Formation and Inhibition Mechanism of Na8SnSi6O18 during the Soda Roasting Process for Preparing Na2SnO3
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Experimental Procedures
2.2.2. Instrument Techniques
3. Results and Discussion
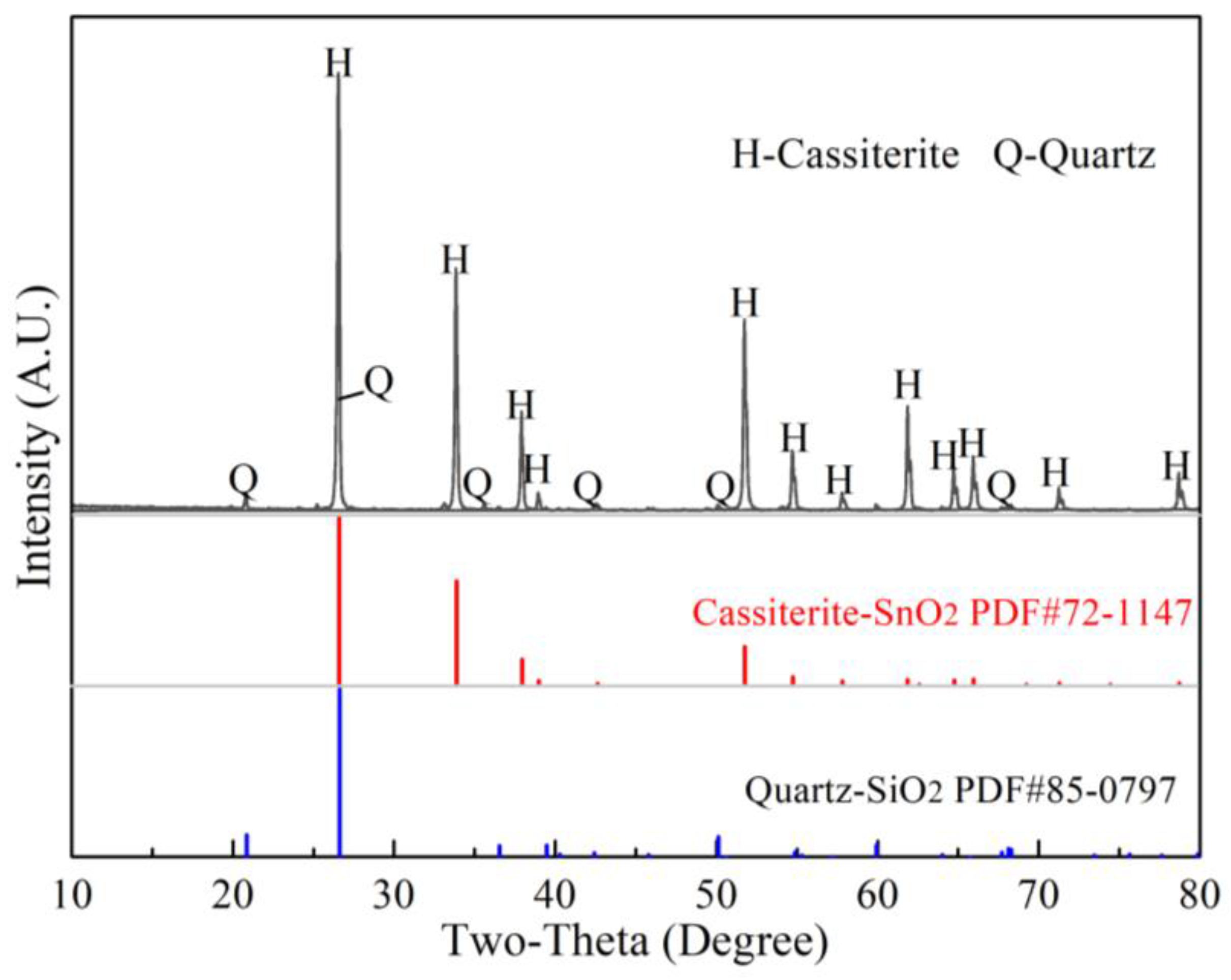
3.1. Phase Analysis for the Products of Soda Roasting
3.2. Effect of Roasting Atmospheres on the Formation of Na8SnSi6O18
3.3. Effect of Intermediate Products on the Formation of Na8SnSi6O18
3.4. Reactions between Na8SnSi6O18 and Na2CO3
3.5. Discussion on the Reaction Mechanism of the SnO2–SiO2–Na2CO3 System
4. Conclusions
- The reactions between Na2CO3 and SnO2/SiO2 proceeded simultaneously during the roasting process, while the formation of Na2SnO3 was promoted under a CO–CO2 atmosphere. Then, Na8SnSi6O18 was easily formed once Na2SnO3 appeared; nonetheless, the reaction between Na2SiO3 and SnO2 was impossible;
- The melting point of Na8SnSi6O18 is only 825 °C, which is much lower than that of Na2CO3, Na2SnO3 and Na2SiO3 in the SnO2–SiO2–Na2CO3 system. Na8SnSi6O18 closely wrapped around the SnO2 particles and restrained the reaction between SnO2 and Na2CO3;
- Na8SnSi6O18 is an unstable compound, and the reaction between Na8SnSi6O18–Na2CO3 can proceed as Na8SnSi6O18 + 3Na2CO3 = 6Na2SiO3 + Na2SnO3 + 3CO2. The reaction was controlled by higher temperatures of above 800 °C as the roasting time was prolonged.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, T.T.; Lee, J.; Moosavi-Khoonsari, E.; Jung, I.-H. Critical evaluation and the thermodynamic optimization of the Sn-O system. Ceram. Int. 2021, 47, 29267–29276. [Google Scholar] [CrossRef]
- Wright, P.A. Extractive Metallurgy of Tin, 2nd ed.; Elsevier Scientific Publishing Company Press: New York, NY, USA, 1982. [Google Scholar]
- Sharma, A.; Das, S.; Das, K. Effect of different electrolytes on the microstructure, corrosion and whisker growth of pulse plated tin coatings. Microelectron. Eng. 2017, 170, 59–68. [Google Scholar] [CrossRef]
- Wang, Y.R.; Tang, R.J.; Yang, C.H.; Xu, T.Y.; Mitsuzaki, N.; Chen, Z.D. Effect of sodium stannate on low temperature electroless Ni-Sn-P deposition and the study of its mechanism. Thin Solid Film. 2019, 669, 72–79. [Google Scholar] [CrossRef]
- Zhang, S.G.; Wei, Y.D.; Yin, S.F.; Luo, S.L.; Au, C.T. Superbasic sodium stannate as catalyst for dehydrogenation, Michael addition and transesterification reactions. Appl. Catal. A-Gen. 2011, 406, 113–118. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Su, Z.J.; Liu, B.B.; You, Z.X.; Li, G.H.; Jiang, T. Sodium stannate preparation from stannic oxide by a novel soda roasting—Leaching process. Hydrometallurgy 2014, 146, 82–88. [Google Scholar] [CrossRef]
- Liu, B.B.; Zhang, Y.B.; Su, Z.J.; Li, G.H.; Jiang, T. Function mechanism of CO-CO2 atmosphere on the formation of Na2SnO3 from SnO2 and Na2CO3 during the roasting process. Powder Technol. 2016, 301, 102–109. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, B.B.; Su, Z.J.; Chen, J.; Li, G.H.; Jiang, T. Effect of Na2CO3 on the preparation of metallic tin from cassiterite roasted under strong reductive atmosphere. J. Min. Met. Sect. B Met. 2016, 52, 9–15. [Google Scholar] [CrossRef]
- Liu, B.B.; Zhang, Y.B.; Su, Z.J.; Li, G.H.; Jiang, T. Phase evolution of tin oxides roasted under CO-CO2 atmospheres in the presence of Na2CO3, Miner. Process. Extr. Met. Rev. 2016, 37, 264–273. [Google Scholar] [CrossRef]
- Liu, B.B.; Zhang, Y.B.; Su, Z.J.; Li, G.H.; Jiang, T. Formation kinetics of Na2SnO3 from SnO2 and Na2CO3 roasted under CO-CO2 atmosphere. Int. J. Miner. Process. 2017, 165, 34–40. [Google Scholar] [CrossRef]
- Liu, W.; Li, W.H.; Han, J.W.; Wu, D.X.; Li, Z.H.; Gu, K.H.; Qin, W.Q. Preparation of calcium stannate from lead refining slag by alkaline leaching-purification-causticization process. Sep. Purif. Technol. 2019, 212, 119–125. [Google Scholar] [CrossRef]
- Wu, D.X.; Liu, W.; Han, J.W.; Jiao, F.; Xu, J.H.; Gu, K.H.; Qin, W.Q. Direct preparation of sodium stannate from lead refining dross after NaOH roasting-water leaching. Sep. Purif. Technol. 2019, 227, 115683. [Google Scholar] [CrossRef]
- Liu, W.; Li, Z.H.; Han, J.W.; Li, W.H.; Wang, X.; Wang, N.; Qin, W.Q. Selective Separation of Arsenic from Lead Smelter Flue Dust by Alkaline Pressure Oxidative Leaching. Minerals 2019, 9, 308. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.X.; Han, J.W.; Liu, W.; Jiao, F.; Qin, W.Q. Preparation of Calcium Stannate from Lead Refining Dross by Roast–Leach–Precipitation Process. Minerals 2019, 9, 283. [Google Scholar] [CrossRef] [Green Version]
- Angadi, S.I.; Sreenivas, T.; Jeon, H.; Baek, S.; Mishra, B.K. A review of cassiterite beneficiation fundamentals and plant practices. Miner. Eng. 2015, 70, 178–200. [Google Scholar] [CrossRef]
- Llorens González, T.; Polonio, F.G.; LópBez Mornito, F.J.; Fernández, A.F.; Contreras, J.L.S.; Moro Benito, M.C. Tin-tantalum-niobium mineralization in the Penouta deposit (NW Spain): Textural features and mineral chemistry to unravel the genesis and evolution of cassiterite and columbite group minerals in a peraluminous system. Ore Geol. Rev. 2017, 81, 79–95. [Google Scholar] [CrossRef]
- Sami, M.; Ntaflos, T.; Mohamed, H.A.; Farahat, E.S.; Hauzenberger, C.; Mahdy, N.M.; Abdelfadil, K.M.; Fathy, D. Origin and Petrogenetic Implications of Spessartine Garnet in Highly-Fractionated Granite from the Central Eastern Desert of Egypt. Acta Geol. Sin. Engl. Ed. 2020, 94, 763–776. [Google Scholar] [CrossRef]
- Sami, M.; El Monsef, M.A.; Abart, R.; Toksoy-Köksal, F.; Abdelfadil, K.M. Unraveling the Genesis of Highly Fractionated Rare-Metal Granites in the Nubian Shield via the Rare-Earth Elements Tetrad Effect, Sr–Nd Isotope Systematics, and Mineral Chemistry. ACS Earth Space Chem. 2022, 6, 2368–2384. [Google Scholar] [CrossRef]
- Su, Z.J.; Zhang, Y.B.; Liu, B.B.; Zhou, Y.L.; Jiang, T.; Li, G.H. Reduction behavior of SnO2 in the tin-bearing iron concentrates under CO-CO2 atmosphere. Part I: Effect of magnetite. Powder Technol. 2016, 292, 251–259. [Google Scholar] [CrossRef]
- Su, Z.J.; Zhang, Y.B.; Liu, B.B.; Lu, M.M.; Li, G.H.; Jiang, T. Extraction and Separation of Tin from Tin-Bearing Secondary Resources: A Review. JOM 2017, 69, 2364–2372. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Han, B.L.; Su, Z.J.; Chen, X.J.; Lu, M.M.; Liu, S.; Liu, J.C.; Jiang, T. Effect of Quartz on the Preparation of Sodium Stannate from Cassiterite Concentrates by Soda Roasting Process. Minerals 2019, 9, 605. [Google Scholar] [CrossRef]
- Lee, J.; Yin, T.T.; Hudon, P.; Jung, I.-H. Phase diagram study of the SnO2-SiO2 system and thermodynamic optimization of the SnO-SnO2-SiO2 system. Ceram. Int. 2021, 48, 4141–4152. [Google Scholar] [CrossRef]
- El-Agawany, F.I.; Tashlykov, O.L.; Mahmoud, K.A.; Rammah, Y.S. The radiation-shielding properties of ternary SiO2-SnO-SnF2 glasses: Simulation and theoretical study. Ceram. Int. 2020, 46, 23369–23378. [Google Scholar] [CrossRef]
- Kang, J.R.; Gu, R.; Guo, X.; Li, J.; Sun, H.C.; Zhang, L.Y.; Jing, R.Y.; Jin, L.; Wei, X.Y. Effect of SnO-P2O5-MgO glass addition on the ionic conductivity of Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte. Ceram. Int. 2021, 48, 157–163. [Google Scholar] [CrossRef]
- Song, L.; Liu, W.H.; Zhao, K.B.; Xin, F.H.; Li, Y.M. Effects of water and carbon dioxide pressure on the adhesion of Na2SiO3 and K2SiO3 binders on silica sand surface: Comparison of experimental data and molecular dynamics simulation. Ceram. Int. 2021, 47, 32648–32656. [Google Scholar] [CrossRef]
- Xia, Y.D.; Mokaya, R. On the synthesis and characterization of ZSM-5/MCM-48 aluminosilicate composite materials. J. Mater. Chem. 2004, 14, 863–870. [Google Scholar] [CrossRef]
- Niphadkar, P.S.; Garade, A.C.; Jha, R.K.; Chandrashekhar, V.R.; Praphulla, N.J. Micro-/meso-porous stannosilicate composites (Sn-MFI/MCM-41) via two-step crystallization process: Process parameter-phase relationship. Microporous Mesoporous Mater. 2010, 136, 115–125. [Google Scholar] [CrossRef]
- Lin, Z.; Rocha, J. Synthesis and characterisation of a stannosilicate with the structure of penkvilksite-1 M. Microporous Mesoporous Mater. 2006, 94, 173–178. [Google Scholar] [CrossRef]
- Santos, T.G.; Silva, A.O.S.; Meneghetti, S.M.P. Comparison of the hydrothermal syntheses of Sn-magadiite using Na2SnO3 and SnCl4·5H2O as the precursors. Appl. Clay Sci. 2019, 183, 105293. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Z.; Liu, S.; Han, B.; Zhang, Y.; Jiang, T. Formation and Inhibition Mechanism of Na8SnSi6O18 during the Soda Roasting Process for Preparing Na2SnO3. Materials 2022, 15, 8718. https://doi.org/10.3390/ma15248718
Su Z, Liu S, Han B, Zhang Y, Jiang T. Formation and Inhibition Mechanism of Na8SnSi6O18 during the Soda Roasting Process for Preparing Na2SnO3. Materials. 2022; 15(24):8718. https://doi.org/10.3390/ma15248718
Chicago/Turabian StyleSu, Zijian, Shuo Liu, Benlai Han, Yuanbo Zhang, and Tao Jiang. 2022. "Formation and Inhibition Mechanism of Na8SnSi6O18 during the Soda Roasting Process for Preparing Na2SnO3" Materials 15, no. 24: 8718. https://doi.org/10.3390/ma15248718



