Recent Progress on Anti-Humidity Strategies of Chemiresistive Gas Sensors
Abstract
:1. Introduction
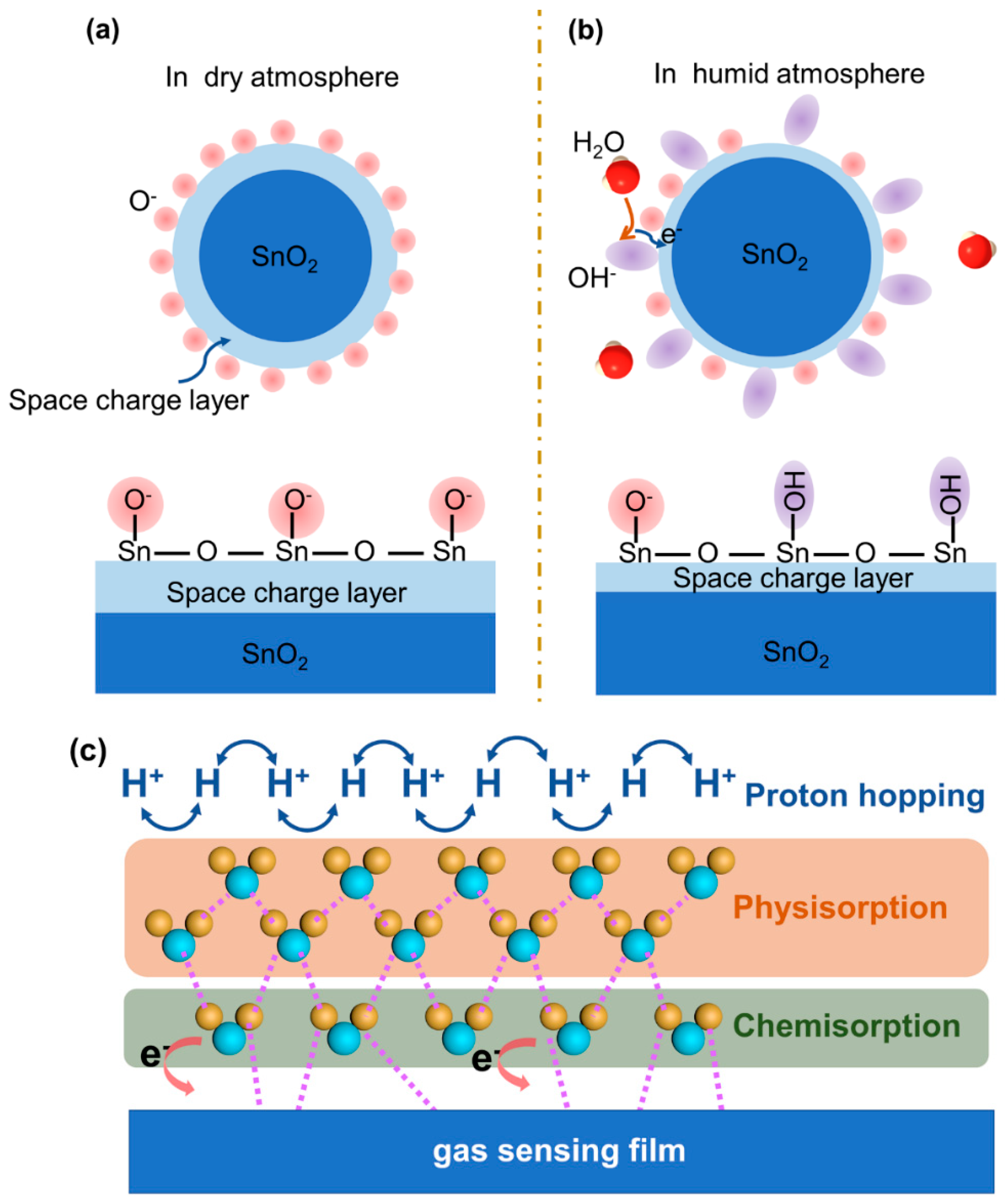
2. Humidity Interference Mechanism
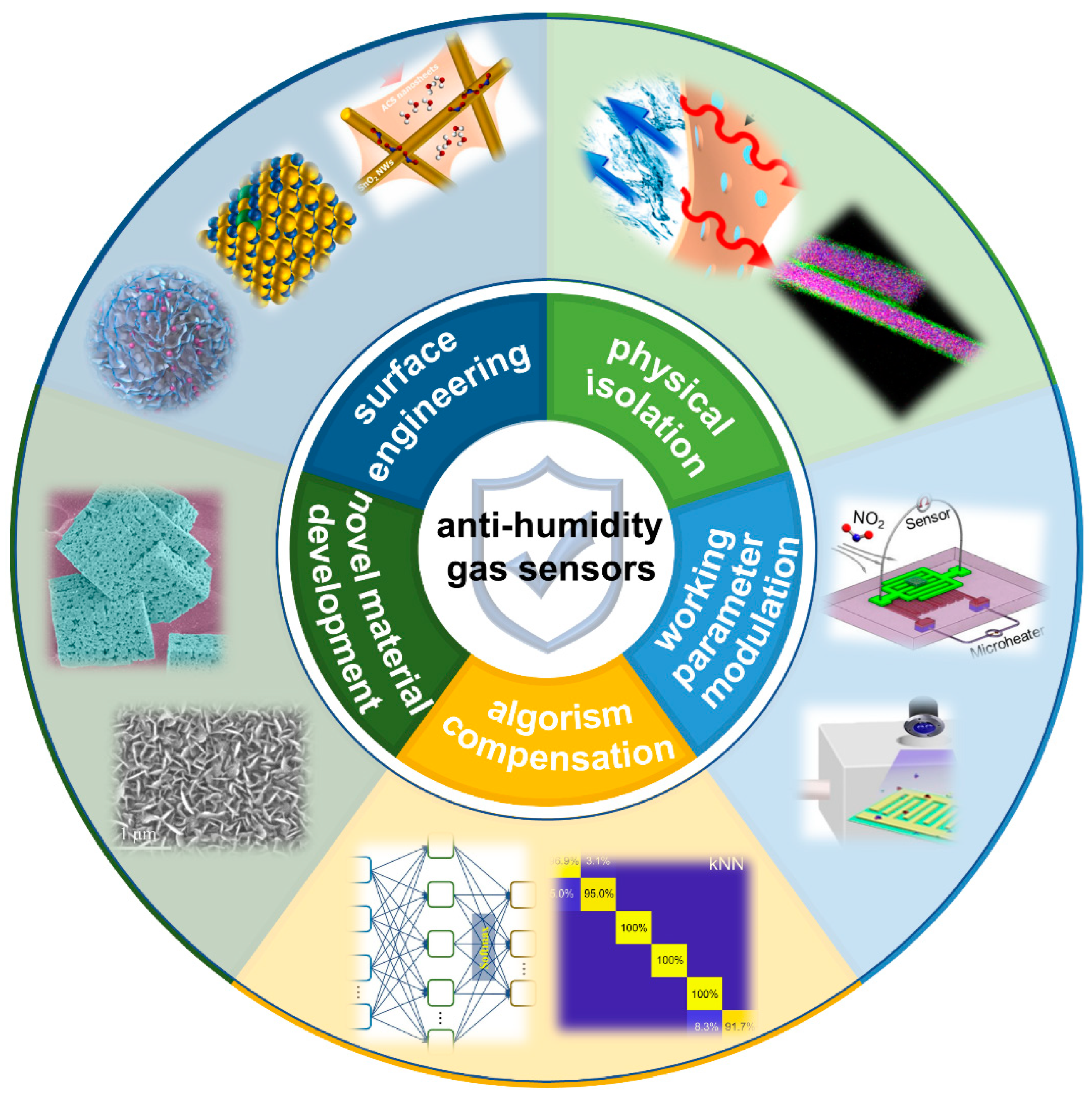
3. Anti-Humidity Strategies
3.1. Surface Engineering
3.1.1. Functionalization of Noble Metals
3.1.2. Element Doping
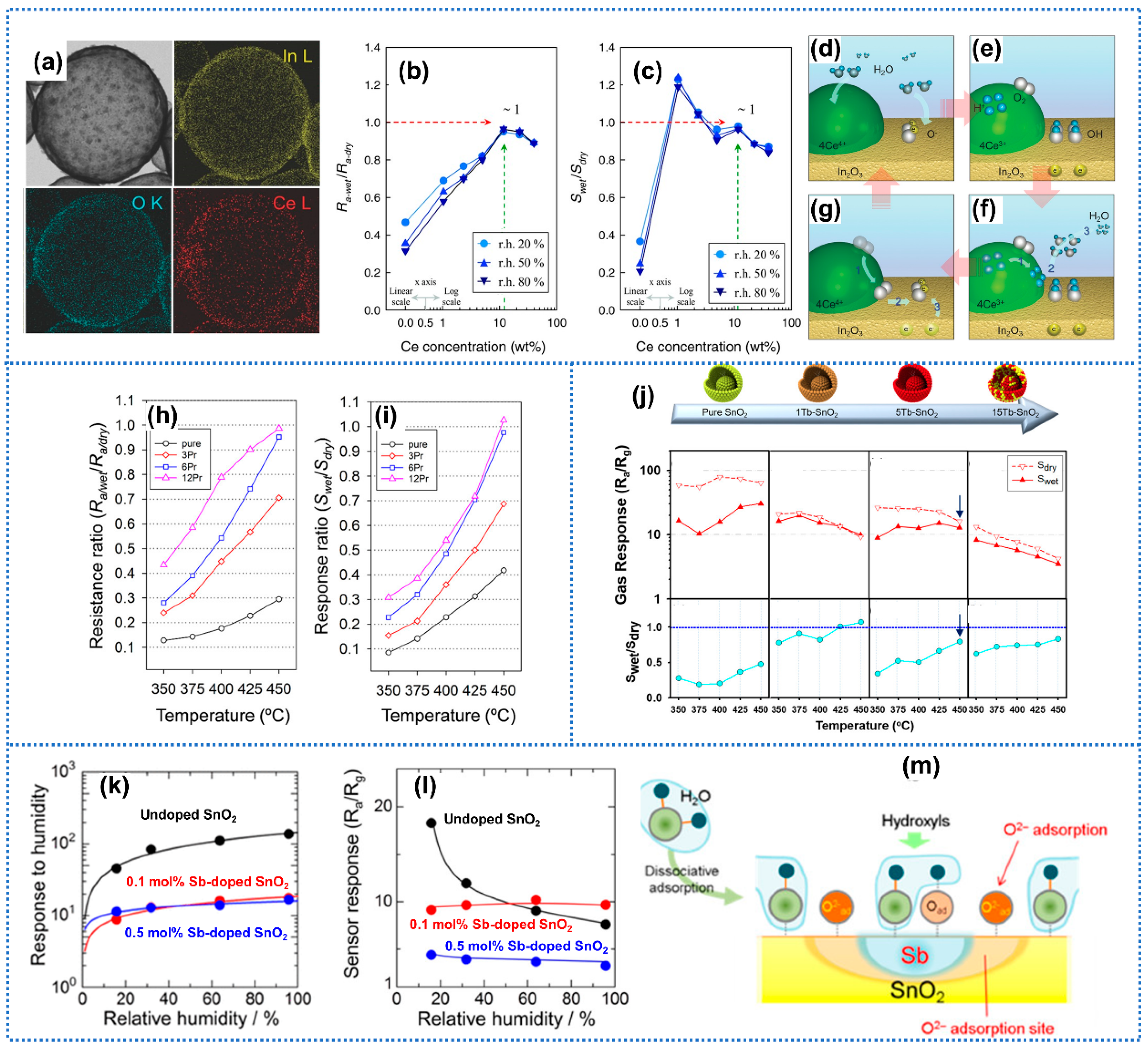
3.1.3. Modification with Hydrophobic Materials
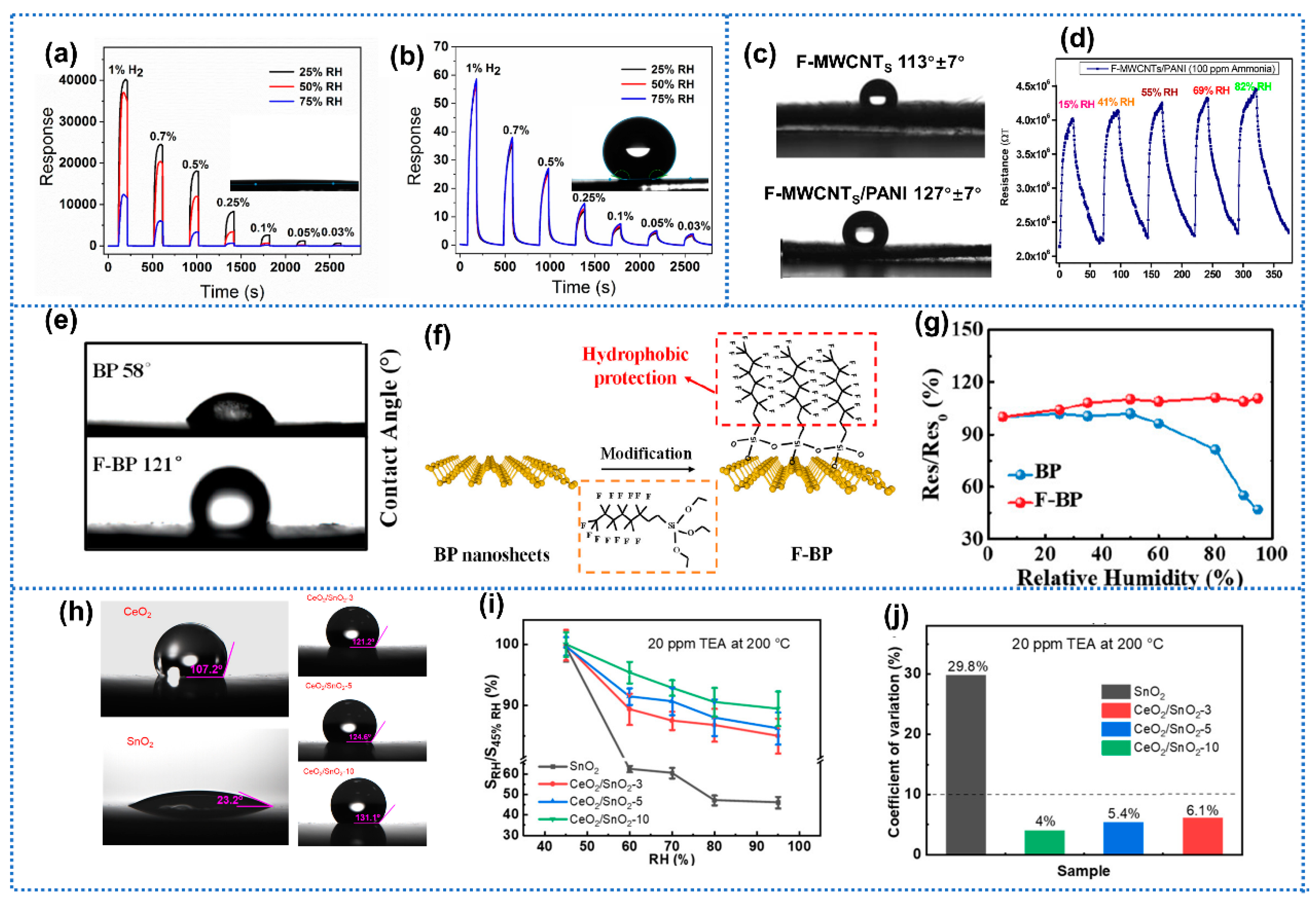
3.1.4. Modification with Hydrophilic Materials
3.1.5. Post-Treatment
3.2. Physical Isolation
3.3. Working Parameter Modulation
3.4. Algorism Compensation
3.5. Novel Material Development
4. Challenges and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alwarappan, S.; Nesakumar, N.; Sun, D.; Hu, T.Y.; Li, C.Z. 2D metal carbides and nitrides (MXenes) for sensors and biosensors. Biosens. Bioelectron. 2022, 205, 113943. [Google Scholar] [CrossRef]
- Chen, W.Y.; Lai, S.N.; Yen, C.C.; Jiang, X.; Peroulis, D.; Stanciu, L.A. Surface functionalization of Ti3C2Tx MXene with highly reliable superhydrophobic protection for volatile organic compounds sensing. ACS Nano 2020, 14, 11490–11501. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro- and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [Green Version]
- Suh, J.-H.; Cho, I.; Kang, K.; Kweon, S.-J.; Lee, M.; Yoo, H.-J.; Park, I. Fully integrated and portable semiconductor-type multi-gas sensing module for IoT applications. Sens. Actuators B Chem. 2018, 265, 660–667. [Google Scholar] [CrossRef]
- Tai, H.; Wang, S.; Duan, Z.; Jiang, Y. Evolution of breath analysis based on humidity and gas sensors: Potential and challenges. Sens. Actuators B Chem. 2020, 318, 128104. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [Green Version]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Chava, R.K.; Cho, H.-Y.; Yoon, J.-M.; Yu, Y.-T. Fabrication of aggregated In2O3 nanospheres for highly sensitive acetaldehyde gas sensors. J. Alloys Compd. 2019, 772, 834–842. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Ma, X.H.; Li, H.Y.; Kweon, S.H.; Jeong, S.Y.; Lee, J.H.; Nahm, S. Highly sensitive and selective PbTiO3 gas sensors with negligible humidity interference in ambient atmosphere. ACS Appl. Mater. Interfaces 2019, 11, 5240–5246. [Google Scholar] [CrossRef] [PubMed]
- Masson, N.; Piedrahita, R.; Hannigan, M. Approach for quantification of metal oxide type semiconductor gas sensors used for ambient air quality monitoring. Sens. Actuators B Chem. 2015, 208, 339–345. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of humidity on gas sensing performance of carbon nanotube gas sensors operated at room temperature. IEEE Sens. J. 2021, 21, 5763–5770. [Google Scholar] [CrossRef]
- Mahdavi, H.; Rahbarpour, S.; Hosseini-Golgoo, S.-M.; Jamaati, H. Reducing the destructive effect of ambient humidity variations on gas detection capability of a temperature modulated gas sensor by calcium chloride. Sens. Actuators B Chem. 2021, 331, 129091. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, Z.; Chen, X.; Huang, C.; Bai, W.; Lu, Z.; Wang, T. Nanomaterial-based gas sensors used for breath diagnosis. J. Mater. Chem. B 2020, 8, 3231–3248. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, L.B.; Ruan, S.; Li, X.; Yu, S.; Li, X.; Jiang, Y.; Yao, Z.; Ye, S.; Wang, C.; et al. Recent development in nanocarbon materials for gas sensor applications. Sens. Actuators B Chem. 2018, 274, 235–267. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T. Insights into the gas sensor materials: Synthesis, performances and devices. Sens. Actuators B Chem. 2022, 371, 132565. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Gonçalves, A.; Pereira, S.; Branquinho, R.; Barquinha, P.; Fortunato, E.; Martins, R. Metal oxide nanostructures for sensor applications. Semicond. Sci. Technol. 2019, 34, 043001. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Deb, J.; Sarkar, U.; Sharma, S. MoS2/MoO3 nanocomposite for selective NH3 detection in a humid environment. ACS Sustain. Chem. Eng. 2021, 9, 7328–7340. [Google Scholar] [CrossRef]
- Zhao, J.; Li, N.; Yu, H.; Wei, Z.; Liao, M.; Chen, P.; Wang, S.; Shi, D.; Sun, Q.; Zhang, G. Highly sensitive MoS2 humidity sensors array for noncontact sensation. Adv. Mater. 2017, 29, 1702076. [Google Scholar] [CrossRef]
- Boehme, I.; Weimar, U.; Barsan, N. Unraveling the surface chemistry of CO sensing with In2O3 based gas sensors. Sens. Actuators B Chem. 2021, 326, 129004. [Google Scholar] [CrossRef]
- Duong, V.T.; Nguyen, C.T.; Luong, H.B.; Nguyen, D.C.; Nguyen, H.L. Ultralow-detection limit ammonia gas sensors at room temperature based on MWCNT/WO3 nanocomposite and effect of humidity. Solid State Sci. 2021, 113, 106534. [Google Scholar] [CrossRef]
- Matatagui, D.; López-Sánchez, J.; Peña, A.; Serrano, A.; del Campo, A.; de la Fuente, O.R.; Carmona, N.; Navarro, E.; Marín, P.; del Carmen Horrillo, M. Ultrasensitive NO2 gas sensor with insignificant NH3-interference based on a few-layered mesoporous graphene. Sens. Actuators B Chem. 2021, 335, 129657. [Google Scholar] [CrossRef]
- Annanouch, F.E.; Haddi, Z.; Vallejos, S.; Umek, P.; Guttmann, P.; Bittencourt, C.; Llobet, E. Aerosol-assisted CVD-grown WO3 nanoneedles decorated with copper oxide nanoparticles for the selective and humidity-resilient detection of H2S. ACS Appl. Mater. Interfaces 2015, 7, 6842–6851. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Zhang, S.; Huang, C.; Guo, X.; Zhu, Y.; Thomas, T.; Guo, H.; Attfield, J.P.; Yang, M. Surface functionalized sensors for humidity-independent gas detection. Angew. Chem. Int. Ed. Engl. 2021, 60, 6561–6566. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Lee, C.S.; Kim, D.H.; Lee, J.H. Flexible room-temperature NH3 sensor for ultrasensitive, selective, and humidity-independent gas detection. ACS Appl. Mater. Interfaces 2018, 10, 27858–27867. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, X.; Jing, S.; Pang, C.; Liu, Q.; Zhang, J. Chemical and electronic modulation via atomic layer deposition of NiO on porous In2O3 films to boost NO2 detection. ACS Appl. Mater. Interfaces 2021, 13, 39621–39632. [Google Scholar] [CrossRef]
- Wang, X.; Han, W.; Yang, J.; Cheng, P.; Wang, Y.; Feng, C.; Wang, C.; Zhang, H.; Sun, Y.; Lu, G. Conductometric ppb-level triethylamine sensor based on macroporous WO3-W18O49 heterostructures functionalized with carbon layers and PdO nanoparticles. Sens. Actuators B Chem. 2022, 361, 131707. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.; Tipparaju, V.V.; Tsow, F.; Xian, X. Mitigation of humidity interference in colorimetric sensing of gases. ACS Sens. 2021, 6, 303–320. [Google Scholar] [CrossRef]
- Li, D.; Tang, Y.; Ao, D.; Xiang, X.; Wang, S.; Zu, X. Ultra-highly sensitive and selective H2S gas sensor based on CuO with sub-ppb detection limit. Int. J. Hydrogen Energy 2019, 44, 3985–3992. [Google Scholar] [CrossRef]
- Sui, L.; Yu, T.; Zhao, D.; Cheng, X.; Zhang, X.; Wang, P.; Xu, Y.; Gao, S.; Zhao, H.; Gao, Y.; et al. In situ deposited hierarchical CuO/NiO nanowall arrays film sensor with enhanced gas sensing performance to H2S. J. Hazard. Mater. 2020, 385, 121570. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Hwang, I.-S.; Park, J.-G.; Choi, K.J.; Park, J.-H.; Lee, J.-H. Novel fabrication of an SnO2 nanowire gas sensor with high sensitivity. Nanotechnology 2008, 19, 095508. [Google Scholar] [CrossRef] [PubMed]
- Navazani, S.; Shokuhfar, A.; Hassanisadi, M.; Askarieh, M.; Di Carlo, A.; Agresti, A. Facile synthesis of a SnO2@rGO nanohybrid and optimization of its methane-sensing parameters. Talanta 2018, 181, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, K.; Wicker, S.; Weimar, U.; Barsan, N. Impact of Pt additives on the surface reactions between SnO2, water vapour, CO and H2; an operando investigation. Phys. Chem. Chem. Phys. 2013, 15, 19151–19158. [Google Scholar] [CrossRef]
- Hao, M.; Zhang, R.; Jia, X.; Gao, X.; Gao, W.; Cheng, L.; Qin, Y. A polymer based self-powered ethanol gas sensor to eliminate the interference of ultraviolet light. Sens. Actuators A Phys. 2021, 332, 113173. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Flexible and low power CO gas sensor with Au-functionalized 2D WS2 nanoflakes. Sens. Actuators B Chem. 2020, 313, 128040. [Google Scholar] [CrossRef]
- Seekaew, Y.; Wisitsoraat, A.; Phokharatkul, D.; Wongchoosuk, C. Room temperature toluene gas sensor based on TiO2 nanoparticles decorated 3D graphene-carbon nanotube nanostructures. Sens. Actuators B Chem. 2019, 279, 69–78. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Xie, G.; Li, J.; Wang, Y.; Liu, X.; Zang, Z. Dual resistance and impedance investigation: Ultrasensitive and stable humidity detection of molybdenum disulfide nanosheet-polyethylene oxide hybrids. ACS Appl. Mater. Interfaces 2021, 13, 25250–25259. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, J.; Xia, B. Humidity-sensing properties of chemically reduced graphene oxide/polymer nanocomposite film sensor based on layer-by-layer nano self-assembly. Sens. Actuators B Chem. 2014, 197, 66–72. [Google Scholar] [CrossRef]
- Bi, H.; Yin, K.; Xie, X.; Ji, J.; Wan, S.; Sun, L.; Terrones, M.; Dresselhaus, M.S. Ultrahigh humidity sensitivity of graphene oxide. Sci. Rep. 2013, 3, 2714. [Google Scholar] [CrossRef]
- Li, Z.; Lou, C.; Lei, G.; Lu, G.; Pan, H.; Liu, X.; Zhang, J. Atomic layer deposition of Rh/ZnO nanostructures for anti-humidity detection of trimethylamine. Sens. Actuators B Chem. 2022, 355, 131347. [Google Scholar] [CrossRef]
- Liu, H.; Liu, B.; Li, P.; Kang, W.; Zhang, Y. High sensitivity and anti-humidity gas sensor for nitrogen dioxide based on Ce/SnO2 nanomaterials. Sens. Actuators A Phys. 2022, 344, 113717. [Google Scholar] [CrossRef]
- Cho, Y.J.; Kwon, Y.J.; Jin, S.; Choi, H.; Lee, J.H.; Yang, S.M.; Choi, S.W.; Jeong, Y.K. Two-Dimensional calcium silicate nanosheets for trapping atmospheric water molecules in humidity-immune gas sensors. J. Hazard. Mater. 2022, 432, 128671. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.-J.; Han, S.D.; Kim, J.-K.; Zhu, J.; Han, W.B.; Chung, J.; Yang, S.M.; Cheng, H.; Kim, D.-H.; Kang, C.-Y.; et al. Biodegradable, flexible silicon nanomembrane-based NOx gas sensor system with record-high performance for transient environmental monitors and medical implants. NPG Asia Mater. 2020, 12, 71. [Google Scholar] [CrossRef]
- Sayegh, S.; Lee, J.-H.; Yang, D.-H.; Weber, M.; Iatsunskyi, I.; Coy, E.; Razzouk, A.; Kim, S.S.; Bechelany, M. Humidity-resistant gas sensors based on SnO2 nanowires coated with a porous alumina nanomembrane by molecular layer deposition. Sens. Actuators B Chem. 2021, 344, 130302. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Ding, H.; Wei, Y.; Huang, W.; Yang, X.; Li, Z.; Qiu, L.; Wang, X. Three-dimensional graphene hydrogel decorated with SnO2 for high-performance NO2 sensing with enhanced immunity to humidity. ACS Appl. Mater. Interfaces 2020, 12, 2634–2643. [Google Scholar] [CrossRef]
- Chen, X.; Hu, J.; Chen, P.; Yin, M.; Meng, F.; Zhang, Y. UV-light-assisted NO2 gas sensor based on WS2/PbS heterostructures with full recoverability and reliable anti-humidity ability. Sens. Actuators B Chem. 2021, 339, 129902. [Google Scholar] [CrossRef]
- Oh, J.; Kim, S.H.; Lee, M.-J.; Hwang, H.; Ku, W.; Lim, J.; Hwang, I.-S.; Lee, J.-H.; Hwang, J.-H. Machine learning-based discrimination of indoor pollutants using an oxide gas sensor array: High endurance against ambient humidity and temperature. Sens. Actuators B Chem. 2022, 364, 131894. [Google Scholar] [CrossRef]
- Wu, T.-C.; Dai, J.; Hu, G.; Yu, W.-B.; Ogbeide, O.; Luca, A.D.; Huang, X.; Su, B.-L.; Li, Y.; Udrea, F.; et al. Machine-intelligent inkjet-printed α-Fe2O3/rGO towards NO2 quantification in ambient humidity. Sens. Actuators B Chem. 2020, 321, 128446. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, C.; Su, M.; Ji, Y.; Xie, L.; Yang, Q.; Du, A.; Zhou, Y.; Yang, J. Highly sensitive, humidity-tolerant and flexible NO2 sensors based on nanoplate Bi2Se3 film. Chin. Chem. Lett. 2022; in press. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.; Li, Y.; Cao, L.; Nan, N.; Li, C.; Yu, L. Tunable NH4F-assisted synthesis of 3D porous In2O3 microcubes for outstanding NO2 gas-sensing performance: Fast equilibrium at high temperature and resistant to humidity at room temperature. ACS Appl. Mater. Interfaces 2021, 13, 14355–14364. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Liu, X.; Zhang, J.; Kumar, M. Room-temperature gas sensors under photoactivation: From metal oxides to 2D materials. Nanomicro Lett. 2020, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, C.; Zheng, B.; Geng, X.; Debliquy, M. Hydrogen sensors based on noble metal doped metal-oxide semiconductor: A review. Int. J. Hydrogen Energy 2017, 42, 20386–20397. [Google Scholar] [CrossRef]
- Nunes Simonetti, E.A.; Cardoso de Oliveira, T.; Enrico do Carmo Machado, Á.; Coutinho Silva, A.A.; Silva dos Santos, A.; de Simone Cividanes, L. TiO2 as a gas sensor: The novel carbon structures and noble metals as new elements for enhancing sensitivity—A review. Ceram. Int. 2021, 47, 17844–17876. [Google Scholar] [CrossRef]
- Koziej, D.; Bârsan, N.; Shimanoe, K.; Yamazoe, N.; Szuber, J.; Weimar, U. Spectroscopic insights into CO sensing of undoped and palladium doped tin dioxide sensors derived from hydrothermally treated tin oxide sol. Sens. Actuators B Chem. 2006, 118, 98–104. [Google Scholar] [CrossRef]
- Pavelko, R.G.; Daly, H.; Hardacre, C.; Vasiliev, A.A.; Llobet, E. Interaction of water, hydrogen and their mixtures with SnO2 based materials: The role of surface hydroxyl groups in detection mechanisms. Phys. Chem. Chem. Phys. 2010, 12, 2639–2647. [Google Scholar] [CrossRef]
- Ma, N.; Suematsu, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of water vapor on Pd-loaded SnO2 nanoparticles gas sensor. ACS Appl. Mater. Interfaces 2015, 7, 5863–5869. [Google Scholar] [CrossRef]
- Ma, N.; Suematsu, K.; Yuasa, M.; Shimanoe, K. Pd size effect on the gas sensing properties of Pd-loaded SnO2 in humid atmosphere. ACS Appl. Mater. Interfaces 2015, 7, 15618–15625. [Google Scholar] [CrossRef]
- Yao, M.-S.; Cao, L.-A.; Hou, G.-L.; Cai, M.-L.; Xiu, J.-W.; Fang, C.-H.; Yuan, F.-L.; Chen, Y.-F. Gold–tin co-sensitized ZnO layered porous nanocrystals: Enhanced responses and anti-humidity. RSC Adv. 2017, 7, 20273–20280. [Google Scholar] [CrossRef]
- Kolmakov, A.; Klenov, D.O.; Lilach, Y.; Stemmer, S.; Moskovits, M. Enhanced gas sensing by individual SnO2 nanowires and nanobelts functionalized with Pd catalyst particles. Nano Lett. 2005, 5, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lou, Z.; Fei, T.; Zhang, T. Templating synthesis of ZnO hollow nanospheres loaded with Au nanoparticles and their enhanced gas sensing properties. J. Mater. Chem. 2012, 22, 4767. [Google Scholar] [CrossRef]
- Yang, M.; Lu, J.; Wang, X.; Zhang, H.; Chen, F.; Sun, J.; Yang, J.; Sun, Y.; Lu, G. Acetone sensors with high stability to humidity changes based on Ru-doped NiO flower-like microspheres. Sens. Actuators B Chem. 2020, 313, 127965. [Google Scholar] [CrossRef]
- Qin, Y.; Zang, J.; Bai, C.; Wang, X. Dual functionalization of aligned silicon nanowires by APTES and nano-Ag to achieve high response to rarefied acetone at high ambient humidity. J. Mater. Sci. Mater. Electron. 2020, 32, 908–922. [Google Scholar] [CrossRef]
- Byoun, Y.; Choi, S.-W.; Tae Byun, Y. Realisation of highly sensitive and selective NO2 detection at room temperature utilizing defect-induced single-walled carbon nanotubes combined with Pt functionalisation. Appl. Surf. Sci. 2022, 590, 153068. [Google Scholar] [CrossRef]
- Choi, K.-I.; Hwang, S.-J.; Dai, Z.; Chan Kang, Y.; Lee, J.-H. Rh-catalyzed WO3 with anomalous humidity dependence of gas sensing characteristics. RSC Adv. 2014, 4, 53130–53136. [Google Scholar] [CrossRef]
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhou, L.; Liu, D.; Liu, F.; Liu, F.; Liang, X.; Yan, X.; Gao, Y.; Lu, G. The role of Ce doping in enhancing sensing performance of ZnO-based gas sensor by adjusting the proportion of oxygen species. Sens. Actuators B Chem. 2018, 273, 991–998. [Google Scholar] [CrossRef]
- Zhou, S.; Lu, Q.; Chen, M.; Li, B.; Wei, H.; Zi, B.; Zeng, J.; Zhang, Y.; Zhang, J.; Zhu, Z.; et al. Platinum-supported cerium-doped indium oxide for highly sensitive triethylamine gas sensing with good antihumidity. ACS Appl. Mater. Interfaces 2020, 12, 42962–42970. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kim, J.S.; Kim, T.H.; Hong, Y.J.; Kang, Y.C.; Lee, J.H. A new strategy for humidity independent oxide chemiresistors: Dynamic self-refreshing of In2O3 sensing surface assisted by layer-by-layer coated CeO2 nanoclusters. Small 2016, 12, 4229–4240. [Google Scholar] [CrossRef]
- Kim, J.S.; Na, C.W.; Kwak, C.H.; Li, H.Y.; Yoon, J.W.; Kim, J.H.; Jeong, S.Y.; Lee, J.H. Humidity-Independent Gas Sensors Using Pr-doped In2O3 macroporous spheres: Role of cyclic Pr(3+)/Pr(4+) redox reactions in suppression of water-poisoning effect. ACS Appl. Mater. Interfaces 2019, 11, 25322–25329. [Google Scholar] [CrossRef]
- Fan, X.; Wang, J.; Sun, C.; Huang, C.; Lu, Y.; Dai, P.; Xu, Y.; He, W. Effect of Pr/Zn on the anti-humidity and acetone-sensing properties of Co3O4 prepared by electrospray. RSC Adv. 2022, 12, 19384–19393. [Google Scholar] [CrossRef]
- Kwak, C.H.; Kim, T.H.; Jeong, S.Y.; Yoon, J.W.; Kim, J.S.; Lee, J.H. Humidity-independent oxide semiconductor chemiresistors using terbium-doped SnO2 yolk-shell spheres for real-time breath analysis. ACS Appl. Mater. Interfaces 2018, 10, 18886–18894. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, K.B.; Li, H.-Y.; Na, C.W.; Lim, K.; Moon, Y.K.; Yoon, J.W.; Lee, J.-H. Pure and Pr-doped Ce4W9O33 with superior hydroxyl scavenging ability: Humidity-independent oxide chemiresistors. J. Mater. Chem. A 2021, 9, 16359–16369. [Google Scholar] [CrossRef]
- Suematsu, K.; Sasaki, M.; Ma, N.; Yuasa, M.; Shimanoe, K. Antimony-doped tin dioxide gas sensors exhibiting high stability in the sensitivity to humidity changes. ACS Sens. 2016, 1, 913–920. [Google Scholar] [CrossRef]
- Suematsu, K.; Ma, N.; Yuasa, M.; Kida, T.; Shimanoe, K. Surface-modification of SnO2 nanoparticles by incorporation of Al for the detection of combustible gases in a humid atmosphere. RSC Adv. 2015, 5, 86347–86354. [Google Scholar] [CrossRef]
- Some, S.; Xu, Y.; Kim, Y.; Yoon, Y.; Qin, H.; Kulkarni, A.; Kim, T.; Lee, H. Highly sensitive and selective gas sensor using hydrophilic and hydrophobic graphenes. Sci. Rep. 2013, 3, 1868. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhou, Y.; Wang, Y.; Zhang, R.; Li, J.; Li, X.; Zang, Z. Conductometric room temperature ammonia sensors based on titanium dioxide nanoparticles decorated thin black phosphorus nanosheets. Sens. Actuators B Chem. 2021, 349, 130770. [Google Scholar] [CrossRef]
- Varughese, S.M.; Bhandaru, N. Durability of submerged hydrophobic surfaces. Soft Matter 2020, 16, 1692–1701. [Google Scholar] [CrossRef]
- Law, K.Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; He, Z.; Wang, P.; Yan, Y.; Ran, J. Hydrophobic modified activated carbon using PDMS for the adsorption of VOCs in humid condition. Sep. Purif. Technol. 2020, 239, 116517. [Google Scholar] [CrossRef]
- Stanton, M.M.; Ducker, R.E.; MacDonald, J.C.; Lambert, C.R.; McGimpsey, W.G. Super-hydrophobic, highly adhesive, polydimethylsiloxane (PDMS) surfaces. J. Colloid Interface Sci. 2012, 367, 502–508. [Google Scholar] [CrossRef]
- Gao, Z.; Song, G.; Zhang, X.; Li, Q.; Yang, S.; Wang, T.; Li, Y.; Zhang, L.; Guo, L.; Fu, Y. A facile PDMS coating approach to room-temperature gas sensors with high humidity resistance and long-term stability. Sens. Actuators B Chem. 2020, 325, 128810. [Google Scholar] [CrossRef]
- Maity, D.; Kumar, R.T.R. Polyaniline anchored MWCNTs on fabric for high performance wearable ammonia sensor. ACS Sens. 2018, 3, 1822–1830. [Google Scholar] [CrossRef]
- Liu, S.; Qin, Y.; Bai, Y. Highly response and humidity-resistant gas sensor based on polyaniline-functionalized Bi2MoO6 with UV activation. Electrochim. Acta 2022, 427, 140863. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Sheng, K.; Chen, C.; Zhou, X.; Dong, B.; Bai, X.; Zhang, S.; Lu, G.; Song, H. APTES-functionalized thin-walled porous WO3 nanotubes for highly selective sensing of NO2 in a polluted environment. J. Mater. Chem. A 2018, 6, 10976–10989. [Google Scholar] [CrossRef]
- Hijazi, M.; Rieu, M.; Stambouli, V.; Tournier, G.; Viricelle, J.-P.; Pijolat, C. Ambient temperature selective ammonia gas sensor based on SnO2-APTES modifications. Sens. Actuators B Chem. 2018, 256, 440–447. [Google Scholar] [CrossRef]
- Talreja, K.; Chauhan, I.; Ghosh, A.; Majumdar, A.; Butola, B.S. Functionalization of silica particles to tune the impact resistance of shear thickening fluid treated aramid fabrics. RSC Adv. 2017, 7, 49787–49794. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Li, X.; Song, Y.; Zhang, R.; Yuan, W.; Xia, D.; Xue, Q.; Yin, F. End Group Modification for Black Phosphorus: Simultaneous Improvement of Chemical Stability and Gas Sensing Performance. ACS Appl. Mater. Interfaces 2021, 13, 50270–50280. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, Y.; Zhao, L. Enhanced humidity resistance of porous SiNWs via OTS functionalization for rarefied NO2 detection. Sens. Actuators B Chem. 2019, 283, 61–68. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, C.; Bai, W.; Ye, W.; Wang, J.; Wei, J.; Wang, Y.; He, J.; Lu, J. Surface functionalization of ion-in-conjugation polymer sensors for humidity-independent gas detection at room temperature. Sens. Actuators B Chem. 2022, 372, 132654. [Google Scholar] [CrossRef]
- Feng, C.; Ni, H.; Chen, J.; Yang, W. Facile Method to Fabricate Highly Thermally Conductive Graphite/PP Composite with Network Structures. ACS Appl. Mater. Interfaces 2016, 8, 19732–19738. [Google Scholar] [CrossRef]
- Gu, F.; Wang, D.; Wang, J.; Wang, P.; Han, D.; Wang, Z.; Qiao, Z.; Hu, Y. Humidity-independent, highly sensitive and selective NO2 sensor based on In2O3 nanoflowers decorated with graphite nanoflakes. IEEE Sens. J. 2022, 22, 14753–14761. [Google Scholar] [CrossRef]
- Al Shboul, A.; Shih, A.; Izquierdo, R. A flexible indium oxide sensor with anti-humidity property for room temperature detection of hydrogen sulfide. IEEE Sens. J. 2021, 21, 9667–9674. [Google Scholar] [CrossRef]
- Singh, S.; Saggu, I.S.; Chen, K.; Xuan, Z.; Swihart, M.T.; Sharma, S. Humidity-tolerant room-temperature selective dual sensing and discrimination of NH3 and no using a WS2/MWCNT Composite. ACS Appl. Mater. Interfaces 2022, 14, 40382–40395. [Google Scholar] [CrossRef]
- Wang, X.; Lu, J.; Han, W.; Cheng, P.; Wang, Y.; Sun, J.; Ma, J.; Sun, P.; Zhang, H.; Sun, Y.; et al. Carbon modification endows WO3 with anti-humidity property and long-term stability for ultrafast H2S detection. Sens. Actuators B Chem. 2022, 350, 130884. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, W.; Chu, Z.; Wang, S.; Chen, L.; Nie, X.; Wang, R.; He, X. High sensitivity, humidity-independent, flexible NO2 and NH3 gas sensors based on SnS2 hybrid functional graphene ink. ACS Appl. Mater. Interfaces 2020, 12, 997–1004. [Google Scholar] [CrossRef]
- Lou, C.; Li, Z.; Yang, C.; Liu, X.; Zheng, W.; Zhang, J. Rational design of ordered porous SnO2/ZrO2 thin films for fast and selective triethylamine detection with humidity resistance. Sens. Actuators B Chem. 2021, 333, 129572. [Google Scholar] [CrossRef]
- Kim, K.; Park, J.K.; Lee, J.; Kwon, Y.J.; Choi, H.; Yang, S.M.; Lee, J.H.; Jeong, Y.K. Synergistic approach to simultaneously improve response and humidity-independence of metal-oxide gas sensors. J. Hazard. Mater. 2022, 424, 127524. [Google Scholar] [CrossRef]
- Zhu, X.; Chang, X.; Tang, S.; Chen, X.; Gao, W.; Niu, S.; Li, J.; Jiang, Y.; Sun, S. Humidity-tolerant chemiresistive gas sensors based on hydrophobic CeO2/SnO2 heterostructure films. ACS Appl. Mater. Interfaces 2022, 14, 25680–25692. [Google Scholar] [CrossRef]
- Kim, H.-R.; Haensch, A.; Kim, I.-D.; Barsan, N.; Weimar, U.; Lee, J.-H. The role of nio doping in reducing the impact of humidity on the performance of SnO2-based gas sensors: Synthesis strategies, and phenomenological and spectroscopic studies. Adv. Funct. Mater. 2011, 21, 4456–4463. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, B.; Wang, X.; Wang, C.; Sun, Y.; Zhang, H.; Shimanoe, K.; Lu, G. MOF-derived porous NiO/NiFe2O4 nanocubes for improving the acetone detection. Sens. Actuators B Chem. 2022, 366, 131985. [Google Scholar] [CrossRef]
- Jin, C.; Choi, M.S.; Lee, K.H.; Choi, S.-W. Gas sensing behavior of p-NiO/n-ZnO composite nanofibers depending on varying p-NiO content: Selectivity and humidity-independence for oxidizing and reducing gas molecules. Sens. Actuators B Chem. 2021, 349, 130813. [Google Scholar] [CrossRef]
- Choi, K.-I.; Kim, H.-J.; Kang, Y.C.; Lee, J.-H. Ultraselective and ultrasensitive detection of H2S in highly humid atmosphere using CuO-loaded SnO2 hollow spheres for real-time diagnosis of halitosis. Sens. Actuators B Chem. 2014, 194, 371–376. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Suematsu, K.; Li, P.; Yu, Z.; Zhang, W.; Hu, J.; Shimanoe, K. Rapid and stable detection of carbon monoxide in changing humidity atmospheres using clustered In2O3/CuO nanospheres. ACS Sens. 2020, 5, 1040–1049. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Cho, H.J.; Kim, N.H.; Kim, I.D. Mesoporous SnO2 nanotubes via electrospinning-etching route: Highly sensitive and selective detection of H2S Molecule. ACS Appl. Mater. Interfaces 2017, 9, 26304–26313. [Google Scholar] [CrossRef]
- Bang, J.H.; Kwon, Y.J.; Lee, J.H.; Mirzaei, A.; Lee, H.Y.; Choi, H.; Kim, S.S.; Jeong, Y.K.; Kim, H.W. Proton-beam engineered surface-point defects for highly sensitive and reliable NO2 sensing under humid environments. J. Hazard. Mater. 2021, 416, 125841. [Google Scholar] [CrossRef]
- Struzzi, C.; Scardamaglia, M.; Casanova-Chafer, J.; Calavia, R.; Colomer, J.F.; Kondyurin, A.; Bilek, M.; Britun, N.; Snyders, R.; Llobet, E.; et al. Exploiting sensor geometry for enhanced gas sensing properties of fluorinated carbon nanotubes under humid environment. Sens. Actuators B Chem. 2019, 281, 945–952. [Google Scholar] [CrossRef]
- Du, B.; Qi, T.; Li, J.; He, Y.; Yang, X. Improving anti-humidity property of In2O3 based NO2 sensor by fluorocarbon plasma treatment. Sens. Actuators B Chem. 2021, 344, 130268. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Zhang, M.; Wu, Z.; Kong, D.; Qian, H.; Su, B. Etching process enhanced H2O2 sensing performance of SnO2/Zn2SnO4 with reliable anti-humidity ability. Anal. Methods 2022, 14, 3335–3344. [Google Scholar] [CrossRef]
- Itoh, T.; Matsubara, I.; Kadosaki, M.; Sakai, Y.; Shin, W.; Izu, N.; Nishibori, M. Effects of high-humidity aging on platinum, palladium, and gold loaded tin oxide--volatile organic compound sensors. Sensors 2010, 10, 6513–6521. [Google Scholar] [CrossRef] [PubMed]
- Rathi, K.; Pal, K. wireless hand-held device based on polylactic acid-protected, highly stable, ctab-functionalized phosphorene for CO2 gas sensing. ACS Appl. Mater. Interfaces 2020, 12, 38365–38375. [Google Scholar] [CrossRef] [PubMed]
- Rocca-Smith, J.R.; Whyte, O.; Brachais, C.-H.; Champion, D.; Piasente, F.; Marcuzzo, E.; Sensidoni, A.; Debeaufort, F.; Karbowiak, T. Beyond biodegradability of poly(lactic acid): Physical and chemical stability in humid environments. ACS Sustain. Chem. Eng. 2017, 5, 2751–2762. [Google Scholar] [CrossRef]
- Komatsuka, T.; Kusakabe, A.; Nagai, K. Characterization and gas transport properties of poly(lactic acid) blend membranes. Desalination 2008, 234, 212–220. [Google Scholar] [CrossRef]
- Yang, L.; Zheng, G.; Cao, Y.; Meng, C.; Li, Y.; Ji, H.; Chen, X.; Niu, G.; Yan, J.; Xue, Y.; et al. Moisture-resistant, stretchable NOx gas sensors based on laser-induced graphene for environmental monitoring and breath analysis. Microsyst. Nanoeng. 2022, 8, 78. [Google Scholar] [CrossRef]
- Liu, W.; Si, X.; Chen, Z.; Xu, L.; Guo, J.; Wei, L.; Cheng, G.; Du, Z. Fabrication of a humidity-resistant formaldehyde gas sensor through layering a molecular sieve on 3D ordered macroporous SnO2 decorated with Au nanoparticles. J. Alloys Compd. 2022, 919, 165788. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Sheng, K.; Zhou, X.; Dong, B.; Lu, G.; Song, H. A highly sensitive and moisture-resistant gas sensor for diabetes diagnosis with Pt@In2O3 nanowires and a molecular sieve for protection. NPG Asia Mater. 2018, 10, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wang, Y.; Wang, Y.; Yu, H.; Zhang, R.; Li, J.; Zang, Z.; Li, X. MXene Ti3C2Tx-derived nitrogen-functionalized heterophase TiO2 homojunctions for room-temperature trace ammonia gas sensing. ACS Appl. Mater. Interfaces 2021, 13, 56485–56497. [Google Scholar] [CrossRef]
- Kondalkar, V.V.; Duy, L.T.; Seo, H.; Lee, K. Nanohybrids of Pt-functionalized Al2O3/ZnO core-shell nanorods for high-performance MEMS-based acetylene gas sensor. ACS Appl. Mater. Interfaces 2019, 11, 25891–25900. [Google Scholar] [CrossRef]
- Wu, J.; Liang, Y.; Zhou, Z.; Wu, Z.; Ding, H.; Huang, W.; Tao, K.; Shi, W.; Yang, B.-R.; Xie, X. Three-dimensional gold nanoparticles-modified graphene hydrogel for high-sensitive NO2 and NH3 detection with enhanced resistance to humidity. Sens. Actuators B Chem. 2021, 344, 130259. [Google Scholar] [CrossRef]
- Yan, M.; Wu, Y.; Hua, Z.; Lu, N.; Sun, W.; Zhang, J.; Fan, S. Humidity compensation based on power-law response for MOS sensors to VOCs. Sens. Actuators B Chem. 2021, 334, 129601. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, C.; Liu, B.; Jiang, Y.; Li, Z.; Tai, H.; Li, X. Self-adaptive temperature and humidity compensation based on improved deep BP neural network for NO2 detection in complex environment. Sens. Actuators B Chem. 2022, 362, 131812. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, Q.; Duan, Z.; Xie, C.; Duan, X.; Li, S.; Ye, Z.; Jiang, Y.; Tai, H. Ag2Te nanowires for humidity-resistant trace-level NO2 detection at room temperature. Sens. Actuators B Chem. 2022, 363, 131790. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Xie, X.; Tao, K.; Liu, C.; Khor, K.A.; Miao, J.; Norford, L.K. 3D superhydrophobic reduced graphene oxide for activated NO2 sensing with enhanced immunity to humidity. J. Mater. Chem. A 2018, 6, 478–488. [Google Scholar] [CrossRef]
- Li, S.; Zhao, C.; Zhou, S.; Zhang, Y.; Zhu, P.; Yu, J. Non-covalent interaction-driven self-assembly of perylene diimide on rGO for room-temperature sensing of triethylamine with enhanced immunity to humidity. Chem. Eng. J. 2020, 385, 123397. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Yang, J.; Han, W.; Ma, J.; Wang, C.; Shimanoe, K.; Zhang, S.; Sun, Y.; Cheng, P.; Wang, Y.; Zhang, H.; et al. Sn doping effect on NiO hollow nanofibers based gas sensors about the humidity dependence for triethylamine detection. Sens. Actuators B Chem. 2021, 340, 129971. [Google Scholar] [CrossRef]
- Drozdowska, K.; Welearegay, T.; Österlund, L.; Smulko, J. Combined chemoresistive and in situ FTIR spectroscopy study of nanoporous NiO films for light-activated nitrogen dioxide and acetone gas sensing. Sens. Actuators B Chem. 2022, 353, 131125. [Google Scholar] [CrossRef]
- Wilson, R.L.; Simion, C.E.; Stanoiu, A.; Taylor, A.; Guldin, S.; Covington, J.A.; Carmalt, C.J.; Blackman, C.S. Humidity-tolerant ultrathin NiO gas-sensing films. ACS Sens. 2020, 5, 1389–1397. [Google Scholar] [CrossRef]
- Miao, J.; Chen, C.; Lin, J.Y.S. Humidity independent hydrogen sulfide sensing response achieved with monolayer film of CuO nanosheets. Sens. Actuators B Chem. 2020, 309, 127785. [Google Scholar] [CrossRef]
- Brown, K.A.; Brittman, S.; Maccaferri, N.; Jariwala, D.; Celano, U. Machine learning in nanoscience: Big data at small scales. Nano Lett. 2020, 20, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, S.M.; Jablonka, K.M.; Smit, B. The role of machine learning in the understanding and design of materials. J. Am. Chem. Soc. 2020, 142, 20273–20287. [Google Scholar] [CrossRef] [PubMed]

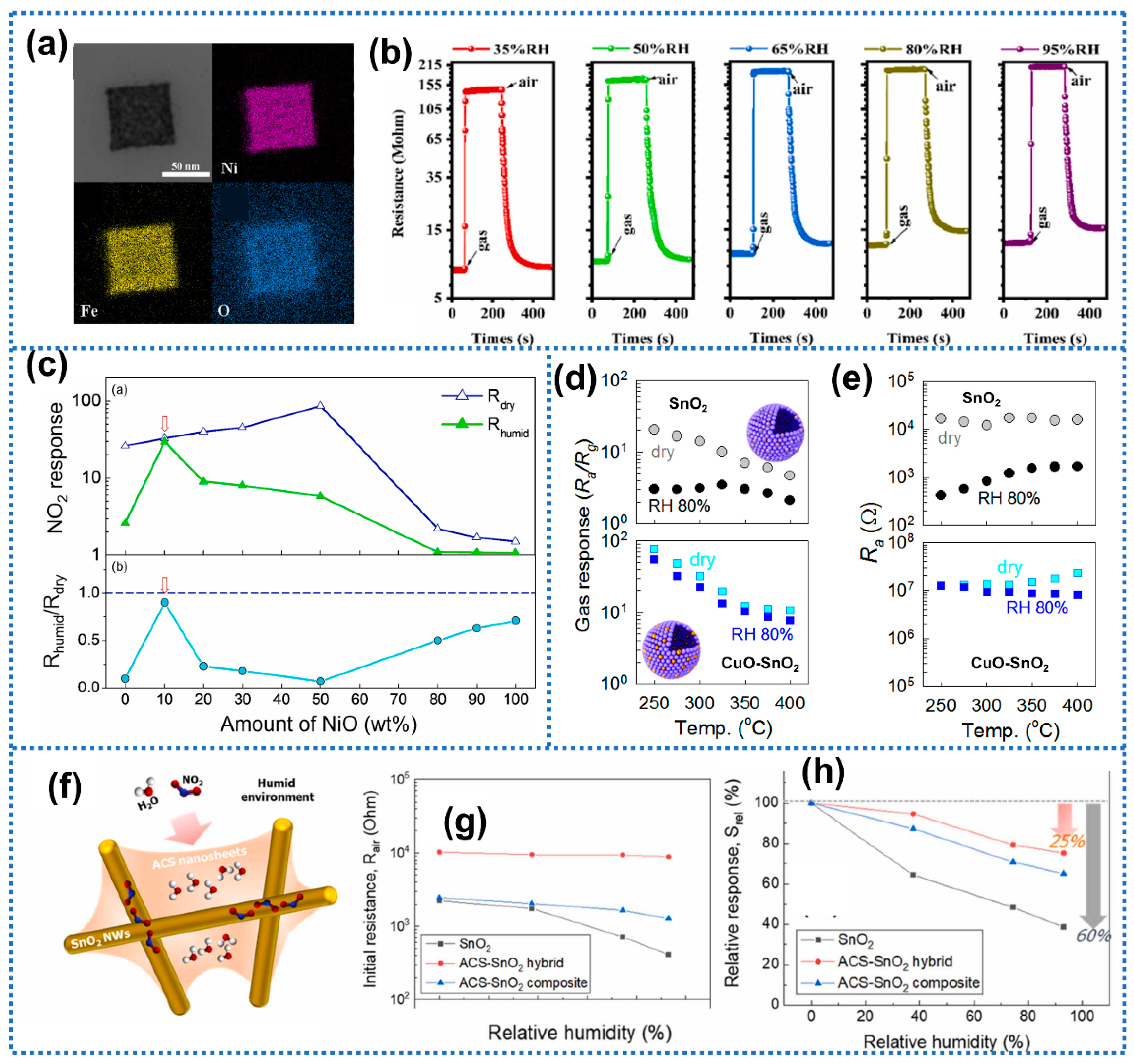

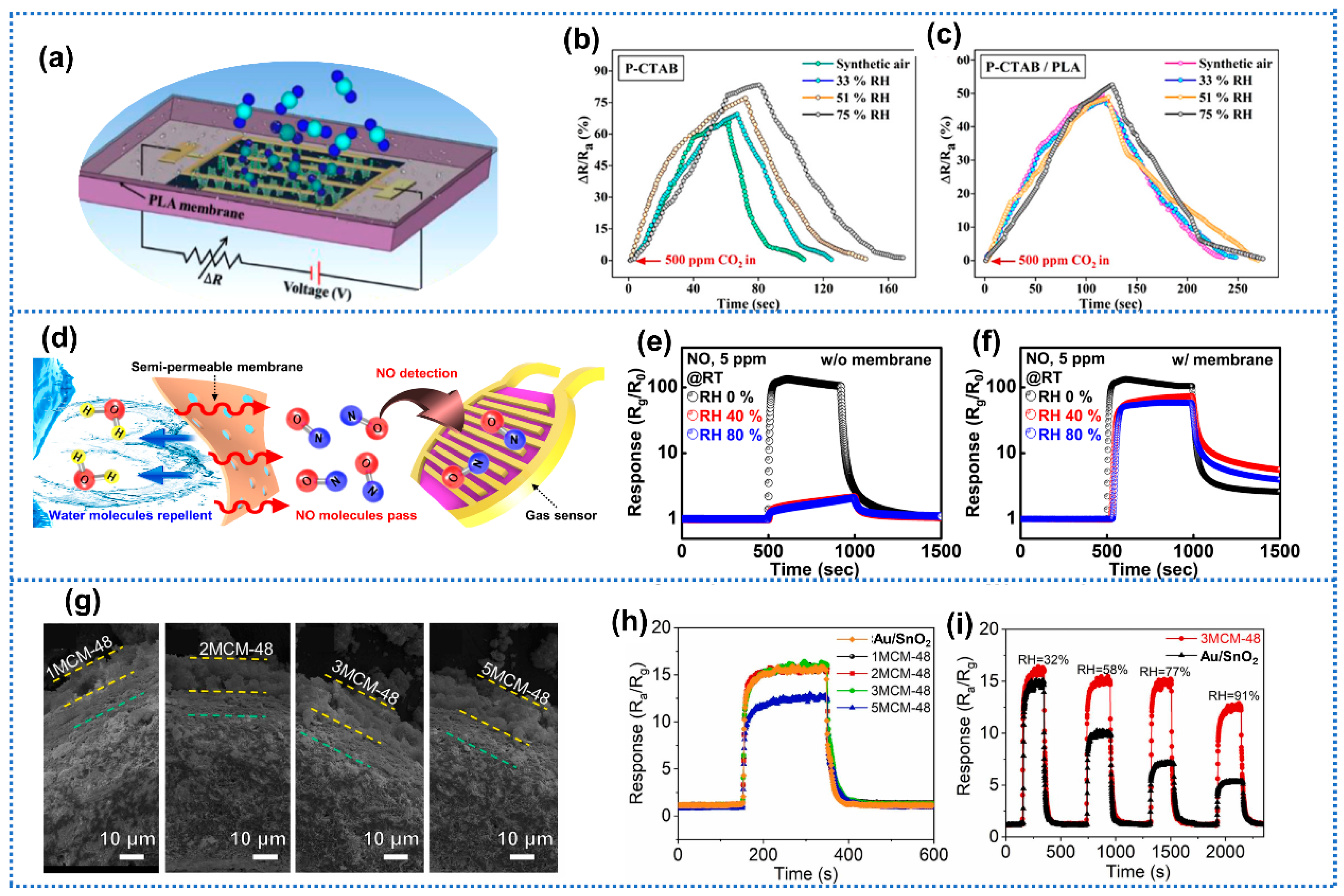
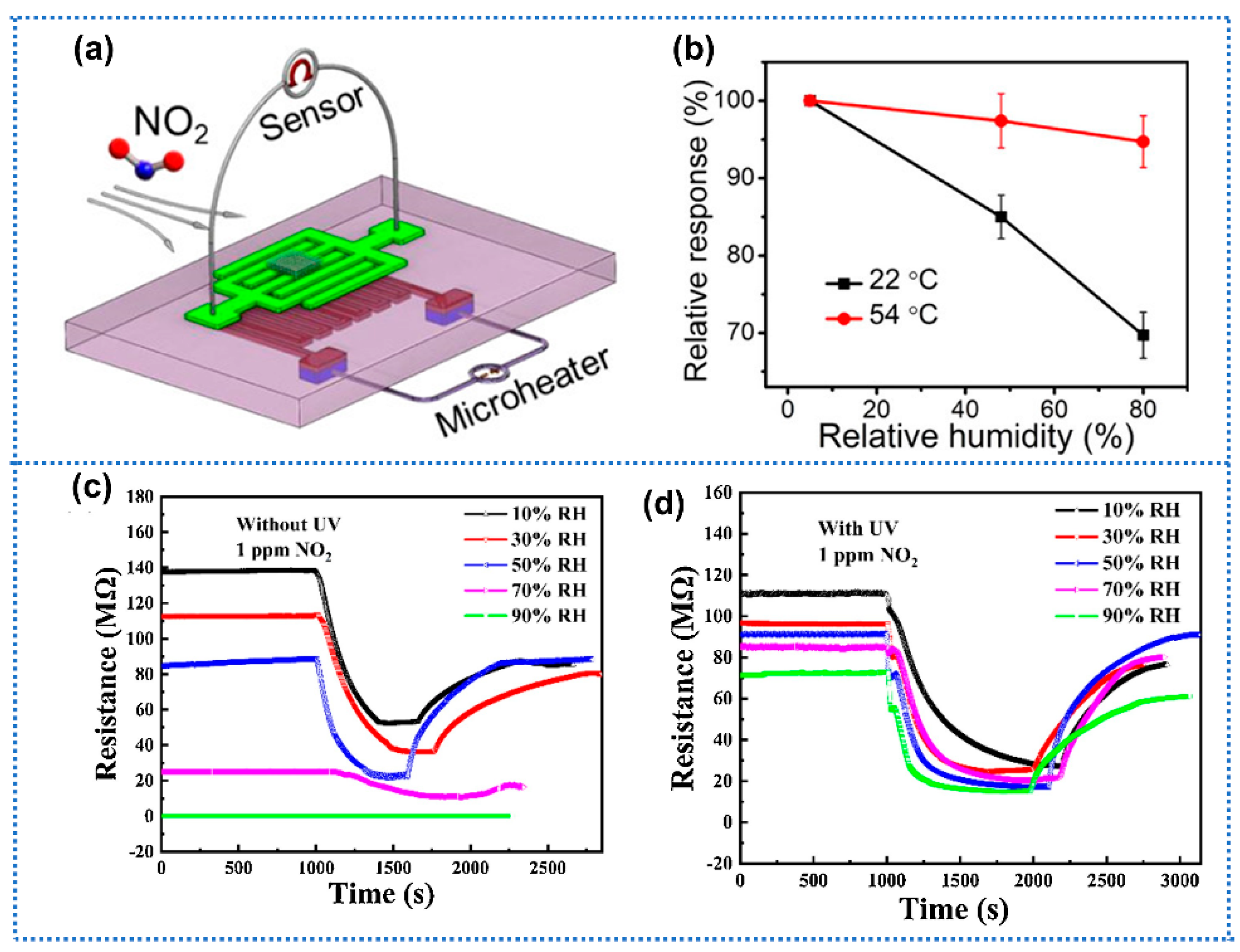
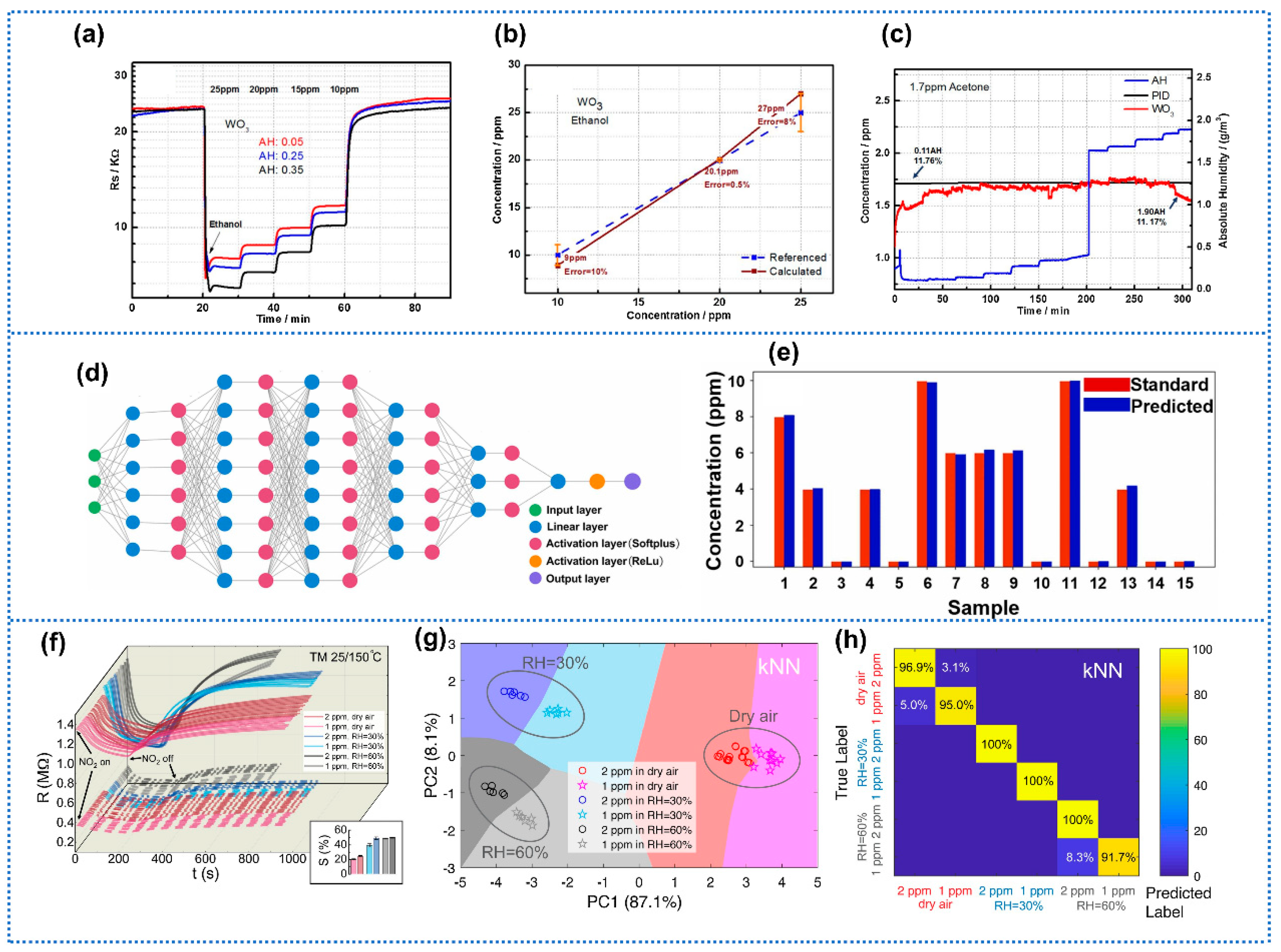
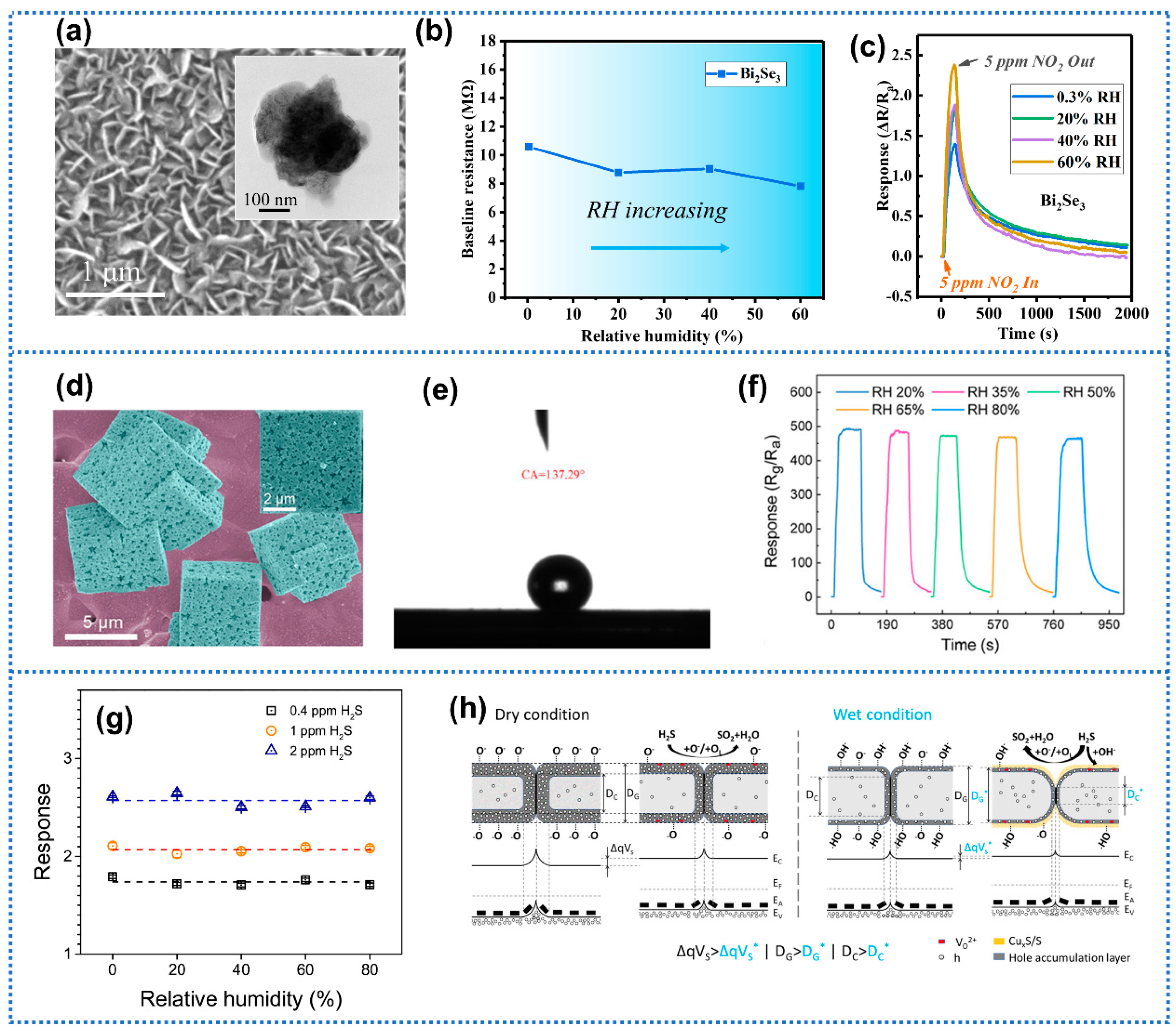
| Anti-Humidity Strategies | Target Gas and OT | Baseline Resistance Drift | Response Drift | Response Decline? * | Ref. | ||
|---|---|---|---|---|---|---|---|
| surface engineering | functionalization of noble metals | Pd-SnO2 | H2 (600 ppm) 300 °C | - | - | Yes | [58] |
| Au-Sn-ZnO | Benzene (49.4 ppm) 350 °C | - | - | No | [60] | ||
| Ru-NiO | Acetone (100 ppm) 200 °C | - | 3% (15–90% RH) | No | [63] | ||
| Ag-APTES/Si nanowires | Acetone (1 ppm) RT | - | - | No | [64] | ||
| Pt-SWCNTs | NO2 (1 ppm) RT | - | - | No | [65] | ||
| Rh-ZnO | TMA (10 ppm) 180 °C | 28.3% (55–90% RH) | No | [41] | |||
| elements doping | Ce-In2O3 | Acetone (10 ppm) 450 °C | 3% (dry–80% RH) | 5% (dry–80% RH) | Yes | [70] | |
| Ce-SnO2 | NO2 (1 ppm) 140 °C | - | - | No | [42] | ||
| Pr-In2O3 | Acetone (20 ppm) 450 °C | ~0% (0–80% RH) | ~0% (0–80% RH) | Yes | [71] | ||
| Pr-Co3O4 | Acetone (50 ppm) 160 °C | 8.82% (30–90% RH) | Yes | [72] | |||
| Tb-SnO2 | Acetone (20 ppm) 450 °C | 23.3% (dry–80% RH) | 20.1% (dry–80% RH) | Yes | [73] | ||
| Pr-Ce-WO3 | TMA (20 ppm) 300 °C | ~0% (dry–80% RH) | ~0% (dry–80% RH) | Yes | [74] | ||
| Sb-SnO2 | H2 (200 ppm) 350 °C | - | - | Yes | [75] | ||
| Al-SnO2 | Ethanol (100 ppm) 250 °C | - | - | Yes | [76] | ||
| modification with hydrophobic materials | PDMS-Pd/TiO2 | H2 (10000 ppm) 25 °C | 20% (25–75% RH) | ~0% (25–75% RH) | Yes | [83] | |
| PDMS-CoSnO3@MOF | NH3 (100 ppm) 160 °C | - | - | No | [25] | ||
| APTES-WO3 | NO2 (1 ppm) 340 °C | - | 19.1% (25–90% RH) | No | [86] | ||
| fluoroalkylsilane-modified BP | NO2 (1 ppm) 25 °C | - | - | No | [89] | ||
| OTS-Si | NO2 (50 ppb) RT | - | 19.3% (25–55% RH) | Yes | [90] | ||
| PANI-MWCNTs | NH3 (100 ppb) 25 °C | - | 19.6% (15–82% RH) | No | [84] | ||
| PANI-Bi2MoO6 | NH3 (1 ppb) RT | 13.5% (40–90% RH) | No | [85] | |||
| PVDF-PNDC | NO2 (1 ppm) RT | - | - | No | [91] | ||
| Graphite-In2O3 | NO2 (1 ppm) 75 °C | - | 7% (20–90% RH) | No | [93] | ||
| Graphite-PS-In2O3 | H2S (100 ppb) RT | - | - | No | [94] | ||
| MWCNT-WS2 | NH3 (1 ppm) 16 °C | - | ~0% (70–90% RH) | - | [95] | ||
| C-WO3 | H2S (100 ppm) 275 °C | 10% (20–98% RH) | No | [96] | |||
| SnS2-S/rGO | NO2 (1 ppm) RT | - | - | No | [97] | ||
| ZrO2-SnO2 | TEA (100 ppm) 190 °C | - | 18% (50–90% RH) | No | [98] | ||
| Y2O3-SnO2 | NO2 (10 ppm) 200 °C | ~0% (0–87% RH) | No | [99] | |||
| CeO2-SnO2 | TEA (20 ppm) 190 °C | 14.1% (45–96% RH) | No | [100] | |||
| composites with hydrophilic materials | NiO-SnO2 | CO (50 ppm) 400 °C | ~0% (dry–25% RH) | - | Yes | [101] | |
| NiO-NiFe2O4 | acetone (100 ppm) 200 °C | 60% (35–95% RH) | 9.5% (35–95% RH) | - | [102] | ||
| NiO-ZnO | NO2 (10 ppm) 350 °C | ~0% (dry–81% RH) | No | [103] | |||
| CuO-SnO2 | H2S (1 ppm) 250 °C | - | - | No | [104] | ||
| CuO-In2O3 | CO (100 ppm) 200 °C | - | 14.4% (25–95% RH) | No | [105] | ||
| ACS-SnO2 | NO2 (10 ppm) 150 °C | - | 25% (dry–93.1% RH) | No | [43] | ||
| SiO2-Cr2O3 | H2S (5 ppm) 170 °C | 9.9% (33–94% RH) | 10% (33–94% RH) | - | [106] | ||
| post-treatment | proton-beam irradiation- ZnO | NO2 (10 ppm) 300 °C | 22.8% (0–75% RH) | No | [107] | ||
| plasma fluorination-CNTs | NO2 (10 ppm) RT | - | - | No | [108] | ||
| fluorocarbon plasma- In2O3 | NO2 (1 ppm) 200 °C | - | ~50% (6–92% RH) | Yes | [109] | ||
| etching-SnO2/Zn2SnO4 | H2O2 (1000 ppm) RT | - | - | No | [110] | ||
| physical isolation | PLA-CTAB/BP | CO2 (500 ppm) RT | - | - | Yes | [112] | |
| PDMS-Si | NO (5 ppm) RT | - | - | Yes | [44] | ||
| PDMS-LIG | NO (1 ppm) RT | - | - | Yes | [115] | ||
| 3MCM-45-Au/SnO2 | Formaldehyde (5 ppm) 110 °C | 17% (32–91% RH) | No | [116] | |||
| SBA-15-Pt/In2O3 | Acetone (1 ppm) 320 °C | - | - | No | [117] | ||
| PTFE-Mxene/TiO2 | NH3 (1 ppm) 20 °C | - | - | - | [118] | ||
| Al2O3-SnO2 | NO2 (100 ppm) 300 °C | - | - | Yes | [45] | ||
| Al2O3-Pt/ZnO | Acetylene (20 ppm) 120 °C | - | 6% (dry–50% RH) | Yes | [119] | ||
| working parameters m modulation | Increasing OT- SnO2/RGOH | NO2 (3 ppm) 54 °C | 9.9% (5–80% RH) | Yes | [46] | ||
| UV illumination WS2/PbS | NO2 (1 ppm) RT | - | 8.8% (10–90% RH) | No | [47] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhou, Y. Recent Progress on Anti-Humidity Strategies of Chemiresistive Gas Sensors. Materials 2022, 15, 8728. https://doi.org/10.3390/ma15248728
Wang Y, Zhou Y. Recent Progress on Anti-Humidity Strategies of Chemiresistive Gas Sensors. Materials. 2022; 15(24):8728. https://doi.org/10.3390/ma15248728
Chicago/Turabian StyleWang, Yanjie, and Yong Zhou. 2022. "Recent Progress on Anti-Humidity Strategies of Chemiresistive Gas Sensors" Materials 15, no. 24: 8728. https://doi.org/10.3390/ma15248728
APA StyleWang, Y., & Zhou, Y. (2022). Recent Progress on Anti-Humidity Strategies of Chemiresistive Gas Sensors. Materials, 15(24), 8728. https://doi.org/10.3390/ma15248728








