Hydrogen Recovery from Waste Aluminum–Plastic Composites Treated with Alkaline Solution
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Blisters
3.2. Sheets
3.3. Lids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.-R.; Feng, T.-T.; Li, Y.; Zhang, H.-M.; Kong, J.-J. What Is the Policy Effect of Coupling the Green Hydrogen Market, National Carbon Trading Market and Electricity Market? Sustainability 2022, 14, 13948. [Google Scholar] [CrossRef]
- Nebel, A.; Cantor, J.; Salim, S.; Salih, A.; Patel, D. The Role of Renewable Energies, Storage and Sector-Coupling Technologies in the German Energy Sector under Different CO2 Emission Restrictions. Sustainability 2022, 14, 10379. [Google Scholar] [CrossRef]
- Li, S.; Kang, Q.; Baeyens, J.; Zhang, H.L.; Deng, Y.M. Hydrogen Production: State of Technology. IOP Conf. Ser. Earth Environ. Sci. 2020, 544, 012011. [Google Scholar] [CrossRef]
- Xu, M.; Qu, J.; Li, M. National Policies, Recent Research Hotspots, and Application of Sustainable Energy: Case of China, USA, and European Countries. Sustainability 2022, 14, 10014. [Google Scholar] [CrossRef]
- Chenic, A.Ș.; Cretu, A.I.; Burlacu, A.; Moroianu, N.; Vîrjan, D.; Huru, D.; Stanef-Puica, M.R.; Enachescu, V. Logical Analysis on the Strategy for a Sustainable Transition of the World to Green Energy—2050. Smart Cities and Villages Coupled to Renewable Energy Sources with Low Carbon Footprint. Sustainability 2022, 14, 8622. [Google Scholar]
- Zhang, M.; Yang, X. The Regulatory Perspectives to China’s Emerging Hydrogen Economy: Characteristics, Challenges, and Solutions. Sustainability 2022, 14, 9700. [Google Scholar]
- González Palencia, J.C.; Itoi, Y.; Araki, M. Design of a Hydrogen Production System Considering Energy Consumption, Water Consumption, CO2 Emissions and Cost. Energies 2022, 15, 7938. [Google Scholar] [CrossRef]
- Deng, Y.; Li, S.; Appels, L.; Dewil, R.; Zhang, H.; Baeyens, J.; Mikulcic, H. Producing hydrogen by catalytic steam reforming of methanol using non-noble metal catalysts. J. Environ. Manag. 2022, 321, 116019. [Google Scholar] [CrossRef]
- Deng, Y.; Dewil, R.; Appels, L.; Van Tulden, F.; Li, S.; Yang, M.; Baeyens, J. Hydrogen-enriched natural gas in a decarbonization perspective. Fuel 2022, 318, 123680. [Google Scholar] [CrossRef]
- Deng, Y.; Dewil, R.; Appels, L.; Li, S.; Baeyens, J.; Degrève, J.; Wang, G. Thermo-chemical water splitting: Selection of priority reversible redox reactions by multi-attribute decision making. Renew. Energy 2021, 170, 800–810. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Nie, J.; Dewil, R.; Baeyens, J.; Deng, Y. The Direct Reduction of Iron Ore with Hydrogen. Sustainability 2021, 13, 8866. [Google Scholar]
- Deng, Y.; Li, S.; Dewil, R.; Appels, L.; Yang, M.; Zhang, H.; Baeyens, J. Water splitting by MnFe2O4/Na2CO3 reversible redox reactions. RSC Adv. 2022, 12, 31392–31401. [Google Scholar] [CrossRef] [PubMed]
- Alshorifi, F.T.; Alswat, A.A.; Salama, R.S. Gold-selenide quantum dots supported onto cesium ferrite nanocomposites for the efficient degradation of rhodamine B. Heliyon 2022, 8, e09652. [Google Scholar] [CrossRef]
- Mannaa, M.A.; Qasim, K.F.; Alshorifi, F.T.; El-Bahy, S.M.; Salama, R.S. Role of NiO Nanoparticles in Enhancing Structure Properties of TiO2 and Its Applications in Photodegradation and Hydrogen Evolution. ACS Omega 2021, 6, 30386–30400. [Google Scholar] [CrossRef] [PubMed]
- Al-Thani, H.; Koç, M.; Isaifan, R.J.; Bicer, Y. A Review of the Integrated Renewable Energy Systems for Sustainable Urban Mobility. Sustainability 2022, 14, 10517. [Google Scholar]
- Duong, P.A.; Ryu, B.; Jung, J.; Kang, H. Design, Modelling, and Thermodynamic Analysis of a Novel Marine Power System Based on Methanol Solid Oxide Fuel Cells, Integrated Proton Exchange Membrane Fuel Cells, and Combined Heat and Power Production. Sustainability 2022, 14, 12496. [Google Scholar] [CrossRef]
- Banihabib, R.; Assadi, M. A Hydrogen-Fueled Micro Gas Turbine Unit for Carbon-Free Heat and Power Generation. Sustainability 2022, 14, 13305. [Google Scholar] [CrossRef]
- Kashkarov, S.; Makarov, D.; Molkov, V. Performance of Hydrogen Storage Tanks of Type IV in a Fire: Effect of the State of Charge. Hydrogen 2021, 2, 386–398. [Google Scholar] [CrossRef]
- Makarov, D.; Shentsov, V.; Kuznetsov, M.; Molkov, V. Hydrogen Tank Rupture in Fire in the Open Atmosphere: Hazard Distance Defined by Fireball. Hydrogen 2021, 2, 134–146. [Google Scholar]
- Zhang, X.; Qiu, G.; Wang, S.; Wu, J.; Peng, Y. Hydrogen Leakage Simulation and Risk Analysis of Hydrogen Fueling Station in China. Sustainability 2022, 14, 12420. [Google Scholar]
- Vlaskin, M.S.; Shkolnikov, E.I.; Bersh, A.V. Oxidation kinetics of micron-sized aluminum powder in high-temperature boiling water. Int. J. Hydrogen Energy 2011, 36, 6484–6495. [Google Scholar] [CrossRef]
- Vlaskin, M.S.; Dudoladov, A.O.; Buryakovskaya, O.A.; Ambaryan, G.N. Modelling of aluminum-fuelled power plant with steam-hydrogen enthalpy utilization. Int. J. Hydrogen Energy 2018, 43, 4623–4631. [Google Scholar] [CrossRef]
- Trowell, K.; Goroshin, S.; Frost, D.; Bergthorson, J. Hydrogen production rates of aluminum reacting with varying densities of supercritical water. RSC Adv. 2022, 12, 12335–12343. [Google Scholar] [CrossRef]
- Trowell, K.A.; Goroshin, S.; Frost, D.L.; Bergthorson, J.M. The use of supercritical water for the catalyst-free oxidation of coarse aluminum for hydrogen production. Sustain. Energy Fuels 2020, 4, 5628–5635. [Google Scholar] [CrossRef]
- Shmelev, V.; Yang, H.; Yim, C. Hydrogen generation by reaction of molten aluminum with water steam. Int. J. Hydrogen Energy 2016, 41, 14562–14572. [Google Scholar] [CrossRef]
- Etminanbakhsh, M.; Reza Allahkaram, S. Reaction of aluminum particles with superheated steam to generate hydrogen gas as a readily usable clean fuel. Fuel 2023, 332, 126011. [Google Scholar] [CrossRef]
- Ouyang, L.; Jiang, J.; Chen, K.; Zhu, M.; Liu, Z. Hydrogen Production via Hydrolysis and Alcoholysis of Light Metal-Based Materials: A Review. Nano-Micro Lett. 2021, 13, 134. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, L.; Zhang, L.; Zhang, X.; Wang, X.; Ge, M.; Li, X.; Zou, M. Preparation of Al-3Ga-3In-3Sn alloy powder by coupling alloying and ball milling and its application on high-rate hydrogen generation at room temperature. Metals 2021, 11, 1704. [Google Scholar] [CrossRef]
- An, Q.; Gao, Q.; Wang, H.; Wei, C.; Li, N. Insight into the indium-related morphology transformation and application for hydrogen production of Al-rich alloys. J. Alloys Compd. 2020, 842, 155864. [Google Scholar] [CrossRef]
- Xiao, F.; Liang, T.; Liu, Z.; Yang, R.; Li, J. Study on the effect of carbon materials with different morphologies on the hydrogen generation performance of aluminum: A strategy to control the hydrogen generation rate of aluminum. J. Alloys Compd. 2021, 879, 160376. [Google Scholar] [CrossRef]
- Xiao, F.; Guo, Y.; Li, J.; Yang, R. Hydrogen generation from hydrolysis of activated aluminum composites in tap water. Energy 2018, 157, 608–614. [Google Scholar] [CrossRef]
- Solovey, V.V.; Zipunnikov, M.M.; Poda, V.B.; Vorobjova, I. Hydrogen generation from water by using alloys based on silicon and aluminium. Vopr. Khimii Khimicheskoi Tekhnol. 2020, 2020, 148–156. [Google Scholar] [CrossRef]
- Eom, K.; Cho, E.; Kwon, H. Feasibility of on-board hydrogen production from hydrolysis of Al-Fe alloy for PEMFCs. Int. J. Hydrogen Energy 2011, 36, 12338–12342. [Google Scholar] [CrossRef]
- Kim, M.; Eom, K.; Kwon, J.; Cho, E.; Kwon, H. On-board hydrogen production by hydrolysis from designed Al-Cu alloys and the application of this technology to polymer electrolyte membrane fuel cells. J. Power Sources 2012, 217, 345–350. [Google Scholar] [CrossRef]
- Eom, K.; Kim, M.; Oh, S.; Cho, E.; Kwon, H. Design of ternary Al-Sn-Fe alloy for fast on-board hydrogen production, and its application to PEM fuel cell. Int. J. Hydrogen Energy 2011, 36, 11825–11831. [Google Scholar] [CrossRef]
- Kahveci, O.; Kaya, M.F. Hydrogen production from Al–Cu alloy using electric vehicle’s waste DC motor coils. Int. J. Hydrogen Energy 2022, 47, 12179–12188. [Google Scholar] [CrossRef]
- Eom, K.S.; Kwon, J.Y.; Kim, M.J.; Kwon, H.S. Design of Al-Fe alloys for fast on-board hydrogen production from hydrolysis. J. Mater. Chem. 2011, 21, 13047–13051. [Google Scholar] [CrossRef]
- Wang, C.; Lin, K.; Liu, Y.; Chen, X.; Zou, H.; Qiu, C.; Yang, S.; Liu, X. Design and fabrication of high activity retention Al-based composite powders for mild hydrogen generation. Materials 2019, 12, 3328. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Du Preez, S.P.; Bessarabov, D.G. The Hydrolysis of Ball-Milled Aluminum–Bismuth–Nickel Composites for On-Demand Hydrogen Generation. Energies 2022, 15, 2356. [Google Scholar] [CrossRef]
- du Preez, S.P.; Bessarabov, D.G. Hydrogen generation of mechanochemically activated Al–Bi–In composites. Int. J. Hydrogen Energy 2017, 42, 16589–16602. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, R.; Gao, W.; Hu, J.; Li, J. Effect of carbon materials and bismuth particle size on hydrogen generation using aluminum-based composites. J. Alloys Compd. 2020, 817, 152800. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, R.; Li, J. Aluminum composites with bismuth nanoparticles and graphene oxide and their application to hydrogen generation in water. Int. J. Hydrogen Energy 2020, 45, 6082–6089. [Google Scholar] [CrossRef]
- Czech, E.; Troczynski, T. Hydrogen generation through massive corrosion of deformed aluminum in water. Int. J. Hydrogen Energy 2010, 35, 1029–1037. [Google Scholar] [CrossRef]
- Manilevich, F.D.; Pirskyy, Y.K.; Kutsyi, A.V.; Berezovets, V.V.; Yartys, V.A. Studies of Mechanochemically Activated Aluminum Powders for Generating Hydrogen from Water. Powder Metall. Met. Ceram. 2021, 60, 268–277. [Google Scholar] [CrossRef]
- Zhu, L.; Zou, M.; Zhang, X.; Zhang, L.; Wang, X.; Song, T.; Wang, S.; Li, X. Enhanced hydrogen generation performance of al-rich alloys by a melting-mechanical crushing-ball milling method. Materials 2021, 14, 7889. [Google Scholar] [CrossRef]
- Jia, Y.; Shen, J.; Meng, H.; Dong, Y.; Chai, Y.; Wang, N. Hydrogen generation using a ball-milled Al/Ni/NaCl mixture. J. Alloys Compd. 2014, 588, 259–264. [Google Scholar] [CrossRef]
- Chen, C.; Guan, X.; Wang, H.; Dong, S.; Luo, P. Hydrogen generation from splitting water with Al–Bi(OH)3 composite promoted by NaCl. Int. J. Hydrogen Energy 2020, 45, 13139–13148. [Google Scholar] [CrossRef]
- Deng, Z.-Y.; Tang, Y.-B.; Zhu, L.-L.; Sakka, Y.; Ye, J. Effect of different modification agents on hydrogen-generation by the reaction of Al with water. Int. J. Hydrogen Energy 2010, 35, 9561–9568. [Google Scholar] [CrossRef]
- Teng, H.-T.; Lee, T.-Y.; Chen, Y.-K.; Wang, H.-W.; Cao, G. Effect of Al(OH)3 on the hydrogen generation of aluminum–water system. J. Power Sources 2012, 219, 16–21. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Y.; Duan, Y.; Hou, L.; Cui, L.; Yang, F.; Zheng, Y.; Li, Y.; Huang, J. Green production of hydrogen by hydrolysis of graphene-modified aluminum through infrared light irradiation. Chem. Eng. J. 2017, 320, 160–167. [Google Scholar] [CrossRef]
- Huang, X.-N.; Lv, C.-J.; Wang, Y.; Shen, H.-Y.; Chen, D.; Huang, Y.-X. Hydrogen generation from hydrolysis of aluminum/graphite composites with a core–shell structure. Int. J. Hydrogen Energy 2012, 37, 7457–7463. [Google Scholar] [CrossRef]
- Yu, M.; Kim, M.; Yoon, B.; Oh, S.; Nam, D.-H.; Kwon, H. Carbon nanotubes/aluminum composite as a hydrogen source for PEMFC. Int. J. Hydrogen Energy 2014, 39, 19416–19423. [Google Scholar] [CrossRef]
- Kumar, D.; Muthukumar, K. An overview on activation of aluminium-water reaction for enhanced hydrogen production. J. Alloys Compd. 2020, 835, 155189. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J.A. Effect of addition of water-soluble salts on the hydrogen generation of aluminum in reaction with hot water. J. Alloys Compd. 2016, 679, 364–374. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, R.; Liu, Z. Active aluminum composites and their hydrogen generation via hydrolysis reaction: A review. Int. J. Hydrogen Energy 2022, 47, 365–386. [Google Scholar] [CrossRef]
- Safyari, M.; Moshtaghi, M. Role of Ultrasonic Shot Peening in Environmental Hydrogen Embrittlement Behavior of 7075-T6 Alloy. Hydrogen 2021, 2, 377–385. [Google Scholar]
- Safyari, M.; Moshtaghi, M.; Kuramoto, S.; Hojo, T. Influence of microstructure-driven hydrogen distribution on environmental hydrogen embrittlement of an Al–Cu–Mg alloy. Int. J. Hydrogen Energy 2021, 46, 37502–37508. [Google Scholar] [CrossRef]
- Alviani, V.N.; Hirano, N.; Watanabe, N.; Oba, M.; Uno, M.; Tsuchiya, N. Local initiative hydrogen production by utilization of aluminum waste materials and natural acidic hot-spring water. Appl. Energy 2021, 293, 116909. [Google Scholar] [CrossRef]
- Martínez-Salazar, A.L.; Melo-Banda, J.A.; Coronel-García, M.A.; González-Barbosa, J.J.; Domínguez-Esquivel, J.M. Hydrogen generation by aluminum alloy corrosion in aqueous acid solutions promoted by nanometal: Kinetics study. Renew. Energy 2020, 146, 2517–2523. [Google Scholar] [CrossRef]
- Alviani, V.N.; Setiani, P.; Uno, M.; Oba, M.; Hirano, N.; Watanabe, N.; Tsuchiya, N.; Saishu, H. Mechanisms and possible applications of the Al–H2O reaction under extreme pH and low hydrothermal temperatures. Int. J. Hydrogen Energy 2019, 44, 29903–29921. [Google Scholar] [CrossRef]
- Hiraki, T.; Takeuchi, M.; Hisa, M.; Akiyama, T. Hydrogen Production from Waste Aluminum at Different Temperatures, with LCA. Mater. Trans. 2005, 46, 1052–1057. [Google Scholar] [CrossRef]
- Moreno-Flores, R.; Loyola-Morales, F.; Valenzuela, E.; Sebastian, P.J. Design and performance evaluation of a prototype hydrogen generator employing hydrolysis of aluminum waste. Clean Technol. Environ. Policy 2022, 1–7. [Google Scholar] [CrossRef]
- Lim, S.T.; Sethupathi, S.; Alsultan, A.G.; Munusamy, Y. Hydrogen Production via Activated Waste Aluminum Cans and Its Potential for Methanation. Energy Fuels 2021, 35, 16212–16221. [Google Scholar] [CrossRef]
- Chen, X.; Wang, C.; Liu, Y.; Zou, H.; Lin, K.; Yang, S.; Lu, Y.; Han, J.; Shi, Z.; Liu, X. Portable water-using H2 production materials converted from waste aluminum. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 1991–1997. [Google Scholar] [CrossRef]
- Yolcular, S.; Karaoglu, S.; Karasoglu, M. Hydrogen generation performance of waste aluminum alloy chips and powders. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 1529–1540. [Google Scholar] [CrossRef]
- Singh, K.; Meshram, A.; Gautam, D.; Jain, A. Hydrogen production using waste aluminium dross: From industrial waste to next-generation fuel. Agron. Res. 2019, 17, 1199–1206. [Google Scholar]
- Kale, M.; Yılmaz, İ.H.; Kaya, A.; Çetin, A.E.; Söylemez, M.S. Pilot-scale hydrogen generation from the hydrolysis of black aluminum dross without any catalyst. J. Energy Inst. 2022, 100, 99–108. [Google Scholar] [CrossRef]
- Tang, W.; Yan, L.; Li, K.; Juan, Y.; Fu, C.; Zhang, J. A comparison study on aluminum-water reaction with different catalysts. Mater. Today Commun. 2022, 31, 103517. [Google Scholar] [CrossRef]
- Belitskus, D. Reaction of Aluminum with Sodium Hydroxide Solution as a Source of Hydrogen. J. Electrochem. Soc. 1970, 117, 1097–1099. [Google Scholar] [CrossRef]
- Salueña-Berna, X.; Marín-Genescà, M.; Massagués Vidal, L.; Dagà-Monmany, J.M. Waste Aluminum Application as Energy Valorization for Hydrogen Fuel Cells for Mobile Low Power Machines Applications. Materials 2021, 14, 7323. [Google Scholar] [CrossRef]
- Haller, M.Y.; Amstad, D.; Dudita, M.; Englert, A.; Häberle, A. Combined heat and power production based on renewable aluminium-water reaction. Renew. Energy 2021, 174, 879–893. [Google Scholar] [CrossRef]
- Jung, C.R.; Kundu, A.; Ku, B.; Gil, J.H.; Lee, H.R.; Jang, J.H. Hydrogen from aluminium in a flow reactor for fuel cell applications. J. Power Sources 2008, 175, 490–494. [Google Scholar] [CrossRef]
- Katsoufis, P.; Doukas, E.; Politis, C.; Avgouropoulos, G.; Lianos, P. Enhanced rate of hydrogen production by corrosion of commercial aluminum. Int. J. Hydrogen Energy 2020, 45, 10729–10734. [Google Scholar] [CrossRef]
- Salueña Berna, X.; Marín-Genescà, M.; Dagà-Monmany, J.M. Analysis of Valorization Process of Aluminum Breakage Scraps to Obtain Green Hydrogen. Metals 2021, 11, 598. [Google Scholar] [CrossRef]
- Martínez, S.S.; Albañil Sánchez, L.; Álvarez Gallegos, A.A.; Sebastian, P.J. Coupling a PEM fuel cell and the hydrogen generation from aluminum waste cans. Int. J. Hydrogen Energy 2007, 32, 3159–3162. [Google Scholar] [CrossRef]
- Wang, E.-D.; Shi, P.-F.; Du, C.-Y.; Wang, X.-R. A mini-type hydrogen generator from aluminum for proton exchange membrane fuel cells. J. Power Sources 2008, 181, 144–148. [Google Scholar] [CrossRef]
- Soler, L.; Candela, A.M.; Macanás, J.; Muñoz, M.; Casado, J. In situ generation of hydrogen from water by aluminum corrosion in solutions of sodium aluminate. J. Power Sources 2009, 192, 21–26. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Peng, R.; Zhang, S.; Huang, X.; Zhao, Z. Highly efficient hydrolysis of magnetic milled powder from waste aluminum (Al) cans with low-concentrated alkaline solution for hydrogen generation. Int. J. Energy Res. 2019, 43, 4797–4806. [Google Scholar] [CrossRef]
- Ho, C.-Y. Hydrolytic reaction of waste aluminum foils for high efficiency of hydrogen generation. Int. J. Hydrogen Energy 2017, 42, 19622–19628. [Google Scholar] [CrossRef]
- Tekade, S.P.; Shende, D.Z.; Wasewar, K.L. Hydrogen Generation in an Annular Micro-Reactor: An Experimental Investigation and Reaction Modelling by Shrinking Core Model (SCM). Int. J. Chem. React. Eng. 2018, 16, 20170202. [Google Scholar] [CrossRef]
- Tekade, S.P.; Shende, D.Z.; Wasewar, K.L. Hydrogen Generation in Water Splitting Reaction Using Aluminum: Effect of NaOH Concentration and Reaction Modelling Using SCM. Int. J. Chem. React. Eng. 2018, 16, 20170250. [Google Scholar] [CrossRef]
- Tekade, S.P.; Pednekar, A.S.; Jadhav, G.R.; Kalekar, S.E.; Shende, D.Z.; Wasewar, K.L. Hydrogen generation through water splitting reaction using waste aluminum in presence of gallium. Int. J. Hydrogen Energy 2020, 45, 23954–23965. [Google Scholar] [CrossRef]
- Meshram, A.; Jain, A.; Rao, M.D.; Singh, K.K. From industrial waste to valuable products: Preparation of hydrogen gas and alumina from aluminium dross. J. Mater. Cycles Waste Manag. 2019, 21, 984–993. [Google Scholar] [CrossRef]
- Tekade, S.P.; Shende, D.Z.; Wasewar, K.L. Hydrogen Generation in an Annular Micro-Reactor: An Experimental Investigation of Water Splitting Reaction Using Aluminum in Presence of Potassium Hydroxide. Int. J. Chem. React. Eng. 2019, 17, 20180104. [Google Scholar] [CrossRef]
- Aleksandrov, Y.A.; Tsyganova, E.; Pisarev, A. Reaction of Aluminum with Dilute Aqueous NaOH Solutions. Russ. J. Gen. Chem. 2003, 73, 689–694. [Google Scholar]
- Barakat, M.A.; El-Sheikh, S.M.; Farghly, F.E. Regeneration of spent alkali from aluminum washing. Sep. Purif. Technol. 2005, 46, 214–218. [Google Scholar] [CrossRef]
- Ambaryan, G.N.; Vlaskin, M.S.; Dudoladov, A.O.; Meshkov, E.A.; Zhuk, A.Z.; Shkolnikov, E.I. Hydrogen generation by oxidation of coarse aluminum in low content alkali aqueous solution under intensive mixing. Int. J. Hydrogen Energy 2016, 41, 17216–17224. [Google Scholar] [CrossRef]
- Lim, S.T.; Sethupathi, S.; Alsultan, A.G.; Leong, L.K.; Taufiq-Yap, Y.H. Hydrogen Gas Production Using Aluminum Waste Cans Powder Produced by Disintegration Method. Key Eng. Mater. 2020, 853, 228–234. [Google Scholar] [CrossRef]
- Porciúncula, C.; Marcilio, N.; Tessaro, I.; Gerchmann, M. Production of hydrogen in the reaction between aluminum and water in the presence of NaOH and KOH. Braz. J. Chem. Eng. 2012, 29, 337–348. [Google Scholar]
- Lluis Soler, J.; Munoz, M.; Casado, J. Aluminium and Aluminium alloy as sources of hydrogen for fuel cell application. J. Power Sources 2007, 169, 144–149. [Google Scholar]
- Kaiser, K.M.A. Recycling of multilayer packaging using a reversible cross-linking adhesive. J. Appl. Polym. Sci. 2020, 137, 49230. [Google Scholar] [CrossRef]
- Mumladze, T.; Tatariants, M.; Rimšaitė, A.; Yousef, S.; Denafas, G. Recycling of multilayer packaging foils by using different organic solvent. Iran. J. Energy Environ. 2019, 10, 38–42. [Google Scholar]
- Cervantes-Reyes, A.; Núñez-Pineda, A.; Barrera-Díaz, C.; Varela-Guerrero, V.; Martínez-Barrera, G.; Cuevas-Yañez, E. Solvent effect in the polyethylene recovery from multilayer postconsumer aseptic packaging. Waste Manag. 2015, 38, 61–64. [Google Scholar] [CrossRef]
- Rodrigues Fernandes, J.; Pereira Amaro, L.; Curti Muniz, E.; Favaro, S.L.; Radovanovic, E. PET depolimerization in supercritical ethanol conditions catalysed by nanoparticles of metal oxides. J. Supercrit. Fluids 2020, 158, 104715. [Google Scholar] [CrossRef]
- Fávaro, S.L.; Freitas, A.R.; Ganzerli, T.A.; Pereira, A.G.B.; Cardozo, A.L.; Baron, O.; Muniz, E.C.; Girotto, E.M.; Radovanovic, E. PET and aluminum recycling from multilayer food packaging using supercritical ethanol. J. Supercrit. Fluids 2013, 75, 138–143. [Google Scholar] [CrossRef]
- Diop, C.I.K.; Lavoie, J.-M.; Huneault, M.A. Separation and Reuse of Multilayer Food Packaging in Cellulose Reinforced Polyethylene Composites. Waste Biomass Valoriz. 2017, 8, 85–93. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhu, Y.; Fang, C.; Xu, D.; Zheng, X. Interactions of the Main Components in Paper-Plastic-Aluminum Complex Packaging Wastes during the Hydrothermal Liquefaction Process. Chem. Eng. Technol. 2021, 44, 1519–1527. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Blázquez, G.; Franco, J.F.; Calero, M.; Martín-Lara, M.A. Recovery, separation and production of fuel, plastic and aluminum from the Tetra PAK waste to hydrothermal and pyrolysis processes. Waste Manag. 2022, 137, 179–189. [Google Scholar] [CrossRef]
- Mu’min, G.F.; Prawisudha, P.; Zaini, I.N.; Aziz, M.; Pasek, A.D. Municipal solid waste processing and separation employing wet torrefaction for alternative fuel production and aluminum reclamation. Waste Manag. 2017, 67, 106–120. [Google Scholar] [CrossRef]
- Chen, X.; Luo, Y.; Bai, X. Upcycling polyamide containing post-consumer Tetra Pak carton packaging to valuable chemicals and recyclable polymer. Waste Manag. 2021, 131, 423–432. [Google Scholar] [CrossRef]
- Huan, S.; Wang, Y.; Peng, J.; Di, Y.; Li, B.; Zhang, L. Recovery of aluminum from waste aluminum alloy by low-temperature molten salt electrolysis. Miner. Eng. 2020, 154, 106386. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Pan, M.-W.; Lo, S.-L. Hydrometallurgical metal recovery from waste printed circuit boards pretreated by microwave pyrolysis. Resour. Conserv. Recycl. 2020, 163, 105090. [Google Scholar] [CrossRef]
- Alvarenga, L.M.; Xavier, T.P.; Barrozo, M.A.S.; Bacelos, M.S.; Lira, T.S. Analysis of Reaction Kinetics of Carton Packaging Pyrolysis. Procedia Eng. 2012, 42, 113–122. [Google Scholar] [CrossRef]
- Kulkarni, A.K.; Daneshvarhosseini, S.; Yoshida, H. Effective recovery of pure aluminum from waste composite laminates by sub- and super-critical water. J. Supercrit. Fluids 2011, 55, 992–997. [Google Scholar] [CrossRef]
- Satish Reddy, M.; Neeraja, D. Aluminum residue waste for possible utilisation as a material: A review. Sādhanā 2018, 43, 124. [Google Scholar] [CrossRef]
- Soares, C.T.d.M.; Ek, M.; Östmark, E.; Gällstedt, M.; Karlsson, S. Recycling of multi-material multilayer plastic packaging: Current trends and future scenarios. Resour. Conserv. Recycl. 2022, 176, 105905. [Google Scholar] [CrossRef]
- He, T.T.; Wang, W.; Chen, D.M.; Yang, K. Effect of Ti on the microstructure and Al–water reactivity of Al-rich alloy. Int. J. Hydrogen Energy 2014, 39, 684–691. [Google Scholar] [CrossRef]
- He, T.; Chen, W.; Wang, W.; Ren, F.; Stock, H.-R. Effect of different Cu contents on the microstructure and hydrogen production of Al–Cu-Ga-In-Sn alloys for dissolvable materials. J. Alloys Compd. 2020, 821, 153489. [Google Scholar] [CrossRef]
- He, T.; Chen, W.; Wang, W.; Du, S.; Deng, S. Microstructure and hydrogen production of the rapidly solidified Al–Mg-Ga-In-Sn alloy. J. Alloys Compd. 2020, 827, 154290. [Google Scholar] [CrossRef]
- Elitzur, S.; Rosenband, V.; Gany, A. Study of hydrogen production and storage based on aluminum–water reaction. Int. J. Hydrogen Energy 2014, 39, 6328–6334. [Google Scholar] [CrossRef]
- Meroueh, L.; Eagar, T.W.; Hart, D.P. Effects of Mg and Si Doping on Hydrogen Generation via Reduction of Aluminum Alloys in Water. ACS Appl. Energy Mater. 2020, 3, 1860–1868. [Google Scholar] [CrossRef]
- Lyndgaard, L.B.; van den Berg, F.; de Juan, A. Quantification of paracetamol through tablet blister packages by Raman spectroscopy and multivariate curve resolution-alternating least squares. Chemom. Intell. Lab. Syst. 2013, 125, 58–66. [Google Scholar] [CrossRef]
- Pedrosa de Oliveira, D.; Costa, J.S.R.; Oliveira-Nascimento, L. Sustainability of blisters for medicines in tablet form. Sustain. Chem. Pharm. 2021, 21, 100423. [Google Scholar] [CrossRef]
- Brunner, A.J. X-ray diffraction pattern of poly(vinyl chloride). J. Polym. Sci. Part B Polym. Lett. 1972, 10, 379–383. [Google Scholar] [CrossRef]
- Mallakpour, S.; Sadeghzadeh, R. Facile and green methodology for surface-grafted Al2O3 nanoparticles with biocompatible molecules: Preparation of the poly(vinyl alcohol)@poly(vinyl pyrrolidone) nanocomposites. Polym. Adv. Technol. 2017, 28, 1719–1729. [Google Scholar] [CrossRef]
- Patrick, S. Practical Guide to Polyvinyl Chloride; iSmithers Rapra Publishing: Shrewsbury, UK, 2005. [Google Scholar]
- Lu, J.; Borjigin, S.; Kumagai, S.; Kameda, T.; Saito, Y.; Yoshioka, T. Practical dechlorination of polyvinyl chloride wastes in NaOH/ethylene glycol using an up-scale ball mill reactor and validation by discrete element method simulations. Waste Manag. 2019, 99, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Kise, H. Dehydrochlorination of poly(vinyl chloride) by aqueous sodium hydroxide solution under two-phase conditions. J. Polym. Sci. Polym. Chem. Ed. 1982, 20, 3189–3197. [Google Scholar] [CrossRef]
- Sata, T.; Tsujimoto, M.; Yamaguchi, T.; Matsusaki, K. Change of anion exchange membranes in an aqueous sodium hydroxide solution at high temperature. J. Membr. Sci. 1996, 112, 161–170. [Google Scholar] [CrossRef]
- Xiong, B.; Zhao, P.; Cai, P.; Zhang, L.; Hu, K.; Cheng, G. NMR spectroscopic studies on the mechanism of cellulose dissolution in alkali solutions. Cellulose 2013, 20, 613–621. [Google Scholar] [CrossRef]
- Braun, J.H.; Baidins, A.; Marganski, R.E. TiO2 pigment technology: A review. Prog. Org. Coat. 1992, 20, 105–138. [Google Scholar] [CrossRef]
- Karaman, A.D.; Özer, B.; Pascall, M.A.; Alvarez, V. Recent Advances in Dairy Packaging. Food Rev. Int. 2015, 31, 295–318. [Google Scholar] [CrossRef]
- Goulas, A.E. Overall migration from commercial coextruded food packaging multilayer films and plastics containers into official EU food simulants. Eur. Food Res. Technol. 2001, 212, 597–602. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R. Review on metal packaging: Materials, forms, food applications, safety and recyclability. J. Food Sci. Technol. 2020, 57, 2377–2392. [Google Scholar] [CrossRef]
- Ceylan Sahin, C.; Erbay, Z.; Koca, N. The physical, microstructural, chemical and sensorial properties of spray dried full-fat white cheese powders stored in different multilayer packages. J. Food Eng. 2018, 229, 57–64. [Google Scholar] [CrossRef]
- Ügdüler, S.; Van Geem, K.M.; Denolf, R.; Roosen, M.; Mys, N.; Ragaert, K.; De Meester, S. Towards closed-loop recycling of multilayer and coloured PET plastic waste by alkaline hydrolysis. Green Chem. 2020, 22, 5376–5394. [Google Scholar] [CrossRef]
- Hadjizadeh, A.; Ajji, A.; Bureau, M.N. Preparation and characterization of NaOH treated micro-fibrous polyethylene terephthalate nonwovens for biomedical application. J. Mech. Behav. Biomed. Mater. 2010, 3, 574–583. [Google Scholar] [CrossRef]
- Purchas, D.; Sutherland, K. Handbook of Filter Media; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Sujathan, S.; Kniggendorf, A.-K.; Kumar, A.; Roth, B.; Rosenwinkel, K.-H.; Nogueira, R. Heat and Bleach: A Cost-Efficient Method for Extracting Microplastics from Return Activated Sludge. Arch. Environ. Contam. Toxicol. 2017, 73, 641–648. [Google Scholar] [CrossRef]
- Gupta, A.; Basu, B. Sustainable Primary Aluminium Production: Technology Status and Future Opportunities. Trans. Indian Inst. Met. 2019, 72, 2135–2150. [Google Scholar] [CrossRef]
- Teichert, M.; Haller, M.; Sick, F. Aluminium redox cycle in comparison to pressurized hydrogen for the energy supply of multi-family houses. Appl. Energy Combust. Sci. 2022, 13, 100098. [Google Scholar] [CrossRef]
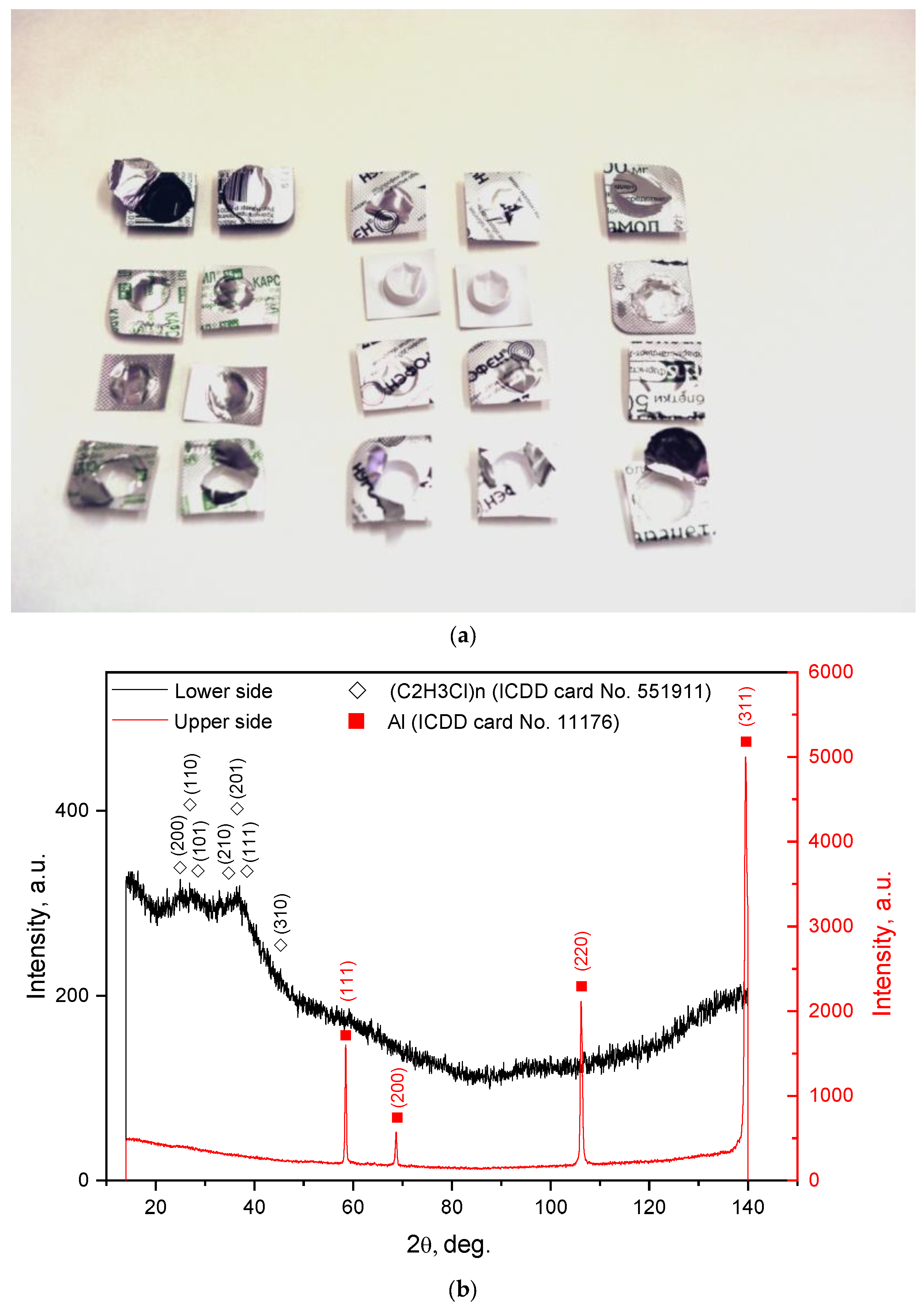
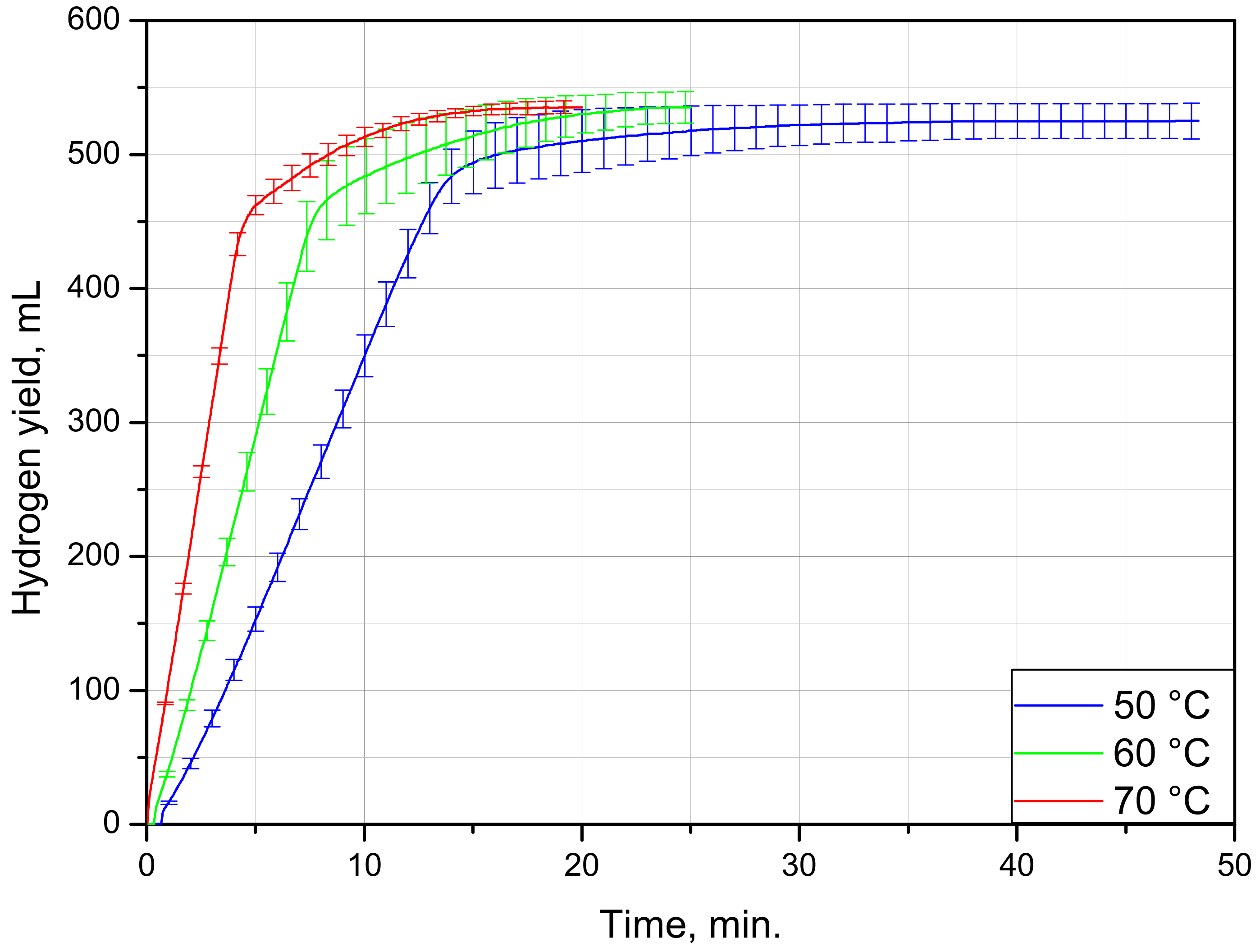
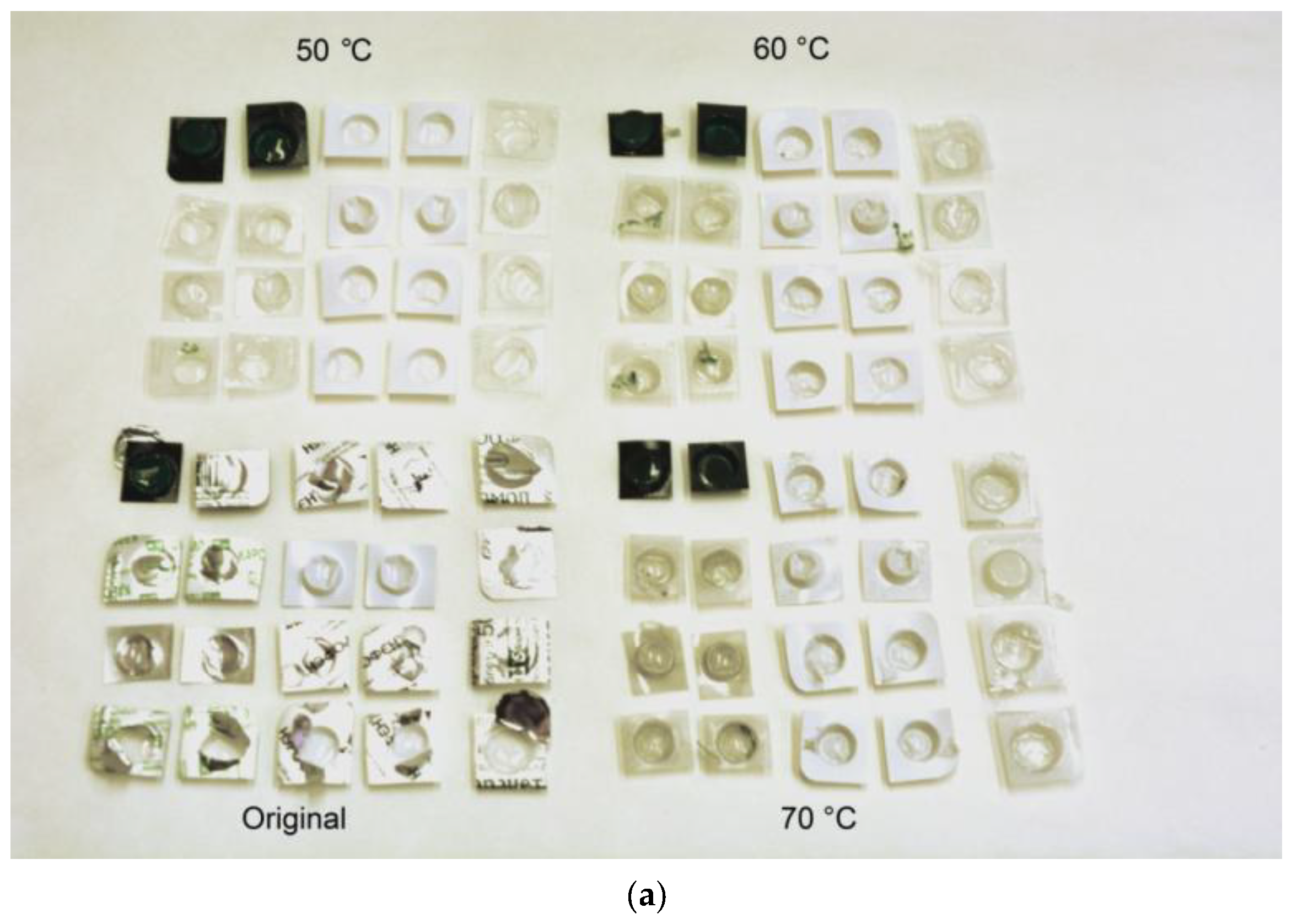
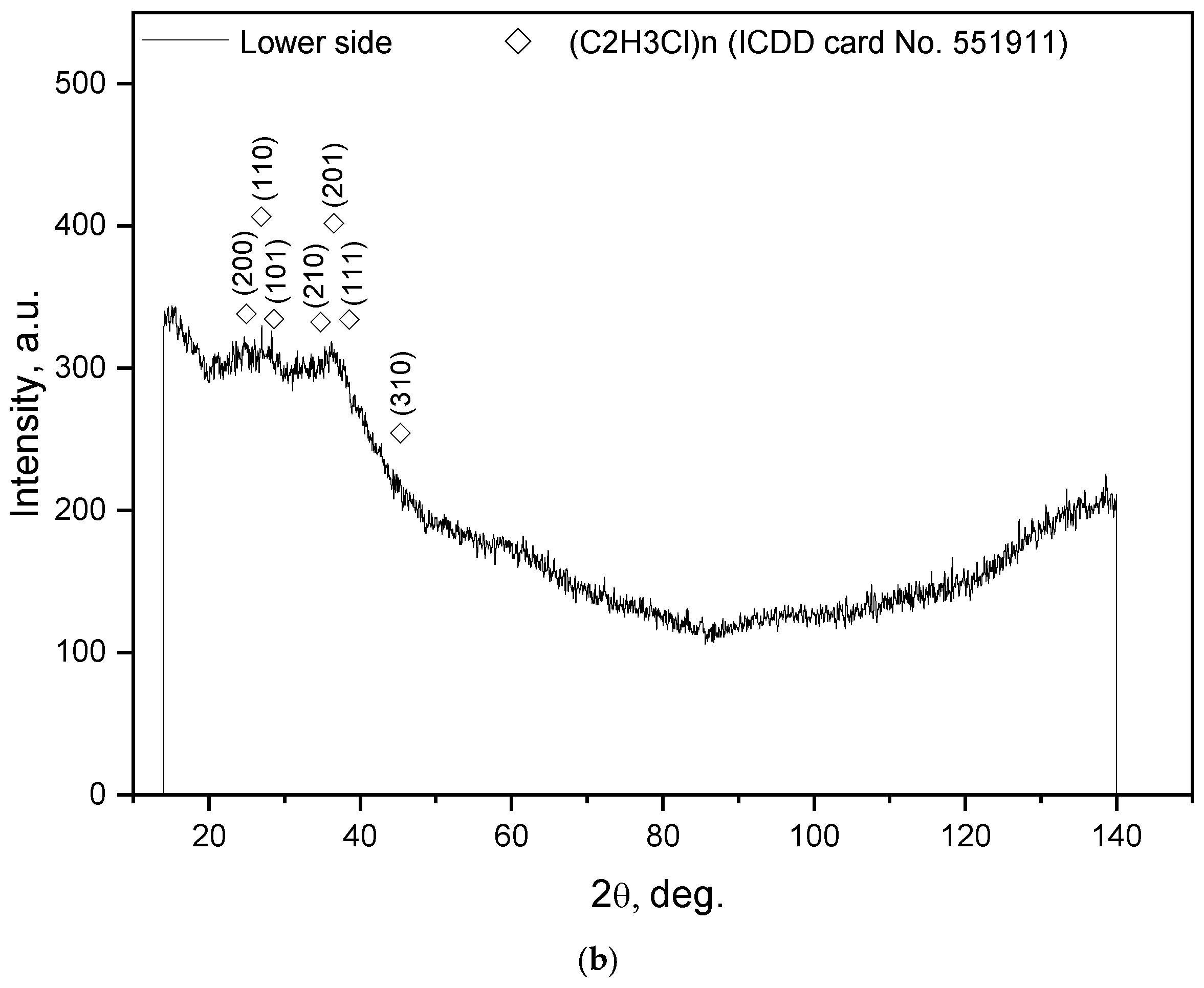
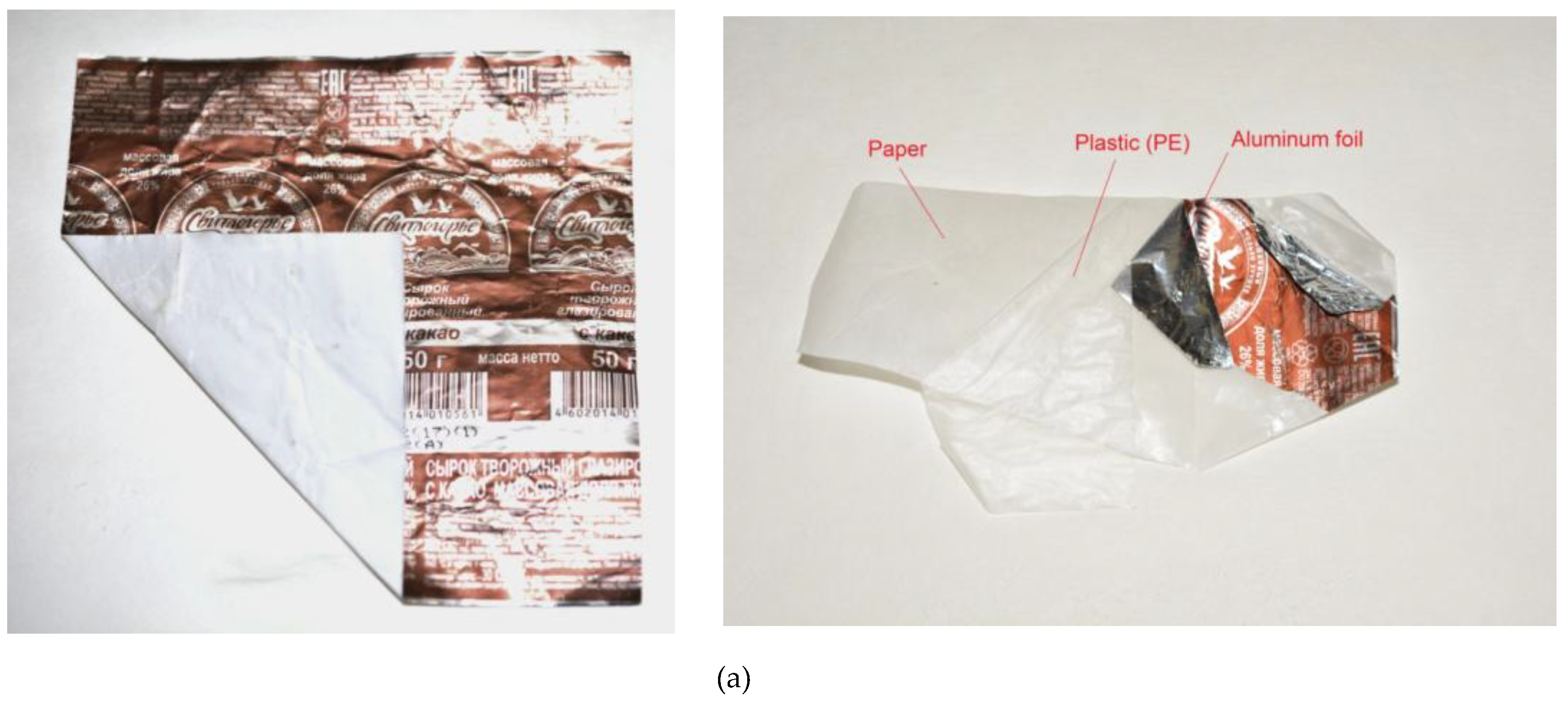
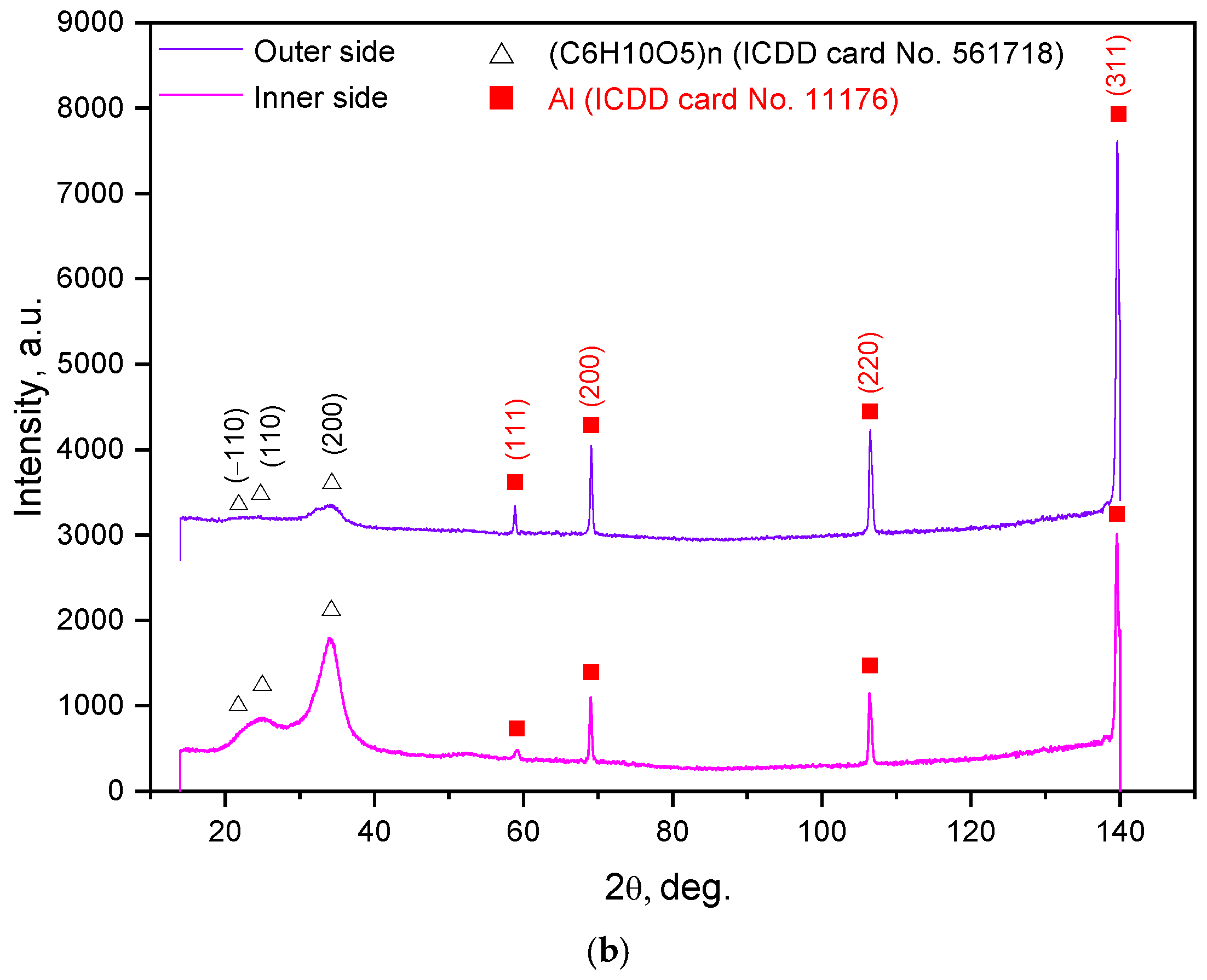
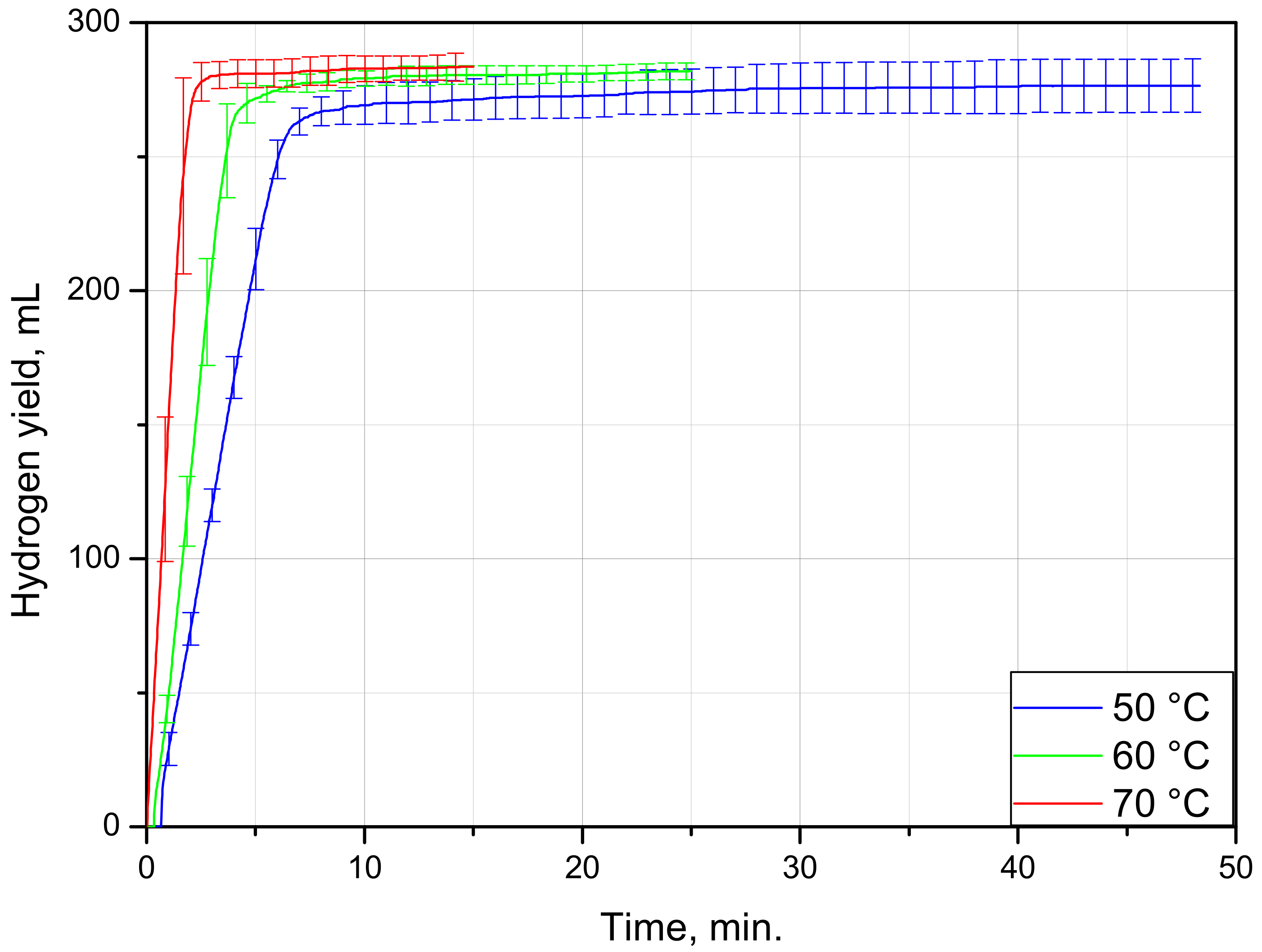

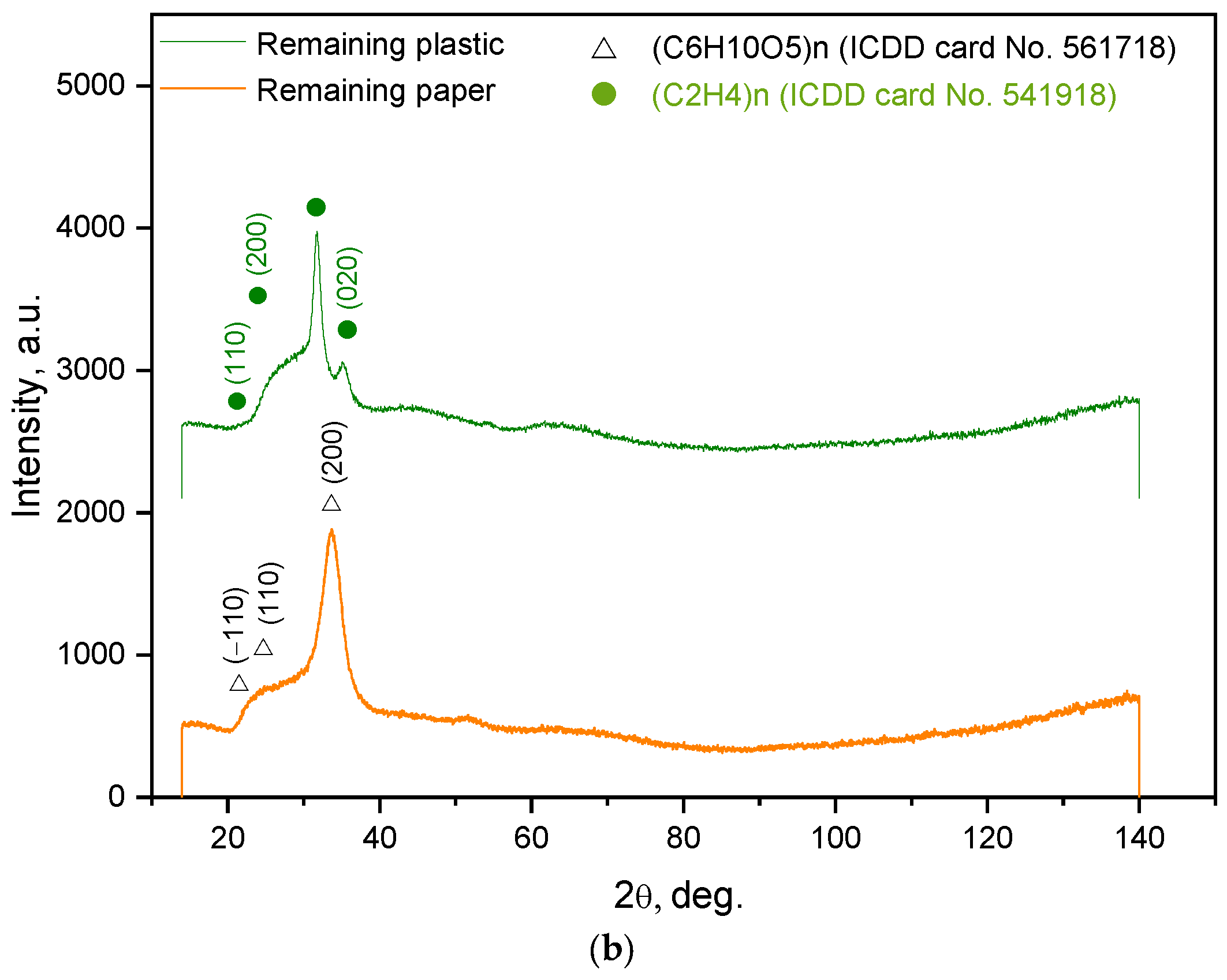

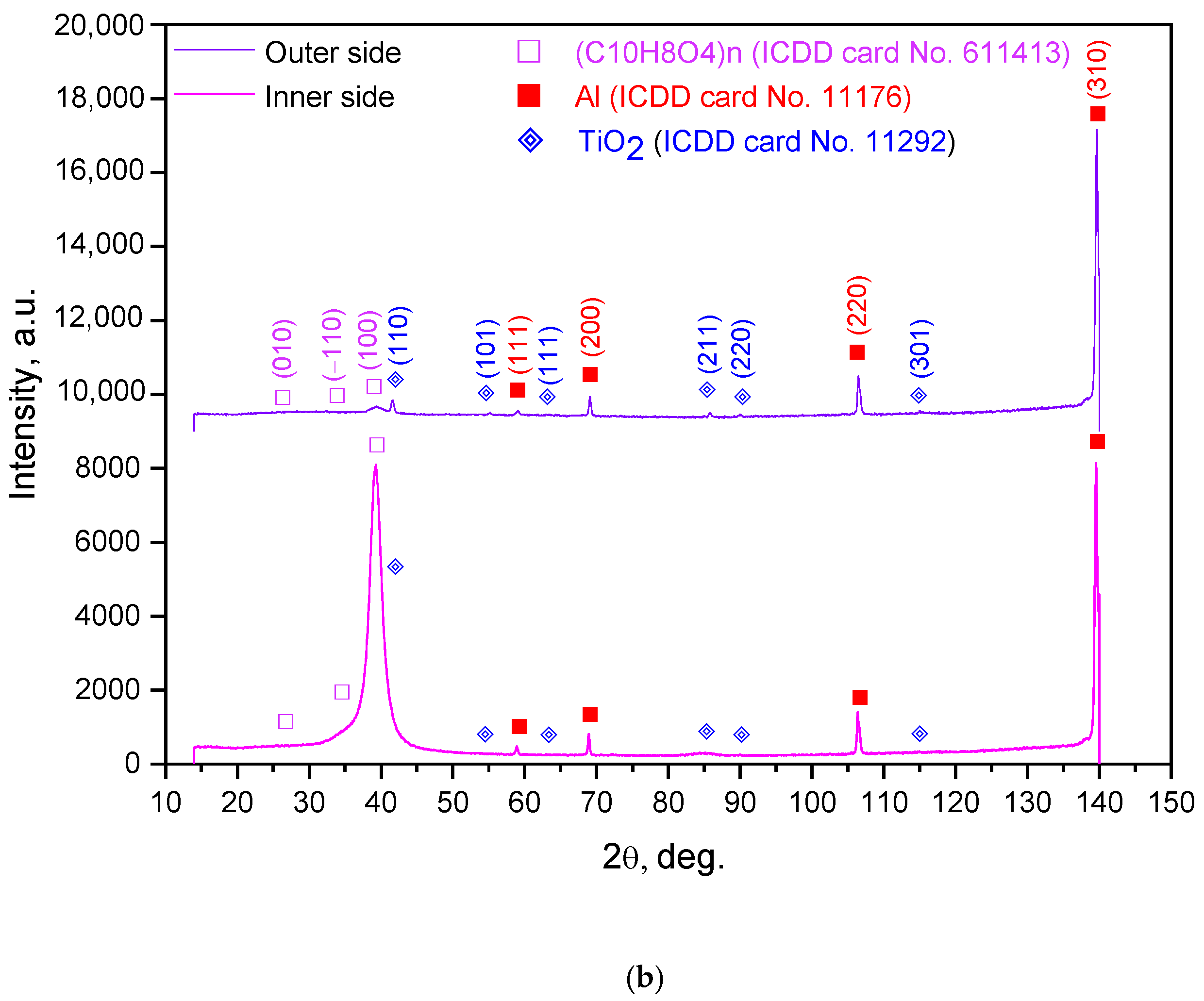
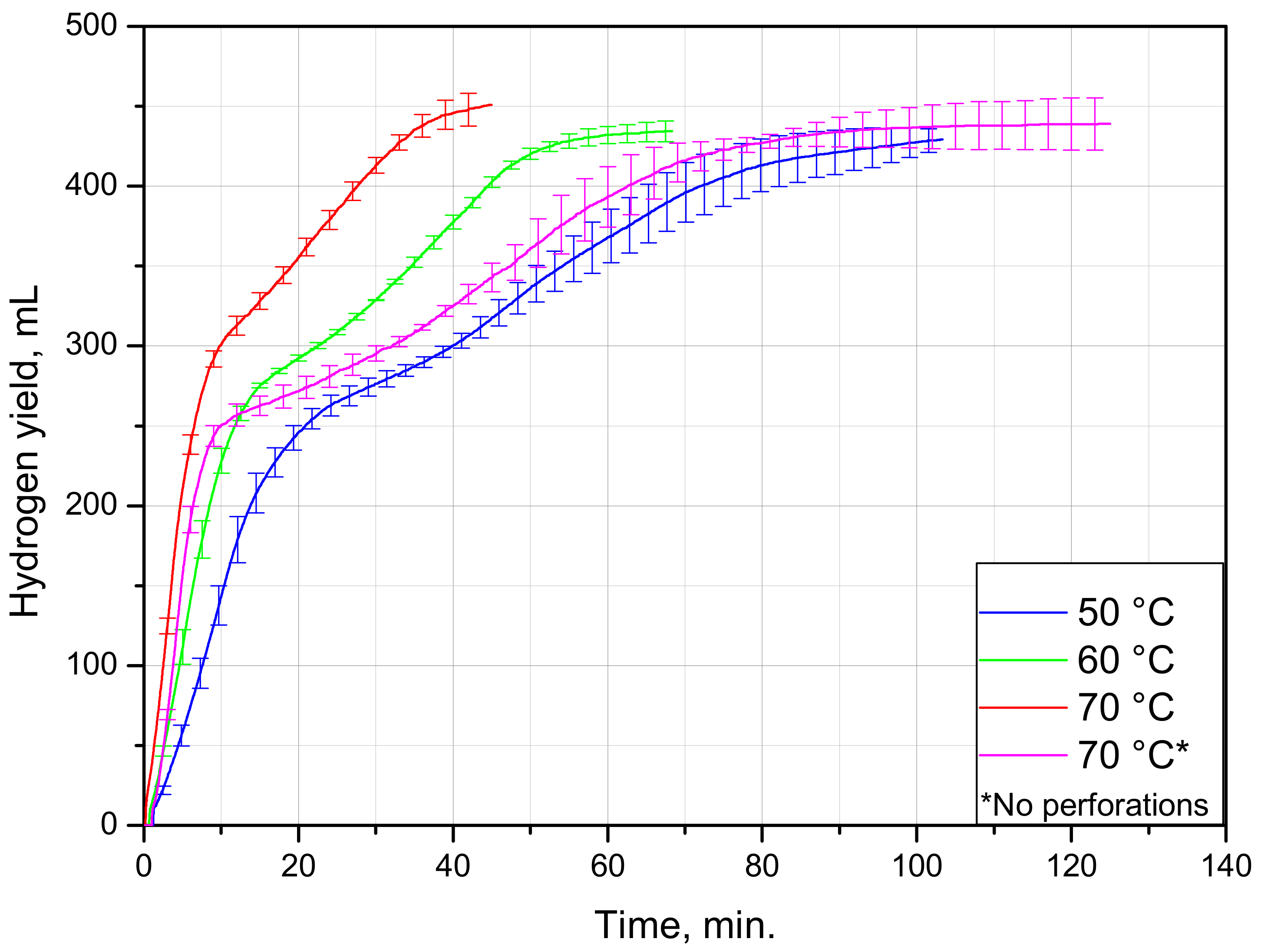


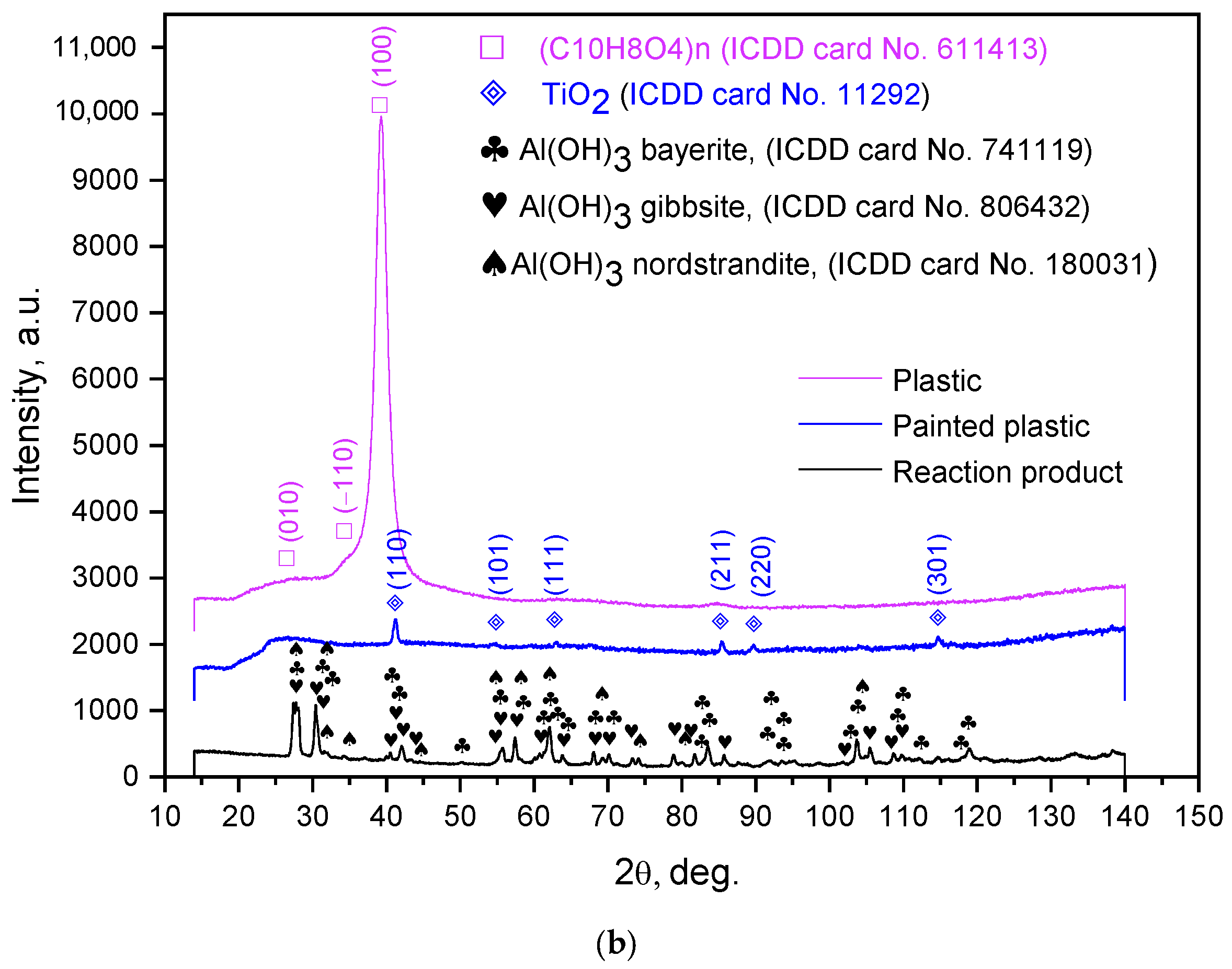
| Sample Mass, g | Foil Surface Area, cm2 | Temperature, °C | H2 Yield, mL | Max. H2 Evolution Rate, mL/g/min |
|---|---|---|---|---|
| 2.9907 | 77.94 | 50 | 540 | 98 |
| 2.8821 | 74.69 | 50 | 519 | 94 |
| 2.9124 | 75.53 | 50 | 516 | 99 |
| 3.0086 | 77.61 | 60 | 546 | 163 |
| 2.9832 | 76.91 | 60 | 536 | 152 |
| 2.9203 | 75.59 | 60 | 523 | 151 |
| 2.9349 | 75.77 | 70 | 537 | 250 |
| 2.9021 | 75.34 | 70 | 530 | 247 |
| 2.9684 | 75.87 | 70 | 539 | 248 |
| Average: 2.945 ± 0.044 | Average: 76.14 ± 1.10 | Average: 532 ± 10 |
| Sample Mass, g | Temperature, °C | Hydrogen Yield, mL | Maximum H2 Evolution Rate, mL/g/min (Average) |
|---|---|---|---|
| 1.1911 | 50 | 286 | 263 |
| 1.1125 | 50 | 277 | 229 |
| 1.0696 | 50 | 266 | 275 |
| 1.1129 | 60 | 278 | 409 |
| 1.1422 | 60 | 283 | 366 |
| 1.1501 | 60 | 284 | 320 |
| 1.1347 | 70 | 280 | 640 |
| 1.1406 | 70 | 281 | 582 |
| 1.1948 | 70 | 290 | 801 |
| Average: 1.139 ± 0.039 | Average: 281 ± 7 |
| Sample Mass, g | Temperature, °C | Hydrogen Yield, mL | Maximum H2 Evolution Rate, mL/g/min. (Average) |
|---|---|---|---|
| 0.5971 | 50 | 432 | 65 |
| 0.6051 | 50 | 434 | 54 |
| 0.5882 | 50 | 422 | 53 |
| 0.5904 | 60 | 434 | 91 |
| 0.5942 | 60 | 428 | 81 |
| 0.6021 | 60 | 442 | 86 |
| 0.5901 | 70 | 453 | 130 |
| 0.5940 | 70 | 460 | 137 |
| 0.6025 | 70 | 440 | 150 |
| 0.5903 * | 70 | 458 * | 133 * |
| 0.5893 * | 70 | 432 * | 130 * |
| 0.5814 * | 70 | 427 * | 138 * |
| Average: 0.594 ± 0.007 | Average: 439 ± 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buryakovskaya, O.A.; Vlaskin, M.S. Hydrogen Recovery from Waste Aluminum–Plastic Composites Treated with Alkaline Solution. Materials 2022, 15, 8699. https://doi.org/10.3390/ma15238699
Buryakovskaya OA, Vlaskin MS. Hydrogen Recovery from Waste Aluminum–Plastic Composites Treated with Alkaline Solution. Materials. 2022; 15(23):8699. https://doi.org/10.3390/ma15238699
Chicago/Turabian StyleBuryakovskaya, Olesya A., and Mikhail S. Vlaskin. 2022. "Hydrogen Recovery from Waste Aluminum–Plastic Composites Treated with Alkaline Solution" Materials 15, no. 23: 8699. https://doi.org/10.3390/ma15238699
APA StyleBuryakovskaya, O. A., & Vlaskin, M. S. (2022). Hydrogen Recovery from Waste Aluminum–Plastic Composites Treated with Alkaline Solution. Materials, 15(23), 8699. https://doi.org/10.3390/ma15238699






