sulphonate on structures and surface morphologies of phosphate coating on AZ91D. Trans. Nonferrous Met. Soc. China 2006, 16, 567–571. [Google Scholar] [CrossRef]
- Chunyan, Z.; Shangju, L.; Baoxing, Y.; Xiaopeng, L.; Xiao-Bo, C.; Tao, Z.; Fuhui, W. Ratio of total acidity to pH value of coating bath: A new strategy towards phosphate conversion coatings with optimized corrosion resistance for magnesium alloys. Corros. Sci. 2019, 150, 279–295. [Google Scholar] [CrossRef]
- Wu, Q.; Yu, B.; Zhou, P.; Zhang, T.; Wang, F. Fabrication of phosphate conversion coatings on rolled AZ31 magnesium alloy: Variation of corrosion resistance on different planes induced by the crystallographic texture. Mater. Chem. Phys. 2021, 273, 125121. [Google Scholar] [CrossRef]
- Rudd, A.L.; Breslin, C.B.; Mansfeld, F. The corrosion protection afforded by rare earth conversion coatings applied to magnesium. Corros. Sci. 2000, 42, 275–288. [Google Scholar] [CrossRef] [Green Version]
- Hughes, A.E.; Gorman, J.D.; Miller, P.R.; Sexton, B.A.; Paterson, P.J.K.; Taylor, R.J. Development of cerium-based conversion coatings on 2024-T3 Al alloy after rare-earth desmutting. Surf. Interface Anal. 2004, 36, 290–303. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Ren, X.; Xu, S.; Yin, L. Cerium-based chemical conversion coating on aluminum alloy to inhibits corrosion in chloride solution. Int. J. Electrochem. Sci. 2015, 10, 9073–9088. [Google Scholar]
- Valdez, B.; Kiyota, S.; Stoytcheva, M.; Zlatev, R.; Bastidas, J.M. Cerium-based conversion coatings to improve the corrosion resistance of aluminium alloy 6061-T6. Corros. Sci. 2014, 87, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Saji, V.S. Review of rare-earth-based conversion coatings for magnesium and its alloys. J. Mater. Res. Technol. 2019, 8, 5012–5035. [Google Scholar] [CrossRef]
- Dabalà, M.; Brunelli, K.; Napolitani, E.; Magrini, M. Cerium-based chemical conversion coating on AZ63 magnesium alloy. Surf. Coat. Technol. 2003, 172, 227–232. [Google Scholar] [CrossRef]
- Uesaka, N.; Shikai, Y.; Takenaka, T. Influence of Conversion Coating Conditions with Rare Earth Elements on Corrosion Resistance of Mg Alloys. ECS Trans. 2009, 16, 39. [Google Scholar] [CrossRef]
- Takenaka, T.; Ono, T.; Narazaki, Y.; Naka, Y.; Kawakami, M. Improvement of corrosion resistance of magnesium metal by rare earth elements. Electrochim. Acta 2007, 53, 117–121. [Google Scholar] [CrossRef]
- Han, B.; Gu, D.; Yang, Y.; Fang, L.; Peng, G.; Yang, C. Preparation ofyttrium-based rare earth conversion coating and its effecton corrosion resistance of AZ91D magnesium alloy. Int. J. Electrochem. Sci. 2017, 12, 374–385. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.C.; Wu, J.F.; Liang, Y.Q.; Ye, S.L.; Li, W.F. Preparation of cerium oxide based environment-friendly chemical conversion coating on magnesium alloy with additives. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2011, 21, 1905–1910. [Google Scholar] [CrossRef]
- Loperena, A.P.; Lehr, I.L.; Saidman, S.B. Formation of a cerium conversion coating on magnesium alloy using ascorbic acid as additive. Characterisation and anticorrosive properties of the formed films. J. Magnes. Alloy. 2016, 4, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Jin, G.; Yang, Y.; Cui, X.; Liu, E.; Wang, Z.; Li, Q. Chrome-free neodymium-based protective coatings for magnesium alloys. Mater. Lett. 2011, 65, 1145–1147. [Google Scholar] [CrossRef]
- Cui, X.; Liu, Z.; Lin, L.; Jin, G.; Wang, H.; Xu, B. Investigation of Carboxylic Acid-Neodymium Conversion Films on Magnesium Alloy. J. Mater. Eng. Perform. 2015, 24, 461–467. [Google Scholar] [CrossRef]
- Saket, M.; Amini, R.; Kardar, P.; Ganjaee, M. The chemical treatment of the AZ31-Magnesium alloy surface by a high-performance corrosion protective praseodymium (III)-based film. Mater. Chem. Phys. 2021, 260, 124113. [Google Scholar] [CrossRef]
- Seifzadeh, D.; Haghighat, A.G. Formation of rare earth-permanganate conversion coating on AZ61 magnesium alloy and its properties. Indian J. Chem. Technol. 2013, 20, 210–216. [Google Scholar]
- Zheng, R.F.; Liang, C.H. Conversion coating treatment for AZ91 magnesium alloys by a permanganate-REMS bath. Mater. Corros. 2007, 58, 193–197. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiong, J.; Yan, F. The preparation and characterization of a nano-CeO2/phosphate composite coating on magnesium alloy AZ91D. Surf. Coat. Technol. 2017, 328, 335–343. [Google Scholar] [CrossRef]
- Mu, S.; Du, J.; Jiang, H.; Li, W. Composition analysis and corrosion performance of a Mo–Ce conversion coating on AZ91 magnesium alloy. Surf. Coat. Technol. 2014, 254, 364–370. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Lin, C.; Zhang, M.; Wu, J. Study of molybdenum/lanthanum-based composite conversion coatings on AZ31 magnesium alloy. Appl. Surf. Sci. 2011, 257, 2838–2842. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, R.; Jiang, S. Microstructure and corrosion resistance of Ce–V conversion coating on AZ31 magnesium alloy. Appl. Surf. Sci. 2015, 341, 166–174. [Google Scholar] [CrossRef]
- Jian, S.-Y.; Tzeng, Y.-C.; Ger, M.-D.; Chang, K.-L.; Shi, G.-N.; Huang, W.-H.; Chen, C.-Y.; Wu, C.-C. The study of corrosion behavior of manganese-based conversion coating on LZ91 magnesium alloy: Effect of addition of pyrophosphate and cerium. Mater. Des. 2020, 192, 108707. [Google Scholar] [CrossRef]
- Lei, L.; Wang, X.; Liu, W.; Tang, Q. Surface evaluation and electrochemical behavior of cerium conversion coating modified with silane on magnesium alloy. Surf. Interface Anal. 2015, 47, 466–473. [Google Scholar] [CrossRef]
- Song, J.; Cui, X.; Jin, G.; Cai, Z.; Liu, E.; Li, X.; Chen, Y.; Lu, B. Self-healing conversion coating with gelatin–chitosan microcapsules containing inhibitor on AZ91D alloy. Surf. Eng. 2018, 34, 79–84. [Google Scholar] [CrossRef]
- Song, J.; Cui, X.; Liu, Z.; Jin, G.; Liu, E.; Zhang, D.; Gao, Z. Advanced microcapsules for self-healing conversion coating on magnesium alloy in Ce(NO3)3 solution with microcapsules containing La(NO3)3. Surf. Coat. Technol. 2016, 307, 500–505. [Google Scholar] [CrossRef]
- Arthanari, S.; Shin, K.S. A simple one step cerium conversion coating formation on to magnesium alloy and electrochemical corrosion performance. Surf. Coat. Technol. 2018, 349, 757–772. [Google Scholar] [CrossRef]
- Castano, C.E.; Maddela, S.; O’Keefe, M.J.; Wang, Y.-M. Effect of Thickness on the Morphology and Corrosion Behavior of Cerium-Based Conversion Coatings on AZ31B Magnesium Alloy. In Magnesium Technology 2012; Mathaudhu, S.N., Sillekens, W.H., Neelameggham, N.R., Hort, N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 247–251. [Google Scholar]
- Gao, H.F.; Tan, H.Q.; Li, J.; Wang, Y.Q.; Xun, J.Q. Synergistic effect of cerium conversion coating and phytic acid conversion coating on AZ31B magnesium alloy. Surf. Coat. Technol. 2012, 212, 32–36. [Google Scholar] [CrossRef]
- Ou, J.; Chen, X. Corrosion resistance of phytic acid/Ce (III) nanocomposite coating with superhydrophobicity on magnesium. J. Alloys Compd. 2019, 787, 145–151. [Google Scholar] [CrossRef]
- Castano, C.E.; O’Keefe, M.J.; Fahrenholtz, W.G. Microstructural evolution of cerium-based coatings on AZ31 magnesium alloys. Surf. Coat. Technol. 2014, 246, 77–84. [Google Scholar] [CrossRef]
- Li, L.; Lei, J.; Yu, S.; Tian, Y.; Jiang, Q.; Pan, F. Formation and characterization of cerium conversion coatings on magnesium alloy. J. Rare Earths 2008, 26, 383–387. [Google Scholar] [CrossRef]
- Sun, J.; Wang, G. Preparation and characterization of a cerium conversion film on magnesium alloy. Anti-Corros. Methods Mater. 2015, 62, 253–258. [Google Scholar] [CrossRef]
- Jamali, S.S.; Moulton, S.E.; Tallman, D.E.; Zhao, Y.; Weber, J.; Wallace, G.G. Self-healing characteristic of praseodymium conversion coating on AZNd Mg alloy studied by scanning electrochemical microscopy. Electrochem. Commun. 2017, 76, 6–9. [Google Scholar] [CrossRef]
- Cui, X.; Yang, Y.; Liu, E.; Jin, G.; Zhong, J.; Li, Q. Corrosion behaviors in physiological solution of cerium conversion coatings on AZ31 magnesium alloy. Appl. Surf. Sci. 2011, 257, 9703–9709. [Google Scholar] [CrossRef]
- Scholes, F.; Soste, C.; Hughes, A.; Hardin, S.; Curtis, P. The role of hydrogen peroxide in the deposition of cerium-based conversion coatings. Appl. Surf. Sci. 2006, 253, 1770–1780. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; Liu, X.; Xie, H.; Liu, F.; Lu, C.; Li, J. Designing for the cerium-based conversion coating with excellent corrosion resistance on Mg–4Y–2Al magnesium alloy. Mater. Corros. 2021, 72, 925–935. [Google Scholar] [CrossRef]
- Yang, X.; Wang, G.; Dong, G.; Gong, F.; Zhang, M. Rare earth conversion coating on Mg–8.5 Li alloys. J. Alloys Compd. 2009, 487, 64–68. [Google Scholar] [CrossRef]
- Ribeiro, E.P.; Couto, A.A.; Oliveira, L.A.d.; Antunes, R.A. Influence of the treatment time on the surface chemistry and corrosion behavior of cerium-based conversion coatings on the AZ91D magnesium alloy. Mater. Res. 2020, 22. [Google Scholar] [CrossRef] [Green Version]
- Kamde, M.A.; Mahton, Y.; Ohodnicki, J.; Roy, M.; Saha, P. Effect of cerium-based conversion coating on corrosion behavior of squeeze cast Mg-4 wt.% Y alloy in 0.1 M NaCl solution. Surf. Coat. Technol. 2021, 421, 127451. [Google Scholar] [CrossRef]
- Mahidashti, Z.; Shahrabi, T.; Ramezanzadeh, B. The role of post-treatment of an ecofriendly cerium nanostructure Conversion coating by green corrosion inhibitor on the adhesion and corrosion protection properties of the epoxy coating. Prog. Org. Coat. 2018, 114, 19–32. [Google Scholar] [CrossRef]
- Saei, E.; Ramezanzadeh, B.; Amini, R.; Kalajahi, M.S. Effects of combined organic and inorganic corrosion inhibitors on the nanostructure cerium based conversion coating performance on AZ31 magnesium alloy: Morphological and corrosion studies. Corros. Sci. 2017, 127, 186–200. [Google Scholar] [CrossRef]
- Nabizadeh, M.; Sarabi, A.A.; Eivaz Mohammadloo, H. Comparative investigation of Cu ion and adipic acid addition on electrochemical and microstructure characteristics of vanadium conversion coating on AZ31 Mg alloy. Surf. Coat. Technol. 2019, 357, 1–11. [Google Scholar] [CrossRef]
- Hamdy, A.S.; Doench, I.; Möhwald, H. Vanadia-based coatings of self-repairing functionality for advanced magnesium Elektron ZE41 Mg–Zn–rare earth alloy. Surf. Coat. Technol. 2012, 206, 3686–3692. [Google Scholar] [CrossRef]
- Hamdy, A.S.; Doench, I.; Möhwald, H. Assessment of a one-step intelligent self-healing vanadia protective coatings for magnesium alloys in corrosive media. Electrochim. Acta 2011, 56, 2493–2502. [Google Scholar] [CrossRef]
- Yang, K.H.; Ger, M.D.; Hwu, W.H.; Sung, Y.; Liu, Y.C. Study of vanadium-based chemical conversion coating on the corrosion resistance of magnesium alloy. Mater. Chem. Phys. 2007, 101, 480–485. [Google Scholar] [CrossRef]
- Ger, M.D.; Yang, K.H.; Hwu, W.H.; Liu, Y.C. Method for Treating Magnesium Alloy by Chemical Conversion. U.S. Patent 6,755,918B2, 29 June 2004. [Google Scholar]
- Sato, T.; Suzuki, M. Chromium-Free Solution for Treating Metal Surfaces. U.S. Patent 8980016B2, 17 March 2015. [Google Scholar]
- Li, K.; Liu, J.; Lei, T.; Xiao, T. Optimization of process factors for self-healing vanadium-based conversion coating on AZ31 magnesium alloy. Appl. Surf. Sci. 2015, 353, 811–819. [Google Scholar] [CrossRef]
- SigmaAldrich. SDS, Sodium metavanadate, CAS-No. 13718-26-8. 2017. [Google Scholar]
- Zhao, C.M.; Yao, Y.W.; Zhou, Y. Study of formation of molybdate conversion coatings on AZ31 magnesium alloy. Trans. IMF 2013, 91, 330–335. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Zhao, C.; Han, Y.; Zhao, C. Preparation and Corrosion Resistance Property of Molybdate Conversion Coatings Containing SiO2 Nanoparticles. J. Electrochem. Soc. 2013, 160, C185–C188. [Google Scholar] [CrossRef]
- Yong, Z.; Zhu, J.; Qiu, C.; Liu, Y. Molybdate/phosphate composite conversion coating on magnesium alloy surface for corrosion protection. Appl. Surf. Sci. 2008, 255, 1672–1680. [Google Scholar] [CrossRef]
- Li, L.; Lei, L. Inhibition of molybdate salt on dissolution of magnesium alloys in phosphoric acid. Surf. Technol. 2007, 36, 16–18. [Google Scholar]
- Yang, L.H.; Zhang, M.L.; Lin, C.G.; Wu, J.H. Effect of additives on structure and corrosion resistance of molybdate conversion coatings deposited on AZ31B Mg alloy. Adv. Mater. Res. 2011, 308–310, 2458–2462. [Google Scholar] [CrossRef]
- Lee, Y.L.; Chu, Y.R.; Chen, F.J.; Lin, C.S. Mechanism of the formation of stannate and cerium conversion coatings on AZ91D magnesium alloys. Appl. Surf. Sci. 2013, 276, 578–585. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, M.; Li, J.; Yu, X.; Niu, Z. Stannate conversion coatings on Mg–8Li alloy. J. Alloys Compd. 2009, 471, 197–200. [Google Scholar] [CrossRef]
- Elsentriecy, H.H.; Azumi, K.; Konno, H. Improvement in stannate chemical conversion coatings on AZ91 D magnesium alloy using the potentiostatic technique. Electrochim. Acta 2007, 53, 1006–1012. [Google Scholar] [CrossRef]
- Makhlouf, A.S.H. Smart stannate-based self-healing coatings for corrosion protection of magnesium alloys. In Handbook of Smart Coatings for Materials Protection; Makhlouf, A.S.H., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 275–286. [Google Scholar] [CrossRef]
- Hamdy, A.S. The effect of surface modification and stannate concentration on the corrosion protection performance of magnesium alloys. Surf. Coat. Technol. 2008, 203, 240–249. [Google Scholar] [CrossRef]
- Feng, Z.; Hurley, B.; Zhu, M.; Yang, Z.; Hwang, J.; Buchheit, R. Corrosion Inhibition of AZ31 Mg Alloy by Aqueous Selenite (SeO32−). J. Electrochem. Soc. 2019, 166, C520. [Google Scholar] [CrossRef]
- Dunstan, B.G.; Dunstan, W.L. Process for Protecting Magnesium and Its Alloys against Corrosion. U.S. Patent 1961030A, 29 May 1934. [Google Scholar]
- Saranya, K.; Kalaiyarasan, M.; Rajendran, N. Selenium conversion coating on AZ31 Mg alloy: A solution for improved corrosion rate and enhanced bio-adaptability. Surf. Coat. Technol. 2019, 378, 124902. [Google Scholar] [CrossRef]
- Feng, Z.; Xu, C.C.; Zhang, D.; Buchheit, R. Corrosion Protective Film Formation on Mg Alloy AZ31 by Exposure to Dilute Selenite Solutions. Materials 2021, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.D.; Corvino, B.S.; Committee, A.I.H. ASM Handbook: Fundamentals, Testing, and Protection. Corrosion; ASM International: Almere, The Netherlands, 2003; Volume 13A. [Google Scholar]
- Spallholz, J.E. On the nature of selenium toxicity and carcinostatic activity. Free Radic. Biol. Med. 1994, 17, 45–64. [Google Scholar] [CrossRef]
- Whanger, P.; Vendeland, S.; Park, Y.-C.; Xia, Y. Metabolism of subtoxic levels of selenium in animals and humans. Ann. Clin. Lab. Sci. 1996, 26, 99–113. [Google Scholar]
- Navarro-Alarcon, M.; López-Martınez, M. Essentiality of selenium in the human body: Relationship with different diseases. Sci. Total Environ. 2000, 249, 347–371. [Google Scholar] [CrossRef]
- Murugan, N.; Kavitha, L.; Shinyjoy, E.; Rajeswari, D.; Vimala, K.; Kannan, S.; Gopi, D. Smart rose flower like bioceramic/metal oxide dual layer coating with enhanced anti-bacterial, anti-cancer, anti-corrosive and biocompatible properties for improved orthopedic applications. RSC Adv. 2015, 5, 85831–85844. [Google Scholar] [CrossRef]
- Rojaee, R.; Fathi, M.; Raeissi, K. Electrophoretic deposition of nanostructured hydroxyapatite coating on AZ91 magnesium alloy implants with different surface treatments. Appl. Surf. Sci. 2013, 285, 664–673. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.; Idris, M.; Abdul-Kadir, M. Synthesis and in vitro degradation evaluation of the nano-HA/MgF2 and DCPD/MgF2 composite coating on biodegradable Mg–Ca–Zn alloy. Surf. Coat. Technol. 2013, 222, 79–89. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Sun, D.; Lin, J.; Meng, F.; Liu, H. Superhydrophobic fluoride conversion coating on bioresorbable magnesium alloy—Fabrication, characterization, degradation and cytocompatibility with BMSCs. J. Magnes. Alloy. 2021, 9, 1246–1260. [Google Scholar] [CrossRef]
- Zhang, L.; Mohammed, E.A.; Adriaens, A. Synthesis and electrochemical behavior of a magnesium fluoride-polydopamine-stearic acid composite coating on AZ31 magnesium alloy. Surf. Coat. Technol. 2016, 307, 56–64. [Google Scholar] [CrossRef]
- Ravichandran, K.; Tsn, S.N. Controlling the rate of degradation of Mg using magnesium fluoride and magnesium fluoride-magnesium phosphate duplex coatings. J. Magnes. Alloy. 2022, 10, 295–312. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Luo, X.; Xiong, P.; Gao, S.; Yan, J.; Li, Y.; Cheng, Y.; Xi, T. A tannic acid-modified fluoride pre-treated Mg–Zn–Y–Nd alloy with antioxidant and platelet-repellent functionalities for vascular stent application. J. Mater. Chem. B 2019, 7, 7314–7325. [Google Scholar] [CrossRef] [PubMed]
- Hernández-López, J.M.; Němcová, A.; Zhong, X.L.; Liu, H.; Arenas, M.A.; Haigh, S.J.; Burke, M.G.; Skeldon, P.; Thompson, G.E. Formation of barrier-type anodic films on ZE41 magnesium alloy in a fluoride/glycerol electrolyte. Electrochim. Acta 2014, 138, 124–131. [Google Scholar] [CrossRef]
- Němcová, A.; Kuběna, I.; Šmíd, M.; Habazaki, H.; Skeldon, P.; Thompson, G.E. Effect of current density and behaviour of second phases in anodizing of a Mg-Zn-RE alloy in a fluoride/glycerol/water electrolyte. J. Solid State Electrochem. 2016, 20, 1155–1165. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Wong, M.H.; Cheng, F.T.; Man, H.C. Characterization and corrosion studies of fluoride conversion coating on degradable Mg implants. Surf. Coat. Technol. 2007, 202, 590–598. [Google Scholar] [CrossRef]
- Gulbrandsen, E.; Taftø, J.; Olsen, A. The passive behaviour of Mg in alkaline fluoride solutions. Electrochemical and electron microscopical investigations. Corros. Sci. 1993, 34, 1423–1440. [Google Scholar] [CrossRef]
- Pereda, M.D.; Alonso, C.; Burgos-Asperilla, L.; del Valle, J.A.; Ruano, O.A.; Perez, P.; Fernández Lorenzo de Mele, M.A. Corrosion inhibition of powder metallurgy Mg by fluoride treatments. Acta Biomater. 2010, 6, 1772–1782. [Google Scholar] [CrossRef]
- Li, Q.; Zhong, X.; Hu, J.; Kang, W. Preparation and corrosion resistance studies of zirconia coating on fluorinated AZ91D magnesium alloy. Prog. Org. Coat. 2008, 63, 222–227. [Google Scholar] [CrossRef]
- Drynda, A.; Hassel, T.; Hoehn, R.; Perz, A.; Bach, F.-W.; Peuster, M. Development and biocompatibility of a novel corrodible fluoride-coated magnesium-calcium alloy with improved degradation kinetics and adequate mechanical properties for cardiovascular applications. J. Biomed. Mater. Res. Part A 2010, 93A, 763–775. [Google Scholar] [CrossRef]
- PARK, H.-Y. Environmentally Friendly Conversion Treatment Method of Magnesium Material, and Magnesium Material Prepared Thereby. European Patent WO2013157760, 3 April 2013. [Google Scholar]
- da Conceição, T.F.; Scharnagl, N. Fluoride conversion coatings for magnesium and its alloys for the biological environment. In Surface Modification of Magnesium and Its Alloys for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2015; pp. 3–21. [Google Scholar] [CrossRef]
- SigmaAldrich. Hydrofluoric Acid, CAS-No. 7664-39-3. 2017. Available online: https://www.spectrumchemical.com/cas/7664-39-3 (accessed on 1 April 2021).
- Milošev, I.; Frankel, G.S. Review—Conversion Coatings Based on Zirconium and/or Titanium. J. Electrochem. Soc. 2018, 165, C127–C144. [Google Scholar] [CrossRef]
- Cano-Iranzo, F.J.; Izagirre-Etxeberria, U.; Zubillaga-Alcorta, O.; Santa, C.M.P.; Lapena, N. Chromium-Free Conversion Coating. U.S. Patent 20130052352A1, 25 October 2016. [Google Scholar]
- Ardelean, H.; Frateur, I.; Marcus, P. Corrosion protection of magnesium alloys by cerium, zirconium and niobium-based conversion coatings. Corros. Sci. 2008, 50, 1907–1918. [Google Scholar] [CrossRef]
- Ardelean, H.; Marcus, P. Composition and Method for Treating Magnesium Alloys. U.S. Patent 7,156,905, 2 January 2007. [Google Scholar]
- Tomlinson, C.E. Chromate-Free Conversion Coatings for Metals. U.S. Patent 5759244A, 2 June 1998. [Google Scholar]
- Greene, J.A.; Vonk, D.R. Conversion Coatings including Alkaline Earth Metal Fluoride Complexes. European Patent WO2003093532A3, 3 June 2004. [Google Scholar]
- Dolan, S.E.; Carlson, L.R. Process for Coating Metal Surfaces. U.S. Patent 2004/0025973 A1, 13 February 2007. [Google Scholar]
- Verdier, S.; van der Laak, N.; Dalard, F.; Metson, J.; Delalande, S. An electrochemical and SEM study of the mechanism of formation, morphology, and composition of titanium or zirconium fluoride-based coatings. Surf. Coat. Technol. 2006, 200, 2955–2964. [Google Scholar] [CrossRef]
- Cerezo, J.; Vandendael, I.; Posner, R.; Lill, K.; de Wit, J.H.W.; Mol, J.M.C.; Terryn, H. Initiation and growth of modified Zr-based conversion coatings on multi-metal surfaces. Surf. Coat. Technol. 2013, 236, 284–289. [Google Scholar] [CrossRef]
- Verdier, S.; Delalande, S.; van der Laak, N.; Metson, J.; Dalard, F. Monochromatized x-ray photoelectron spectroscopy of the AM60 magnesium alloy surface after treatments in fluoride-based Ti and Zr solutions. Surf. Interface Anal. 2005, 37, 509–516. [Google Scholar] [CrossRef]
- Chen, X.; Li, G.; Lian, J.; Jiang, Q. An organic chromium-free conversion coating on AZ91D magnesium alloy. Appl. Surf. Sci. 2008, 255, 2322–2328. [Google Scholar] [CrossRef]
- Chen, X.; Li, G.; Lian, J.; Jiang, Q. Study of the formation and growth of tannic acid based conversion coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2009, 204, 736–747. [Google Scholar] [CrossRef]
- Yi, A.; Du, J.; Wang, J.; Mu, S.; Zhang, G.; Li, W. Preparation and characterization of colored Ti/Zr conversion coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2015, 276, 239–247. [Google Scholar] [CrossRef]
- Gou, J.; Sun, M.; Ma, X.; Tang, G.; Zhang, Y. Effects of temperature and pH value on the morphology and corrosion resistance of titanium-containing conversion coating. Appl. Surf. Sci. Adv. 2021, 3, 100060. [Google Scholar] [CrossRef]
- Reck, J.; Wang, Y.-M.; Kuo, H.-H.H. Development of Zirconium-based Conversion Coatings for the Pretreatment of AZ91D Magnesium Alloy Prior to Electrocoating. In Magnesium Technology 2011; Springer: Berlin/Heidelberg, Germany, 2011; pp. 523–529. [Google Scholar]
- Andreatta, F.; Turco, A.; de Graeve, I.; Terryn, H.; de Wit, J.H.W.; Fedrizzi, L. SKPFM and SEM study of the deposition mechanism of Zr/Ti based pre-treatment on AA6016 aluminum alloy. Surf. Coat. Technol. 2007, 201, 7668–7685. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Zhang, C.; Zhang, T.; Yu, B.; Meng, G.; Shao, Y.; Wang, F.; Liu, L. Protection of AA5083 by a zirconium-based conversion coating. J. Electrochem. Soc. 2016, 163, C576. [Google Scholar] [CrossRef]
- Eivaz Mohammadloo, H.; Sarabi, A.A.; Mohammad Hosseini, R.; Sarayloo, M.; Sameie, H.; Salimi, R. A comprehensive study of the green hexafluorozirconic acid-based conversion coating. Prog. Org. Coat. 2014, 77, 322–330. [Google Scholar] [CrossRef]
- Zhu, W.; Li, W.; Mu, S.; Fu, N.; Liao, Z. Comparative study on Ti/Zr/V and chromate conversion treated aluminum alloys: Anti-corrosion performance and epoxy coating adhesion properties. Appl. Surf. Sci. 2017, 405, 157–168. [Google Scholar] [CrossRef]
- Sharifi Golru, S.; Attar, M.M.; Ramezanzadeh, B. Effects of surface treatment of aluminium alloy 1050 on the adhesion and anticorrosion properties of the epoxy coating. Appl. Surf. Sci. 2015, 345, 360–368. [Google Scholar] [CrossRef]
- Fockaert, L.; Pletincx, S.; Boelen, B.; Hauffman, T.; Terryn, H.; Mol, J. Effect of zirconium-based conversion treatments of zinc, aluminium and magnesium on the chemisorption of ester-functionalized molecules. Appl. Surf. Sci. 2020, 508, 145199. [Google Scholar] [CrossRef]
- Fockaert, L.; Pletincx, S.; Ganzinga-Jurg, D.; Boelen, B.; Hauffman, T.; Terryn, H.; Mol, J. Chemisorption of polyester coatings on zirconium-based conversion coated multi-metal substrates and their stability in aqueous environment. Appl. Surf. Sci. 2020, 508, 144771. [Google Scholar]
- Kuznetsov, Y.I. Organic corrosion inhibitors: Where are we now? A review. part III. Passivation and the role of the chemical structure of organophosphates. Int. J. Corros. Scale Inhib. 2017, 6, 209–239. [Google Scholar] [CrossRef]
- Jianrui, L.; Yina, G.; Weidong, H. Study on the corrosion resistance of phytic acid conversion coating for magnesium alloys. Surf. Coat. Technol. 2006, 201, 1536–1541. [Google Scholar] [CrossRef]
- Pan, F.; Yang, X.; Zhang, D. Chemical nature of phytic acid conversion coating on AZ61 magnesium alloy. Appl. Surf. Sci. 2009, 255, 8363–8371. [Google Scholar] [CrossRef]
- Cui, X.; Li, Q.; Li, Y.; Wang, F.; Jin, G.; Ding, M. Microstructure and corrosion resistance of phytic acid conversion coatings for magnesium alloy. Appl. Surf. Sci. 2008, 255, 2098–2103. [Google Scholar] [CrossRef]
- Gao, X.; Lu, K.; Xu, L.; Xu, H.; Lu, H.; Gao, F.; Hou, S.; Ma, H. Excellent anti-corrosive pretreatment layer on iron substrate based on three-dimensional porous phytic acid/silane hybrid. Nanoscale 2016, 8, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Pak, S.-N.; Jiang, Z.; Yao, Z.; Ju, J.-M.; Ju, K.-S.; Pak, U.-J. Fabrication of environmentally friendly anti-corrosive composite coatings on AZ31B Mg alloy by plasma electrolytic oxidation and phytic acid/3-aminopropyltrimethoxysilane post treatment. Surf. Coat. Technol. 2017, 325, 579–587. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, C.; Zhang, M.; Huang, X.; Jiang, X. Phytic acid conversion coating on Mg-Li alloy. J. Alloys Compd. 2009, 485, 789–793. [Google Scholar] [CrossRef]
- Cui, X.; Li, Y.; Li, Q.; Jin, G.; Ding, M.; Wang, F. Influence of phytic acid concentration on performance of phytic acid conversion coatings on the AZ91D magnesium alloy. Mater. Chem. Phys. 2008, 111, 503–507. [Google Scholar] [CrossRef]
- Hernández-Alvarado, L.A.; Hernández, L.S.; Garrido, J.; Rivera-Villalobos, S.; Escudero, M.L. Statistical design of the parameters involved in the obtainment of a biodegradable phytate coating over AZ31 alloy for possible endoprosthetic temporary applications. Surf. Coat. Technol. 2017, 325, 473–481. [Google Scholar] [CrossRef]
- Ye, C.; Zheng, Y.; Wang, S.; Xi, T.; Li, Y. In vitro corrosion and biocompatibility study of phytic acid modified WE43 magnesium alloy. Appl. Surf. Sci. 2012, 258, 3420–3427. [Google Scholar] [CrossRef]
- Bauman, A.T.; Chateauneuf, G.M.; Boyd, B.R.; Brown, R.E.; Murthy, P.P.N. Conformational inversion processes in phytic acid: NMR spectroscopic and molecular modeling studies. Tetrahedron Lett. 1999, 40, 4489–4492. [Google Scholar] [CrossRef]
- ECHA. Candidate List of Substances of Very High Concern for Authorisation; ECHA Location: Helsinki, Finland, 2017. [Google Scholar]
- Zhang, B.; Yao, R.; Li, L.; Li, M.; Yang, L.; Liang, Z.; Yu, H.; Zhang, H.; Luo, R.; Wang, Y. Bionic Tea Stain–Like, All-Nanoparticle Coating for Biocompatible Corrosion Protection. Adv. Mater. Interfaces 2019, 6, 1900899. [Google Scholar]
- Zhang, B.; Yao, R.; Li, L.; Wang, Y.; Luo, R.; Yang, L.; Wang, Y. Green tea polyphenol induced Mg2+-rich multilayer conversion coating: Toward enhanced corrosion resistance and promoted in situ endothelialization of AZ31 for potential cardiovascular applications. ACS Appl. Mater. Interfaces 2019, 11, 41165–41177. [Google Scholar] [CrossRef]
- Abatti, G.P.; Pires, A.T.N.; Spinelli, A.; Scharnagl, N.; da Conceição, T.F. Conversion coating on magnesium alloy sheet (AZ31) by vanillic acid treatment: Preparation, characterization and corrosion behavior. J. Alloys Compd. 2018, 738, 224–232. [Google Scholar] [CrossRef]
- Frignani, A.; Grassi, V.; Zucchi, F.; Zanotto, F. Mono-carboxylate conversion coatings for AZ31 Mg alloy protection. Mater. Corros. 2011, 62, 995–1002. [Google Scholar] [CrossRef]
- Sun, J.; Howlett, P.C.; MacFarlane, D.R.; Lin, J.; Forsyth, M. Synthesis and physical property characterisation of phosphonium ionic liquids based on P(O)2(OR)2− and P(O)2(R)2− anions with potential application for corrosion mitigation of magnesium alloys. Electrochim. Acta 2008, 54, 254–260. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Latham, J.-A.; MacFarlane, D.R.; Howlett, P.C.; Forsyth, M. A review of ionic liquid surface film formation on Mg and its alloys for improved corrosion performance. Electrochim. Acta 2013, 110, 501–510. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Petro, R.; Schlesinger, M.; Song, G.-L. Ionic liquid treatments for enhanced corrosion resistance of magnesium-based substrates. In Modern Plating, 5th ed.; Schlesingher, M., Paunovic, M., Eds.; Wiley: New York, NY, USA, 2010; pp. 665–686. [Google Scholar]
- Elsentriecy, H.H.; Qu, J.; Luo, H.; Meyer, H.M.; Ma, C.; Chi, M. Improving corrosion resistance of AZ31B magnesium alloy via a conversion coating produced by a protic ammonium-phosphate ionic liquid. Thin Solid Film. 2014, 568, 44–51. [Google Scholar] [CrossRef]
- Forsyth, M.; Howlett, P.C.; Tan, S.K.; MacFarlane, D.R.; Birbilis, N. An ionic liquid surface treatment for corrosion protection of magnesium alloy AZ31. Electrochem. Solid State Lett. 2006, 9, B52. [Google Scholar] [CrossRef]
- Caporali, S.; Ghezzi, F.; Giorgetti, A.; Lavacchi, A.; Tolstogouzov, A.; Bardi, U. Interaction between an imidazolium based ionic liquid and the AZ91D magnesium alloy. Adv. Eng. Mater. 2007, 9, 185–190. [Google Scholar] [CrossRef]
- Birbilis, N.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. Exploring corrosion protection of Mg via ionic liquid pretreatment. Surf. Coat. Technol. 2007, 201, 4496–4504. [Google Scholar] [CrossRef]
- Santos, A.G.; Ribeiro, B.D.; Alviano, D.S.; Coelho, M.A.Z. Toxicity of ionic liquids toward microorganisms interesting to the food industry. RSC Adv. 2014, 4, 37157–37163. [Google Scholar] [CrossRef]
- Howlett, P.C.; Zhang, S.; MacFarlane, D.R.; Forsyth, M. An investigation of a phosphinate-based ionic liquid for corrosion protection of magnesium alloy AZ31. Aust. J. Chem. 2007, 60, 43–46. [Google Scholar] [CrossRef]
- Forsyth, M.; Neil, W.C.; Howlett, P.C.; Macfarlane, D.R.; Hinton, B.R.; Rocher, N.; Kemp, T.F.; Smith, M.E. New insights into the fundamental chemical nature of ionic liquid film formation on magnesium alloy surfaces. ACS Appl. Mater. Interfaces 2009, 1, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Howlett, P.C.; Khoo, T.; Mooketsi, G.; Efthimiadis, J.; Macfarlane, D.R.; Forsyth, M. The effect of potential bias on the formation of ionic liquid generated surface films on Mg alloys. Electrochim. Acta 2010, 55, 2377–2383. [Google Scholar] [CrossRef]
- Latham, J.-A.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. Electrochemical reactivity of trihexyl (tetradecyl) phosphonium bis (2, 4, 4-trimethylpentyl) phosphinate ionic liquid on glassy carbon and AZ31 magnesium alloy. Electrochim. Acta 2011, 56, 5328–5334. [Google Scholar] [CrossRef]
- Zheng, Y.; Peng, R.; Jin, H.; Luo, Y.; Ping, Y. Film formation on magnesium alloy AZ31B in 1-butyl-3-methylimidazolium dibutylphosphate ionic liquid. Surf. Coat. Technol. 2017, 325, 539–547. [Google Scholar] [CrossRef]
- Howlett, P.C.; Efthimiadis, J.; Hale, P.; van Riessen, G.A.; MacFarlane, D.R.; Forsyth, M. Characterization of the magnesium alloy AZ31 surface in the ionic liquid trihexyl (tetradecyl) phosphonium bis (trifluoromethanesulfonyl) amide. J. Electrochem. Soc. 2010, 157, C392. [Google Scholar] [CrossRef]
- Latham, J.-A.; Howlett, P.C.; MacFarlane, D.R.; Somers, A.; Forsyth, M. Anodising AZ31 in a phosphonium ionic liquid: Corrosion protection through composite film deposition. J. Electrochem. Soc. 2012, 159, C539. [Google Scholar] [CrossRef] [Green Version]
- Elsentriecy, H.H.; Luo, H.; Meyer, H.M.; Grado, L.L.; Qu, J. Effects of pretreatment and process temperature of a conversion coating produced by an aprotic ammonium-phosphate ionic liquid on magnesium corrosion protection. Electrochim. Acta 2014, 123, 58–65. [Google Scholar] [CrossRef]
- Latham, J.-A.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. Passive film formation in dilute ionic liquid solutions on magnesium Alloy AZ31. Electrochem. Commun. 2012, 19, 90–92. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.; Wang, X.; Tu, J. Deep eutectic solvents (DESs)-derived advanced functional materials for energy and environmental applications: Challenges, opportunities, and future vision. J. Mater. Chem. A 2017, 5, 8209–8229. [Google Scholar] [CrossRef]
- Guo, L.; Gu, C.; Feng, J.; Guo, Y.; Jin, Y.; Tu, J. Hydrophobic epoxy resin coating with ionic liquid conversion pretreatment on magnesium alloy for promoting corrosion resistance. J. Mater. Sci. Technol. 2020, 37, 9–18. [Google Scholar] [CrossRef]
- Guo, L.; Gu, C.; Zhang, J.; Wang, X.; Wang, K.; Jin, Y.; Tu, J. A black conversion coating produced by hot corrosion of magnesium with deep eutectic solvent membrane. Surf. Coat. Technol. 2019, 357, 833–840. [Google Scholar] [CrossRef]
- Vaccari, A. Clays and catalysis: A promising future. Appl. Clay Sci. 1999, 14, 161–198. [Google Scholar] [CrossRef]
- Visser, P.; Liu, Y.; Zhou, X.; Hashimoto, T.; Thompson, G.E.; Lyon, S.B.; van der Ven, L.G.; Mol, A.J.; Terryn, H.A. The corrosion protection of AA2024-T3 aluminium alloy by leaching of lithium-containing salts from organic coatings. Faraday Discuss. 2015, 180, 511–526. [Google Scholar] [CrossRef]
- Liu, Y.; Visser, P.; Zhou, X.; Lyon, S.B.; Hashimoto, T.; Curioni, M.; Gholinia, A.; Thompson, G.E.; Smyth, G.; Gibbon, S.R.; et al. Protective Film Formation on AA2024-T3 Aluminum Alloy by Leaching of Lithium Carbonate from an Organic Coating. J. Electrochem. Soc. 2016, 163, C45–C53. [Google Scholar] [CrossRef] [Green Version]
- Poznyak, S.K.; Tedim, J.; Rodrigues, L.M.; Salak, A.N.; Zheludkevich, M.L.; Dick, L.F.; Ferreira, M.G. Novel inorganic host layered double hydroxides intercalated with guest organic inhibitors for anticorrosion applications. ACS Appl. Mater. Interfaces 2009, 1, 2353–2362. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Poznyak, S.K.; Rodrigues, L.M.; Raps, D.; Hack, T.; Dick, L.F.; Nunes, T.; Ferreira, M.G.S. Active protection coatings with layered double hydroxide nanocontainers of corrosion inhibitor. Corros. Sci. 2010, 52, 602–611. [Google Scholar] [CrossRef]
- Montemor, M.F.; Snihirova, D.V.; Taryba, M.G.; Lamaka, S.V.; Kartsonakis, I.A.; Balaskas, A.C.; Kordas, G.C.; Tedim, J.; Kuznetsova, A.; Zheludkevich, M.L.; et al. Evaluation of self-healing ability in protective coatings modified with combinations of layered double hydroxides and cerium molibdate nanocontainers filled with corrosion inhibitors. Electrochim. Acta 2012, 60, 31–40. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Mamidipally, S.B.; Schmutz, P.; Guan, H. Active corrosion protection in Ce-modified hydrotalcite conversion coatings. Corrosion 2002, 58, 3–14. [Google Scholar] [CrossRef]
- Ba, Z.; Dong, Q.; Zhang, X.; Qiang, X.; Cai, Z.; Luo, X. Cerium-based modification treatment of Mg-Al hydrotalcite film on AZ91D Mg alloy assisted with alternating electric field. J. Alloys Compd. 2017, 695, 106–113. [Google Scholar] [CrossRef]
- Dong, Q.; Ba, Z.; Jia, Y.; Chen, Y.; Lv, X.; Zhang, X.; Wang, Z. Effect of solution concentration on sealing treatment of Mg-Al hydrotalcite film on AZ91D Mg alloy. J. Magnes. Alloy. 2017, 5, 320–325. [Google Scholar] [CrossRef]
- Newman, S.P.; Jones, W. Synthesis, characterization and applications of layered double hydroxides containing organic guests. New J. Chem. 1998, 22, 105–115. [Google Scholar] [CrossRef]
- McMurray, H.N.; Williams, G. Inhibition of Filiform Corrosion on Organic-Coated Aluminum Alloy by Hydrotalcite-Like Anion-Exchange Pigments. Corrosion 2004, 60, 219–228. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N. Inhibition of filiform corrosion on organic-coated AA2024-T3 by smart-release cation and anion-exchange pigments. Electrochim. Acta 2012, 69, 287–294. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, P.; Wu, J.; Chen, F.; Li, Y.; Zhang, Y.; Zuo, Y.; Qi, Y. Enhancement of anticorrosion protection via inhibitor-loaded ZnAlCe-LDH nanocontainers embedded in sol–gel coatings. J. Coat. Technol. Res. 2017, 15, 303–313. [Google Scholar] [CrossRef]
- Stimpfling, T.; Vialat, P.; Hintze-Bruening, H.; Keil, P.; Shkirskiy, V.; Volovitch, P.; Ogle, K.; Leroux, F. Amino Acid Interleaved Layered Double Hydroxides as Promising Hybrid Materials for AA2024 Corrosion Inhibition. Eur. J. Inorg. Chem. 2016, 2016, 2006–2016. [Google Scholar] [CrossRef]
- Guo, L.; Wu, W.; Zhou, Y.; Zhang, F.; Zeng, R.; Zeng, J. Layered double hydroxide coatings on magnesium alloys: A review. J. Mater. Sci. Technol. 2018, 34, 1455–1466. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Zhang, S.; Yuan, B.; Zheng, Z.; Pan, F. A Novel Approach to Fabricate Protective Layered Double Hydroxide Films on the Surface of Anodized Mg-Al Alloy. Adv. Mater. Interfaces 2017, 4, 1700163. [Google Scholar] [CrossRef]
- Wu, L.; Pan, F.; Liu, Y.; Zhang, G.; Tang, A.; Atrens, A. Influence of pH on the growth behaviour of Mg–Al LDH films. Surf. Eng. 2017, 34, 674–681. [Google Scholar] [CrossRef]
- Wu, L.; Yang, D.; Zhang, G.; Zhang, Z.; Zhang, S.; Tang, A.; Pan, F. Fabrication and characterization of Mg-M layered double hydroxide films on anodized magnesium alloy AZ31. Appl. Surf. Sci. 2017, 431, 177–186. [Google Scholar] [CrossRef]
- Peng, F.; Wang, D.; Tian, Y.; Cao, H.; Qiao, Y.; Liu, X. Sealing the Pores of PEO Coating with Mg-Al Layered Double Hydroxide: Enhanced Corrosion Resistance, Cytocompatibility and Drug Delivery Ability. Sci. Rep. 2017, 7, 8167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrova, E.; Serdechnova, M.; Shulha, T.; Lamaka, S.V.; Wieland, D.C.F.; Karlova, P.; Blawert, C.; Starykevich, M.; Zheludkevich, M.L. Use of synergistic mixture of chelating agents for in situ LDH growth on the surface of PEO-treated AZ91. Sci. Rep. 2020, 10, 8645. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liang, J.; Peng, Z.; Liu, B. Electrochemical deposition and characterization of Zn-Al layered double hydroxides (LDHs) films on magnesium alloy. Appl. Surf. Sci. 2014, 313, 834–840. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. In situ growth of Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy. Corros. Sci. 2011, 53, 3281–3288. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. Study of the in situ growth mechanism of Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy. Corros. Sci. 2012, 63, 148–158. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.-G.; Zeng, R.-C.; Li, S.-Q.; Cui, H.-Z.; Song, L.; Han, E.-H. Corrosion resistance of Mg–Al-LDH coating on magnesium alloy AZ31. Surf. Coat. Technol. 2014, 258, 1152–1158. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. Influence of alloying elements and microstructure on the formation of hydrotalcite film on Mg alloys. Corros. Sci. 2015, 93, 90–99. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. Modifications of the hydrotalcite film on AZ31 Mg alloy by phytic acid: The effects on morphology, composition and corrosion resistance. Corros. Sci. 2013, 74, 130–138. [Google Scholar] [CrossRef]
- Nakamura, K.; Tsunakawa, M.; Shimada, Y.; Serizawa, A.; Ishizaki, T. Formation mechanism of Mg-Al layered double hydroxide-containing magnesium hydroxide films prepared on Ca-added flame-resistant magnesium alloy by steam coating. Surf. Coat. Technol. 2017, 328, 436–443. [Google Scholar] [CrossRef]
- Kamiyama, N.; Panomsuwan, G.; Yamamoto, E.; Sudare, T.; Saito, N.; Ishizaki, T. Effect of treatment time in the Mg(OH)2/Mg–Al LDH composite film formed on Mg alloy AZ31 by steam coating on the corrosion resistance. Surf. Coat. Technol. 2016, 286, 172–177. [Google Scholar] [CrossRef]
- Lin, J.K.; Uan, J.Y. Formation of Mg,Al-hydrotalcite conversion coating on Mg alloy in aqueous HCO3−/CO32− and corresponding protection against corrosion by the coating. Corros. Sci. 2009, 51, 1181–1188. [Google Scholar] [CrossRef]
- Ferreira, M.; Zheludkevich, M.; Tedim, J.; Gandubert, V.; Schmidt-Hansberg, T.; Hack, T.; Nixon, S.; Raps, D.; Becker, D.; Schröder, S. Process for Coating Metallic Surfaces with Coating Compositions Containing Particles of a Layered Double Hydroxide. U.S. Patent 20150079298A1, 19 March 2015. [Google Scholar]
- Zeng, R.-C.; Liu, Z.-G.; Zhang, F.; Li, S.-Q.; Cui, H.-Z.; Han, E.-H. Corrosion of molybdate intercalated hydrotalcite coating on AZ31 Mg alloy. J. Mater. Chem. A 2014, 2, 13049–13057. [Google Scholar] [CrossRef]
- Shulha, T.N.; Serdechnova, M.; Lamaka, S.V.; Wieland, D.C.F.; Lapko, K.N.; Zheludkevich, M.L. Chelating agent-assisted in situ LDH growth on the surface of magnesium alloy. Sci. Rep. 2018, 8, 16409. [Google Scholar] [CrossRef] [PubMed]
- Shulha, T.; Serdechnova, M.; Iuzviuk, M.; Zobkalo, I.; Karlova, P.; Scharnagl, N.; Wieland, D.; Lamaka, S.; Yaremchenko, A.; Blawert, C. In situ formation of LDH-based nanocontainers on the surface of AZ91 magnesium alloy and detailed investigation of their crystal structure. J. Magnes. Alloy. 2021, 10, 1268–1285. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.-W.; Shan, D.-Y.; Han, E.-H. Properties of dawsonite conversion film on AZ31 magnesium alloy. Trans. Nonferrous Met. Soc. China 2011, 21, 936–942. [Google Scholar] [CrossRef]
- Jin, Q.; Tian, G.; Li, J.; Zhao, Y.; Yan, H. The study on corrosion resistance of superhydrophobic magnesium hydroxide coating on AZ31B magnesium alloy. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 8–16. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.; Song, L.; Zeng, R.; Li, S.; Cui, H. Fabrication of the Superhydrophobic Surface on Magnesium Alloy and Its Corrosion Resistance. J. Mater. Sci. Technol. 2015, 31, 1139–1143. [Google Scholar] [CrossRef]
- Ng, W.; Wong, M.; Cheng, F. Stearic acid coating on magnesium for enhancing corrosion resistance in Hanks’ solution. Surf. Coat. Technol. 2010, 204, 1823–1830. [Google Scholar] [CrossRef]
- Zhou, M.; Pang, X.; Wei, L.; Gao, K. Insitu grown superhydrophobic Zn–Al layered double hydroxides films on magnesium alloy to improve corrosion properties. Appl. Surf. Sci. 2015, 337, 172–177. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.; Zeng, R.; Song, L.; Guo, L.; Huang, X. Corrosion resistance of the superhydrophobic Mg (OH)2/Mg-Al layered double hydroxide coatings on magnesium alloys. Metals 2016, 6, 85. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Dai, X.; Xiong, J. Study on the preparation and corrosion resistance of hydroxyapatite/stearic acid superhydrophobic composite coating on magnesium alloy surface. AIP Adv. 2019, 9, 045314. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xiao, X.; Liu, E.; Yu, S.; Yin, X.; Wang, J.; Zhu, G.; Li, Q.; Li, J. Fabrication of superhydrophobic needle-like Ca-P coating with anti-fouling and anti-corrosion properties on AZ31 magnesium alloy. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126568. [Google Scholar] [CrossRef]
- Gupta, R.; Mensah-Darkwa, K.; Sankar, J.; Kumar, D. Enhanced corrosion resistance of phytic acid coated magnesium by stearic acid treatment. Trans. Nonferrous Met. Soc. China 2013, 23, 1237–1244. [Google Scholar] [CrossRef]
- Yang, N.; Li, Q.; Chen, F.; Cai, P.; Tan, C.; Xi, Z. A solving-reprecipitation theory for self-healing functionality of stannate coating with a high environmental stability. Electrochim. Acta 2015, 174, 1192–1201. [Google Scholar] [CrossRef]
- Kuang, J.; Ba, Z.; Li, Z.; Jia, Y.; Wang, Z. Fabrication of a superhydrophobic Mg-Mn layered double hydroxides coating on pure magnesium and its corrosion resistance. Surf. Coat. Technol. 2019, 361, 75–82. [Google Scholar] [CrossRef]
- Williams, G.; Ap Llwyd Dafydd, H.; Subramanian, R.; McMurray, H.N. The influence of chloride ion concentration on passivity breakdown in magnesium. Corrosion 2017, 73, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Merino, M.; Pardo, A.; Arrabal, R.; Merino, S.; Casajús, P.; Mohedano, M. Influence of chloride ion concentration and temperature on the corrosion of Mg–Al alloys in salt fog. Corros. Sci. 2010, 52, 1696–1704. [Google Scholar] [CrossRef]
- Jin, H.; Yang, X.; Wang, M. Chemical conversion coating on AZ31B magnesium alloy and its corrosion tendency. Acta Metall. Sin. (Engl. Lett.) 2009, 22, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Van Phuong, N.; Gupta, M.; Moon, S. Enhanced corrosion performance of magnesium phosphate conversion coating on AZ31 magnesium alloy. Trans. Nonferrous Met. Soc. China 2017, 27, 1087–1095. [Google Scholar] [CrossRef]
- Zhao, H.; Cai, S.; Ding, Z.; Zhang, M.; Li, Y.; Xu, G. A simple method for the preparation of magnesium phosphate conversion coatings on a AZ31 magnesium alloy with improved corrosion resistance. RSC Adv. 2015, 5, 24586–24590. [Google Scholar] [CrossRef]
- Han, B.; Gu, D.; Yang, Y.; Fang, L.; Peng, G.; Yang, C. Preparation and phosphating of yttrium-based chemical conversion coatings on AZ91D magnesium alloy for corrosion protection. Int. J. Electrochem. Sci. 2016, 11, 10779–10794. [Google Scholar] [CrossRef]
- Rocca, E.; Juers, C.; Steinmetz, J. Corrosion behaviour of chemical conversion treatments on as-cast Mg-Al alloys: Electrochemical and non-electrochemical methods. Corros. Sci. 2010, 52, 2172–2178. [Google Scholar] [CrossRef]
- BRUSCIOTTI, F.; Izagirre, E.U.; Unzurrunzaga, I.A. Conversion Coating Process for Magnesium Alloys. European Patent EP2944707A1, 22 March 2017. Available online: https://www.google.de/patents/EP2944707B2944701?cl=en (accessed on 22 March 2017).
- Xiong, Q.; Xiong, J.P.; Zhou, Y.; Yan, F. The study of a phosphate conversion coating on magnesium alloy AZ91D: III. Nano-particle modification. Int. J. Electrochem. Sci. 2017, 12, 4238–4250. [Google Scholar] [CrossRef] [Green Version]
- Van Phuong, N.; Moon, S.; Chang, D.; Lee, K.H. Effect of microstructure on the zinc phosphate conversion coatings on magnesium alloy AZ91. Appl. Surf. Sci. 2013, 264, 70–78. [Google Scholar] [CrossRef]
- Su, H.Y.; Li, W.J.; Lin, C.S. Effect of acid pickling pretreatment on the properties of cerium conversion coating on AZ31 magnesium alloy. J. Electrochem. Soc. 2012, 159, C219–C225. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; He, X.; Sun, F. Effect of cerium additive on aluminum-based chemical conversion coating on AZ91D magnesium alloy. Appl. Surf. Sci. 2013, 280, 467–473. [Google Scholar] [CrossRef]
- Lin, C.S.; Lin, H.C.; Lin, K.M.; Lai, W.C. Formation and properties of stannate conversion coatings on AZ61 magnesium alloys. Corros. Sci. 2006, 48, 93–109. [Google Scholar] [CrossRef]
- Hamdy, A.S.; Butt, D.P. Novel smart stannate based coatings of self-healing functionality for AZ91D magnesium alloy. Electrochim. Acta 2013, 97, 296–303. [Google Scholar] [CrossRef]
- Liang, C.H.; Zheng, R.F.; Huang, N.B.; Xu, L.S. Conversion coating treatment for AZ31 magnesium alloys by a phytic acid bath. J. Appl. Electrochem. 2009, 39, 1857. [Google Scholar] [CrossRef]
- Gupta, R.K.; Mensah-Darkwa, K.; Kumar, D. Effect of Post Heat Treatment on Corrosion Resistance of Phytic Acid Conversion Coated Magnesium. J. Mater. Sci. Technol. 2013, 29, 180–186. [Google Scholar] [CrossRef]
- Ely, M.; Światowska, J.; Seyeux, A.; Zanna, S.; Klein, L.; Marcus, P. Effect of post-treatment on trivalent chromium protection (TCP) on aluminium alloy 2024-T3. J. Electrochem. Soc. 2017, 164, C276. [Google Scholar] [CrossRef]







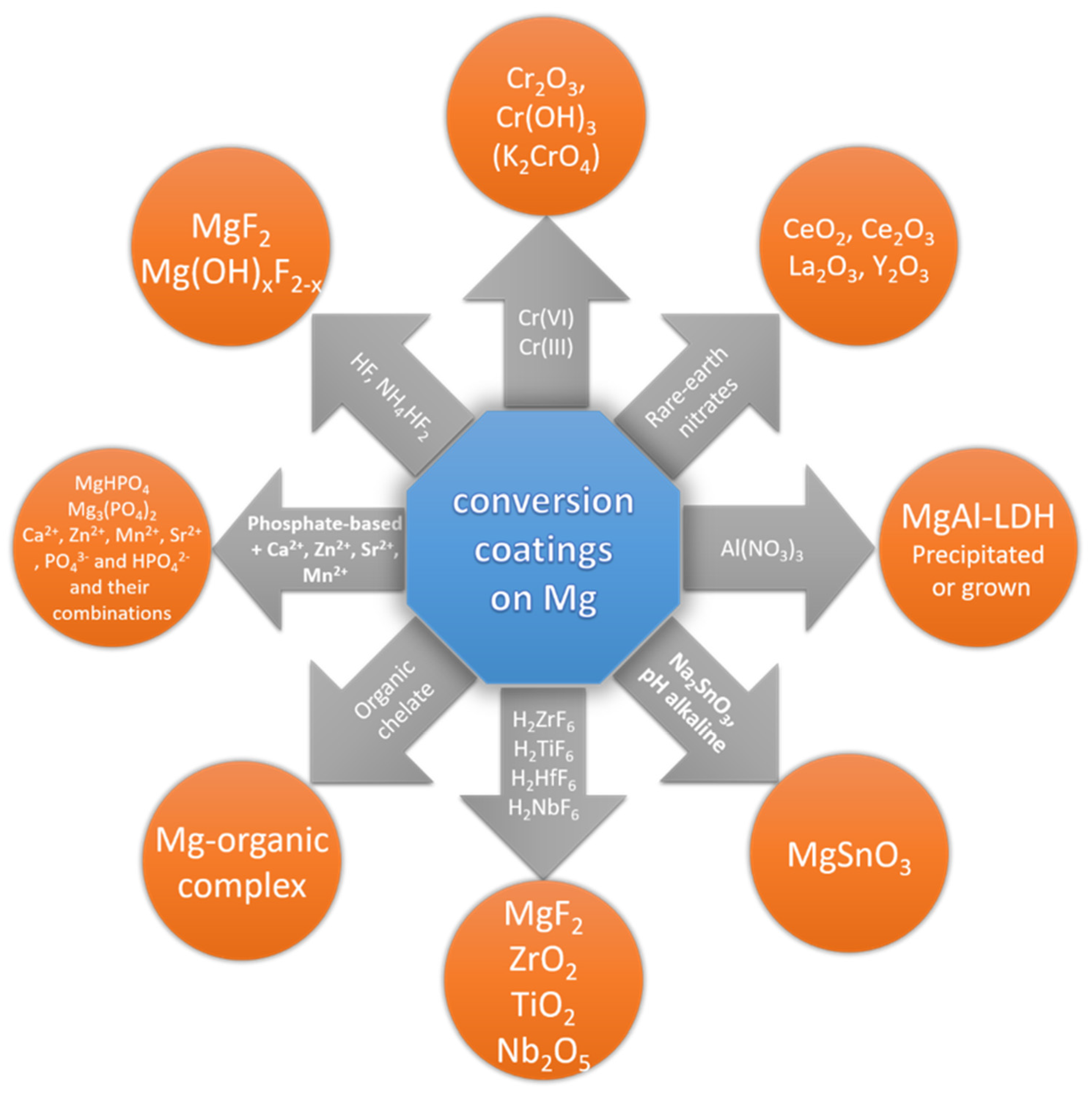










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaghefinazari, B.; Wierzbicka, E.; Visser, P.; Posner, R.; Arrabal, R.; Matykina, E.; Mohedano, M.; Blawert, C.; Zheludkevich, M.; Lamaka, S. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: PART I—Pre-Treatment and Conversion Coating. Materials 2022, 15, 8676. https://doi.org/10.3390/ma15238676
Vaghefinazari B, Wierzbicka E, Visser P, Posner R, Arrabal R, Matykina E, Mohedano M, Blawert C, Zheludkevich M, Lamaka S. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: PART I—Pre-Treatment and Conversion Coating. Materials. 2022; 15(23):8676. https://doi.org/10.3390/ma15238676
Chicago/Turabian StyleVaghefinazari, Bahram, Ewa Wierzbicka, Peter Visser, Ralf Posner, Raúl Arrabal, Endzhe Matykina, Marta Mohedano, Carsten Blawert, Mikhail Zheludkevich, and Sviatlana Lamaka. 2022. "Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: PART I—Pre-Treatment and Conversion Coating" Materials 15, no. 23: 8676. https://doi.org/10.3390/ma15238676
APA StyleVaghefinazari, B., Wierzbicka, E., Visser, P., Posner, R., Arrabal, R., Matykina, E., Mohedano, M., Blawert, C., Zheludkevich, M., & Lamaka, S. (2022). Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: PART I—Pre-Treatment and Conversion Coating. Materials, 15(23), 8676. https://doi.org/10.3390/ma15238676












