Durability and Corrosion Resistance Test of Adhesive Joints Using Two Adherence Promoters for the Connection of Aerospace Aluminum Alloys
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials for Research
2.2. Preparation of the Specimens
- The BR127 primer was annealed at 120 °C for 30 min.
- The EW-5000 AS primer was baked at 120 °C for 60 min.
2.3. Research Methodology
2.4. Wedge Test
2.5. Standard Test Method for Apparent Shear Strength of Single-Lap-Joint Adhesively Bonded Metal Specimens by Tension Loading
2.6. Corrosion Resistance Tests
3. Research Results
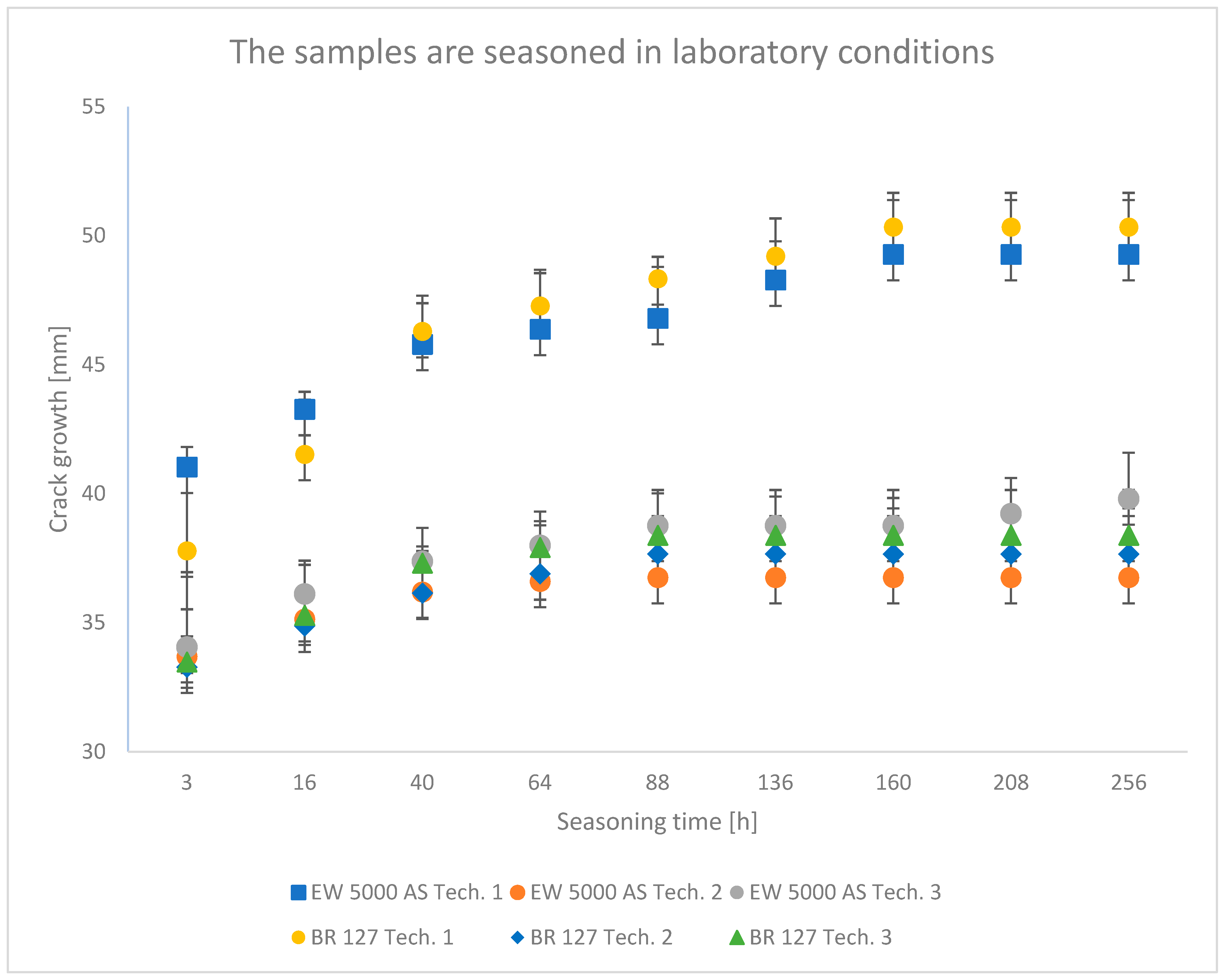
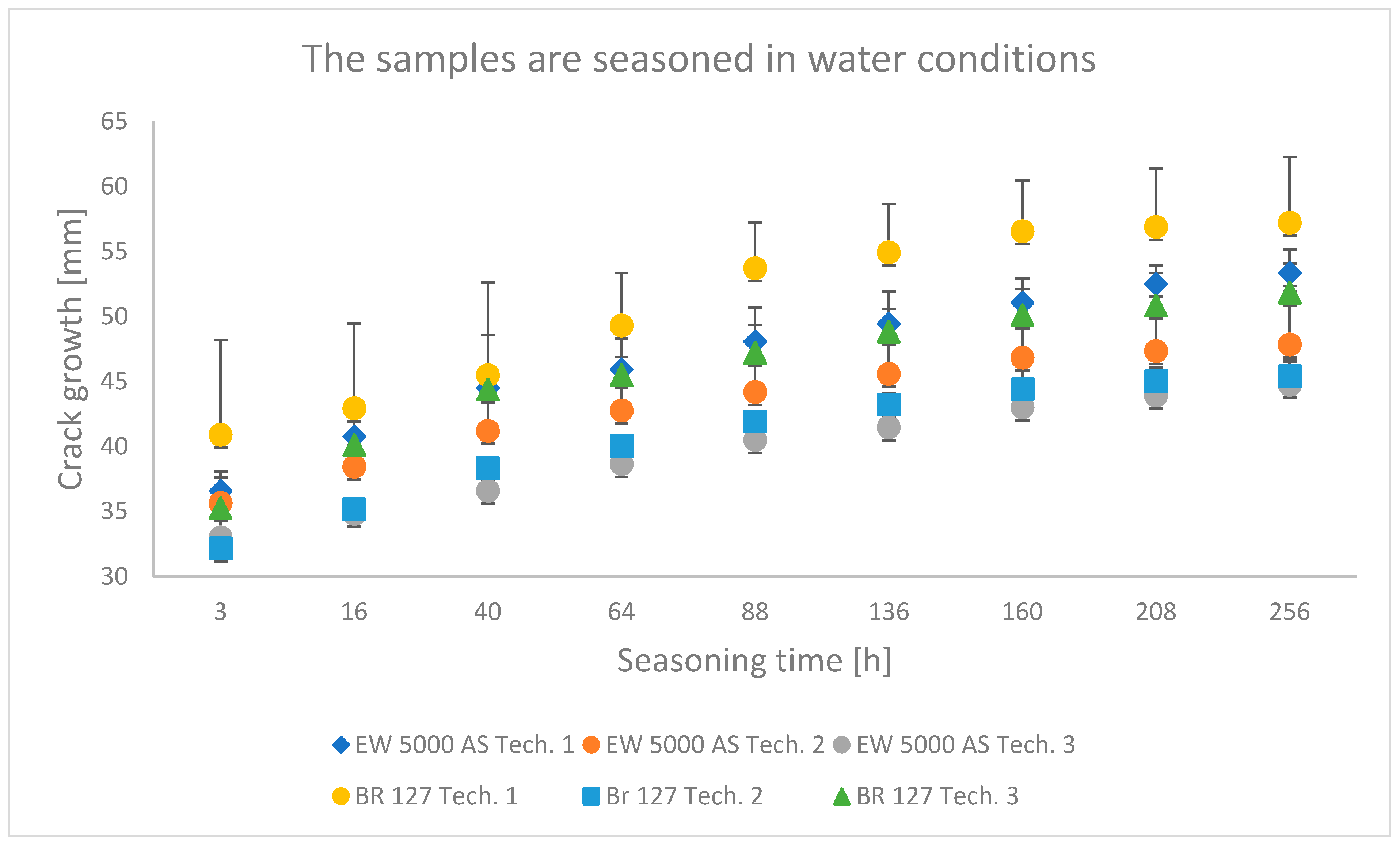
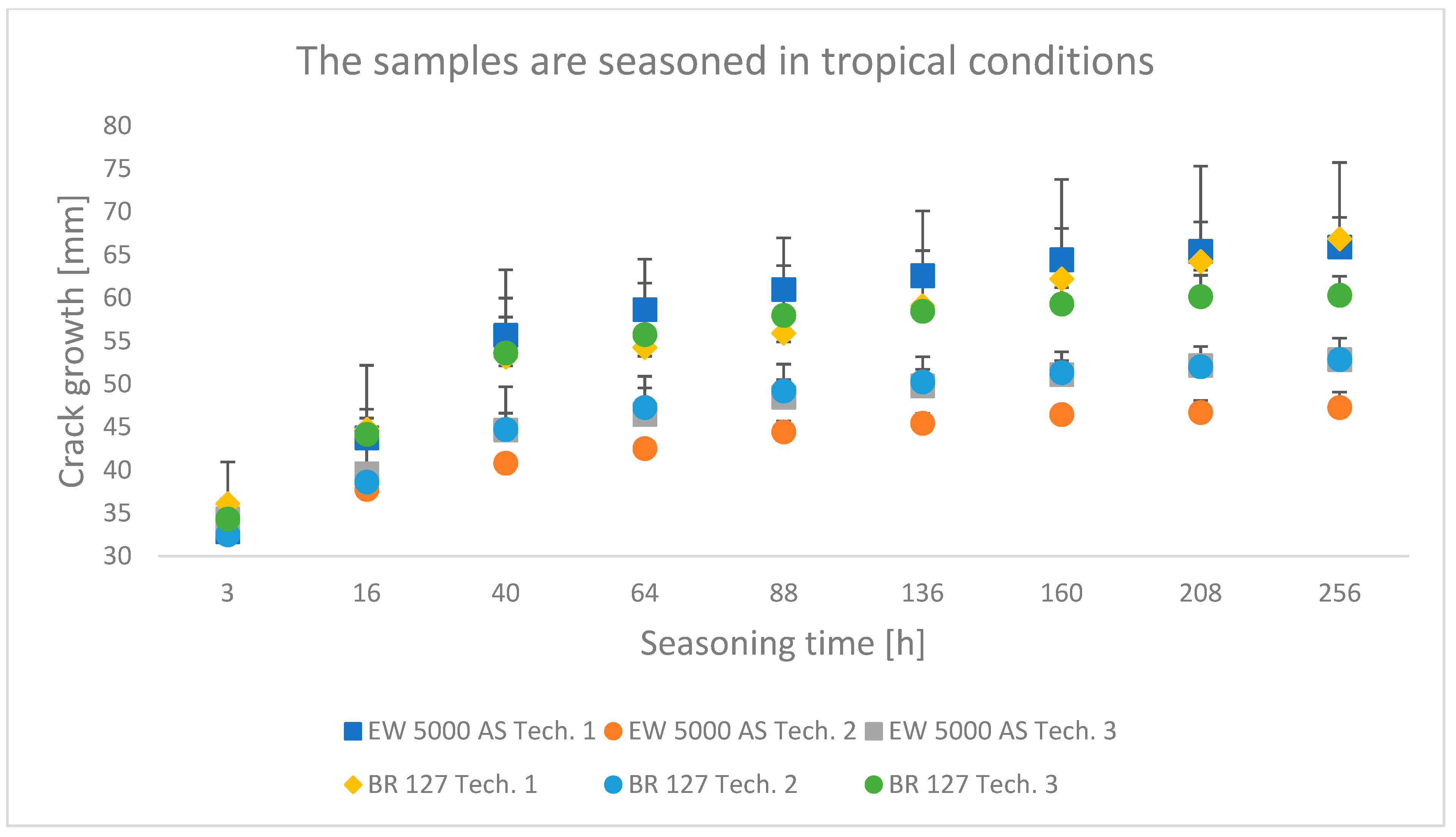
3.1. Wedge Test Results
3.2. Results of Apparent Shear Strength of Single-Lap-Joint Adhesively Bonded Metal Specimens by Tension Loading
3.3. Corrosion Test Results
4. Analysis of Research Results
4.1. Analysis of the Research Results of the Wedge Test
4.2. Analysis of the Research Results of Apparent Shear Strength of Single-Lap-Joint Adhesively Bonded Metal Specimens by Tension Loading
4.3. Analysis of the Research Results in the Corrosion Test
5. Conclusions
- The wedge test showed that the second surface preparation technology used, which assumes heating and hardening of the adhesive in one operation, provides the best bond durability. In addition, in the case of the BR 127 primer, the conclusion is confirmed by the results of shear strength tests.
- The results of the tensile shear strength of samples made using the EW-5000 AS primer show that these samples have higher strength properties in each tested seasoning condition compared to samples produced using the BR 127 primer. Therefore, it can be stated that the EW-5000AS primer can be a replacement for the BR 127 primer.
- The shear strength value of the samples seasoning in water or tropical conditions decreased by about 15% for both primers compared to laboratory conditions. The least aggressive environment for seasoning was laboratory conditions, which constituted a specific reference necessary for properly comparing the obtained test results.
- The results of the corrosion resistance tests of the undercoats protecting the substrate showed, in the case of the tested samples and the test environment used, increased corrosion susceptibility after 264 h of exposure to brine spray.
- The expected benefits for aviation include using substances without harmful chemical components for bonding, i.e., chromium VI at the appropriate level of mechanical properties and reliability of glued aircraft structures, as well as providing additional guidance for users in the aviation industry.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Petrie, E.M. Adhesive Bonding of Aluminum Alloys. Met. Finish. 2007, 105, 49–56. [Google Scholar] [CrossRef]
- Brockmann, W.; Hennemann, O.D.; Kollek, H.; Matz, C. Adhesion in bonded aluminium joints for aircraft construction. Int. J. Adhes. Adhes. 1986, 6, 115–143. [Google Scholar] [CrossRef]
- Higgins, A. Adhesive bonding of aircraft structures. Int. J. Adhes. Adhes. 2000, 20, 367–376. [Google Scholar] [CrossRef]
- Tserpes, K. Adhesive Bonding of Aircraft Structures. In Revolutionizing Aircraft Materials and Processes 2020; Pantelakis, S., Tserpes, K., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Kłonica, M. Analysis of the effect of selected factors on the strength of adhesive joints. IOP Conf. Ser. Mater. Sci. Eng. 2018, 393, 012041. [Google Scholar] [CrossRef]
- Kłonica, M.; Bielawski, R.; Żokowski, M. Comparative Tests of Shear Strength of Adhesive Lap Joints after Thermal Shocks. Adv. Sci. Technol. Res. J. 2021, 15, 364–375. [Google Scholar] [CrossRef]
- Kłonica, M.; Chatys, R.; Blumbergs, I. Strength Analysis of Lap Joints in Aerospace Structural Materials. Adv. Sci. Technol. Res. J. 2022, 16, 147–155. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Handbook of Adhesives and Surface Preparation Technology, Applications and Manufacturing, a Volume in Plastics Design Library; William Andrew Publishing: Norwich, NY, USA, 2011. [Google Scholar] [CrossRef]
- Baker, A.A.; Nezhad, H.Y. 15—Adhesively bonded repairs to highly loaded structure. In Woodhead Publishing Series in Welding and Other Joining Technologies, Adhesive Bonding, 2nd ed.; Adams, R.D., Ed.; Woodhead Publishing: Cambridge, UK, 2021; pp. 437–497. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shukla, D.K. Effect of stress rate on shear strength of aluminium alloy single lap joints bonded with epoxy/nanoalumina adhesives. Int. J. Adhes. Adhes. 2020, 99, 102587. [Google Scholar] [CrossRef]
- Chabot, K.A.; Brescia, J.A. Evaluation of primers for adhesively-bonded aircraft repair. J. Adhes. Sci. Technol. 1993, 7, 1183–1194. [Google Scholar] [CrossRef]
- Mazza, J.J.; Storage, K.M. The Evaluation of Adhesive Bond Primers for Repair Bonding of Aluminum Alloys. In Proceedings of the 42nd ISTC, Salt Lake City, UT, USA, 11–14 October 2010. [Google Scholar]
- Zbierski, P. An alternative to chromate anti-corrosion coatings in aviation. Des. Eng. Struct. 2018, 128, 5. (In Polish) [Google Scholar]
- Kohli, D.; Dickerson, E. Water Based Primer Compositions. U.S. Patent 6,475,621, 11 May 2002. [Google Scholar]
- Wegman, R.F.; Twisk, J.V. Surface Preparation Techniques for Adhesive Bonding; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Sachdeva, Y.P. Corrosion Resistant Waterborne Adhesive Primers. U.S. Patent 5,260,357, 9 November 1993. [Google Scholar]
- Teschendorf, A.F. Waterborne Epoxy Derived Adhesive Primer. Patent WO 94/02543, 3 February 1994. [Google Scholar]
- Guan, H.; Buchheit, R.G. Corrosion protection of aluminum alloy 2024-T3 by vanadate conversion Coatings. Corrosion 2004, 60, 284. [Google Scholar] [CrossRef]
- Lewis, K.; Park, C.; Aklian, J. Non-Chromate Corrosion Inhibitors for Alumimun Alloys. U.S. Patent 5,951,747, 14 September 1999. [Google Scholar]
- Shah, K.; Kohli, D. Water Based Non-Chromated Primers for Structural Bonding Applications. Patent US WO 2010/117757, 14 October 2010. [Google Scholar]
- Zhao, Y.; De Grace, H. BR® 179 Non-Chromate Bonding Primer for Aerospace Structural Bonding Applications. In Proceedings of the SAMPE neXus, Online, June 29–July 1 2021; Society for the Advancement of Material and Process Engineering: Covina, CA, USA, 2021. [Google Scholar] [CrossRef]
- Schröder, S. Extreme requirements for corrosion protection in aviation. Varnishing 2012, 4, 78. [Google Scholar]
- Barosso, J.M. Commission Regulation (EU) No 301/2014 of 25 March 2014 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) as Regards to chromium VI Compounds. Off. J. Eur. Union 2014, 90, 1–3. [Google Scholar]
- Ridera, A.; Chalkley, P. Durability of an off-optimum cured aluminum joint. Int. J. Adhes. Adhes. 2004, 24, 95–106. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Pidaparti, R.M. Static characteristics and fatigue behavior of composite-repaired aluminum plates. Compos. Struct. 2002, 56, 151–155. [Google Scholar] [CrossRef]
- Zabłocka, M.; Sałaciński, M.; Synaszko, P. The Effect of Cure Cycle Time on the Properties of Epoxy-Bonded Joints. Fatigue Aircr. Struct. 2012, 1, 157–161. [Google Scholar] [CrossRef][Green Version]
- McCray, D.B.; Mazza, J.J. Optimization of sol-gel surface preparations for repair bonding of aluminum alloys. In Proceedings of the SAMPE 2000, Long Beach, CA, USA, 21–25 May 2000. [Google Scholar]
- Chu, C.; Zheng, H.; Yang, C.; Damron, M. Sol-Gel Coating as a Surface Pretreatment Process for Adhesive Bonding of Aluminum Alloys. SAE Tech. Pap. 1997, 970020, 1–10. [Google Scholar] [CrossRef]
- Gonzalez, E.; Vejar, N.; Solis, R.; Muñoz, L.; Encinas, M.V.; Paez, M. Sol-Gel Films: Corrosion Protection Coating for Aluminium Alloy. In Sol-Gel Method: Design and Synthesis of New Materials with Interesting Physical, Chemical and Biological Properties; Aguilar, G., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Deskin, R. Technical Data Sheet BR® 127 PRIMER, 15 January 2013, New Jersey, USA. Available online: https://www.heatcon.com/wp-content/uploads/2015/08/HCS2406-011_-BR-127-Corrosion-Inhibiting-Primer.pdf (accessed on 25 October 2021).
- Technical Data Sheet 3M™ Scotch-Weld™ Structural Adhesive Primer EW-5000 AS–05.2006. Available online: https://multimedia.3m.com/mws/media/391046O/3mtm-scotch-weldtm-structural-adh-primer-ew-5000-as.pdf (accessed on 25 October 2021).
- Zhao, J.; Xia, L.; Sehgal, A.; Lu, D.; McCreery, R.; Frankel, G. Effects of chromate and chromate conversion coatings on corrosion of aluminum alloy 2024-T3. Surf. Coat. Technol. 2001, 140, 51–57. [Google Scholar] [CrossRef]
- Surowska, B. Selected Issues of Corrosion and Protection against Corrosion; Lublin University of Technology: Lublin, Poland, 2002. [Google Scholar]
- Data Sheets: 3M™ Scotch-Weld™ Structural Adhesive Film AF 163-2. Available online: https://multimedia.3m.com/mws/media/282041O/3m-scotch-weld-structural-adhesive-film-af-163-2-af-163-3.pdf (accessed on 1 February 2021).
- Data Sheets: 3M™ Surface Pre-Treatment AC-130-2. Available online: https://multimedia.3m.com/mws/media/1063834O/3m-surface-pre-treatment-ac-130-2-application-guide.pdf (accessed on 1 February 2021).
- PN-EN 573-1:2005; Standard Aluminium and Aluminium Alloys—Chemical Composition and Form of Wrought Products—Part 1: Numerical Designation System. ISO: Geneva, Switzerland, 2005.
- Available online: https://www.theworldmaterial.com/2024-aluminum-alloy/ (accessed on 20 March 2022).
- ASTM D3762–03(2010); Standard Test Method for Adhesive-Bonded Surface Durability of Aluminum (Wedge Test). ASTM: West Conshohocken, PA, USA, 2010.
- ASTM D1002-10(2019); Standard Test Method for Apparent Shear Strength of Single-Lap-Joint Adhesively Bonded Metal Specimens by Tension Loading (Metal-to-Metal). ASTM: West Conshohocken, PA, USA, 2019.
- Adams, R.D.; Cowap, J.W.; Farquharson, G. The relative merits of the Boeing wedge test and the double cantilever beam test for assessing the durability of adhesively bonded joints, with particular reference to the use of fracture mechanics. Int. J. Adhes. Adhes. 2009, 29, 609–620. [Google Scholar] [CrossRef]
- Bellini, C.; Parodo, G.; Polini, W.; Sorrentino, L. Influence of Hydrothermal Ageing on Single Lap Bonded CFRP Joints; University of Cassino and Southern Lazio, Fratturaed Integrità Strutturale: Cassino, Italy, 2018; Volume 45. [Google Scholar]
- PN-EN ISO 9227:2017-06; Standard Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. ISO: Geneva, Switzerland, 2017.
- Storage, K.; Mazza, J.; Smith, J. Evaluation of Adhesive Bond Primers for Repair Bonding of Aluminum; DoD Joint Committee on Tactical Shelters (JOCOTAS), Materials and Manufacturing Directorate: Panama City Beach, FL, USA, 2011. [Google Scholar]
- Synaszko, P.; Salacinski, M.; Ciezak, P. The an Approach to Damage Detection in Metal Sandwich Structures with Composite-Metal Patch Bonded Repair; SAE Technical Paper 2017-01-2050; SAE: Warrendalem, PA, USA, 2017. [Google Scholar] [CrossRef]
- Sałaciński, M.; Synaszko, P.; Stefaniuk, M.; Dragan, K. Monitoring of Crack Growth in a Structure Under a Composite Patch. Fatigue Aircr. Struct. 2011, 2011, 103–111. [Google Scholar] [CrossRef][Green Version]
- Hosoi, A.; Suzuki, T.; Kosugi, K.; Atsumi, T.; Shimamura, Y.; Tsuda, T.; Kawada, H. Very High-Cycle Fatigue Characteristics of Cross-Ply CFRP Laminates in Transverse Crack Initiation. In ICAF 2019—Structural Integrity in the Age of Additive Manufacturing; Niepokolczycki, A., Komorowski, J., Eds.; ICAF 2019; Lecture Notes in Mechanical Engineering; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Kamińska, P.; Synaszko, P.; Ciężak, P.; Dragan, K. Analysis of the Corrosion Resistance of Aircraft Structure Joints with Double-Sided Rivets and Single-Sided Rivets. Fatigue Aircr. Struct. 2020, 2020, 57–68. [Google Scholar] [CrossRef]
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 on the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) and Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94, as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. The Official Journal of the European Union EUR-Lex, Luksemburg. 2006. Available online: http://data.europa.eu/eli/reg/2006/1907/2022-10-14 (accessed on 15 March 2022).


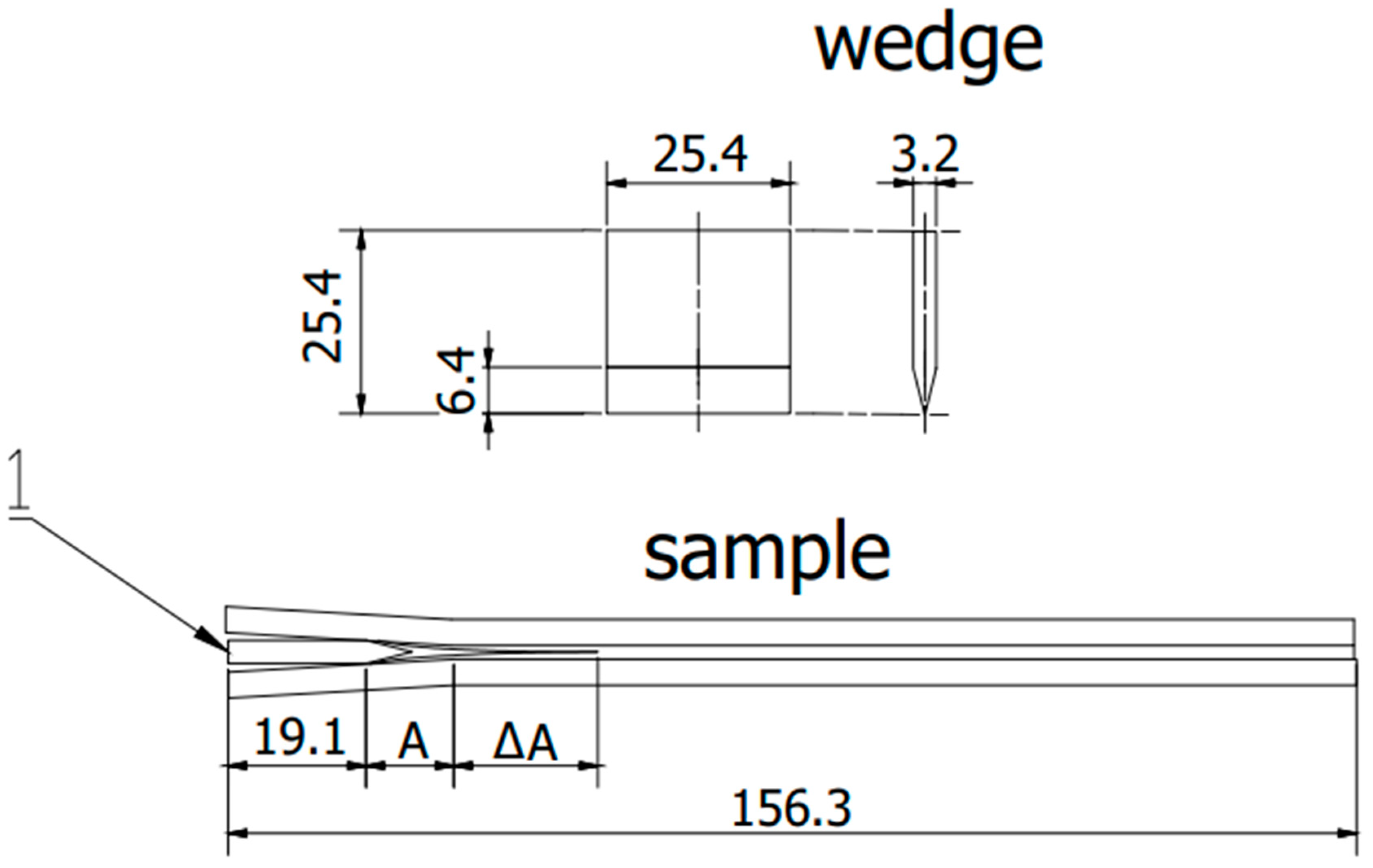
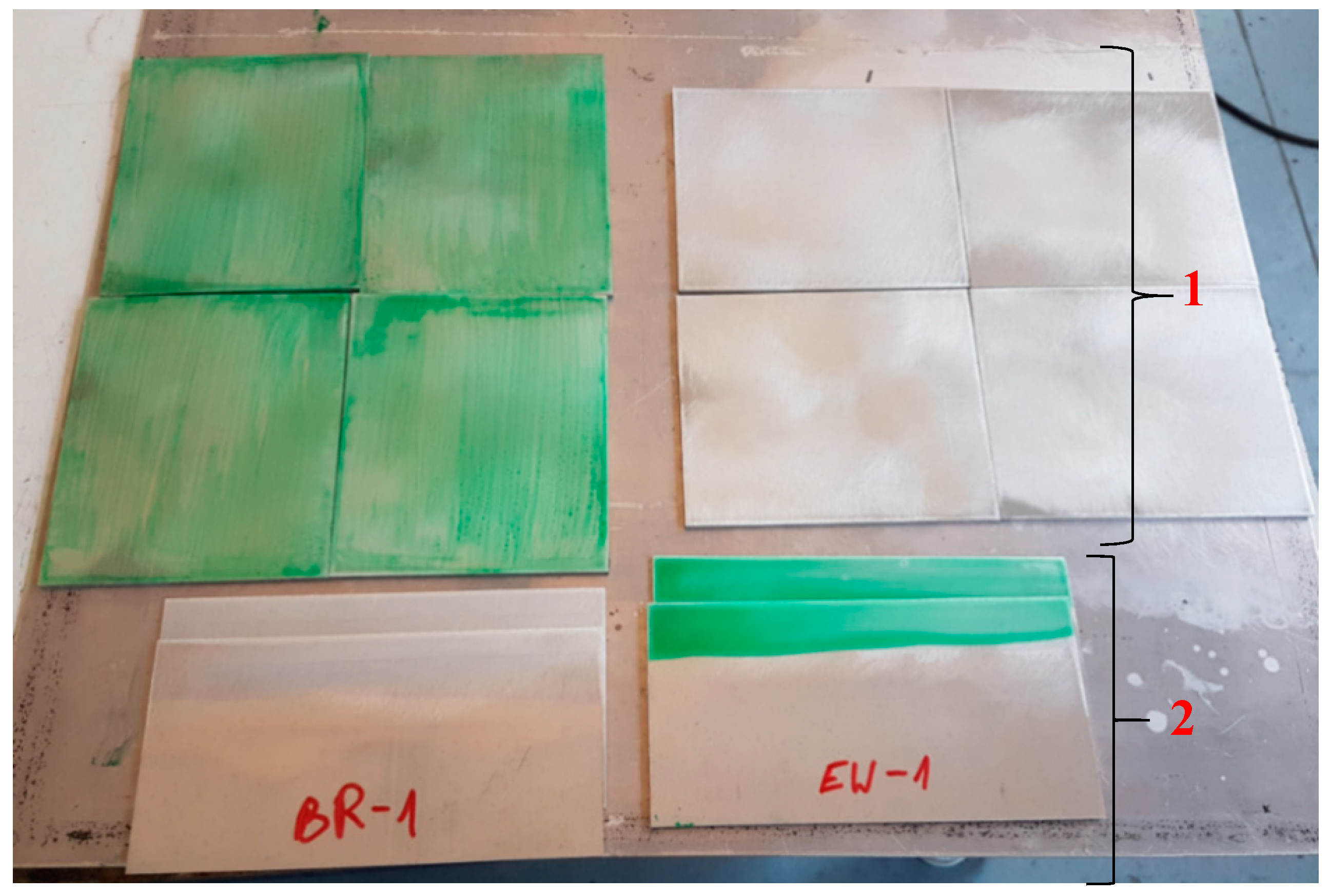
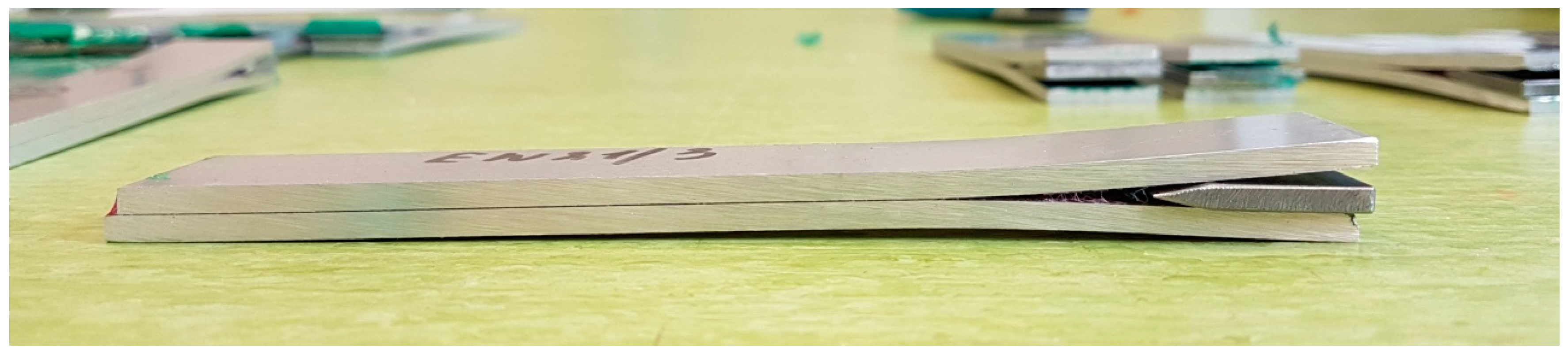








| Component | wt. % | Component | wt. % |
|---|---|---|---|
| Al | 90.7–94.7 | Cr | Max. 0.1 |
| Cu | 3.8–4.9 | Fe | Max. 0.5 |
| Mg | 1.2–1.8 | Mn | 0.3–0.9 |
| Si | Max. 0.5 | Ti | Max. 0.15 |
| Zn | Max. 0.25 | Other, each | Max. 0.05 |
| Other, total | Max. 0.15 |
| Mechanical and Physical Property | Value |
|---|---|
| Yield Strength, [MPa] | 345 |
| Tensile Strength, [MPa] | 483 |
| Modulus of Elasticity, [GPa] | 73.1 |
| Elongation, [%] | 15 |
| Hardness, HB | 123 |
| Thermal Conductivity, [W/mK] | 121 |
| Density, [g/cm3] | 2.78 |
| Melting Point, [°F] | 935–1180 |
| BR 127 | EW-5000 AS | |||
|---|---|---|---|---|
| Composition | % by wt. | Composition | % by wt. | |
| Information on Ingredients |
| 60–75 |
| 50–70 |
| 10–30 |
| 10–20 | |
| 1–5 |
| 1–6 | |
| 1–5 |
| 1–5 | |
| 1–5 |
| 1–5 | |
| 1–5 |
| 1–5 | |
| 0.1–1 |
| 1–5 | |
| 1–5 | |||
| 0.5–1.5 | |||
| <1 | |||
| <0.75 | |||
| <0.1 | |||
| Volatile Organic Compounds [g/L] | 780–800 | 80–84 | ||
| Density [g/cm3] | 0.875 | 1.04–1.09 | ||
| Specific Gravity [g/cm3] | 0.88 | 1.06 | ||
| Flash Point [°F] | 29 | 108.5 | ||
| Boiling Point [°F] | 176 | 212 | ||
| Flammable Limits [% by Vol] | LEL: 1.8; UEL: 10.0 | LEL: 1.5; UEL: 12.7 | ||
| Vapor Pressure [mmHg] | 86 | 15 | ||
| Solubility in Water | Slight | Complete | ||
| Technology | Method of Applying BR-127 or EW-5000 AS Primer |
|---|---|
| Tech. 1 | Hardening of the primer in accordance with the recommendations of the 3M manufacturer [22,23] |
| Tech. 2 | Hardening of the primer and the adhesive film in one operation |
| Tech. 3 | Primer curing in room conditions |
| Primer | Kind of Technology | Seasoning Method | Number of Samples | ||
|---|---|---|---|---|---|
| Wedge Test | Tensile Shear Test | Corrosion Test | |||
| Samples without primer | X | X | X | X | 4 |
| BR 127 | Tech. 1 | Laboratory conditions | 2 | 4 | 4 |
| Tropical conditions | 3 | 5 | |||
| Water conditions | 3 | 5 | |||
| Tech. 2 | Laboratory conditions | 2 | 4 | ||
| Tropical conditions | 3 | 5 | |||
| Water conditions | 3 | 5 | |||
| Tech. 3 | Laboratory conditions | 2 | 4 | ||
| Tropical conditions | 3 | 5 | |||
| Water conditions | 3 | 5 | |||
| EW 5000 AS | Tech. 1 | Laboratory conditions | 2 | 4 | 4 |
| Tropical conditions | 3 | 5 | |||
| Water conditions | 3 | 5 | |||
| Tech. 2 | Laboratory conditions | 2 | 4 | ||
| Tropical conditions | 3 | 5 | |||
| Water conditions | 3 | 5 | |||
| Tech. 2 | Laboratory conditions | 2 | 4 | ||
| Tropical conditions | 3 | 5 | |||
| Water conditions | 3 | 5 | |||
| Primer Application Technology and Sample Seasoning Conditions | EW-5000 AS | BR 127 |
|---|---|---|
| TECHNOLOGY 1 | Image and nature of the destruction | Image and nature of the destruction |
| Seasoned in laboratory conditions |  Cohesive failure |  The initial cohesive character of the crack turned into adhesive during the test, constituting about 30% of the total surface |
| Seasoned in tropical conditions |  The adhesive nature of the destruction occurs on about 30% of the tested sample surface |  During the test, the cohesive character of the destruction was transformed into the adhesive one, occurring on 50% of the surface |
| Seasoned in water |  Adhesive nature of the destruction |  During the test, the cohesive character of the destruction was transformed into the adhesive one, occurring on 50% of the surface |
| TECHNOLOGY 2 | ||
| Seasoned in laboratory conditions |  The initial, cohesive character of the crack during the test turned into adhesive, which occurs in about 60% of the entire joint |  The first phase of the crack is about 60% and has a cohesive character, then turns into an adhesive character |
| Seasoned in tropical conditions |  Adhesive character, which accounts for approximately 60% of the total joint |  The samples show an adhesive cracking character in the area of about 60% |
| Seasoned in water |  The adhesive–cohesive character of failure occurs in the ratio of 50: 50% |  The samples show an adhesive character of cracking in the area of about 40% of the surface |
| TECHNOLOGY 3 | ||
| Seasoned in laboratory conditions |  A cohesive crack in the first stage of the test turns into an adhesive failure |  The samples are characterized by a 70% cohesive crack, which changed into adhesive character during the test |
| Seasoned in tropical conditions |  The adhesive–cohesive character of failure occurring in the ratio of 50: 50% |  Adhesive character of destruction on the surface of about 60% |
| Seasoned in water |  Adhesive character of destruction on the surface of about 30% |  The adhesive–cohesive character of failure occurring in the ratio of 50: 50% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chomiak, M.; Sałaciński, M.; Gołębiowski, F.; Broda, P. Durability and Corrosion Resistance Test of Adhesive Joints Using Two Adherence Promoters for the Connection of Aerospace Aluminum Alloys. Materials 2022, 15, 8733. https://doi.org/10.3390/ma15248733
Chomiak M, Sałaciński M, Gołębiowski F, Broda P. Durability and Corrosion Resistance Test of Adhesive Joints Using Two Adherence Promoters for the Connection of Aerospace Aluminum Alloys. Materials. 2022; 15(24):8733. https://doi.org/10.3390/ma15248733
Chicago/Turabian StyleChomiak, Monika, Michał Sałaciński, Filip Gołębiowski, and Piotr Broda. 2022. "Durability and Corrosion Resistance Test of Adhesive Joints Using Two Adherence Promoters for the Connection of Aerospace Aluminum Alloys" Materials 15, no. 24: 8733. https://doi.org/10.3390/ma15248733
APA StyleChomiak, M., Sałaciński, M., Gołębiowski, F., & Broda, P. (2022). Durability and Corrosion Resistance Test of Adhesive Joints Using Two Adherence Promoters for the Connection of Aerospace Aluminum Alloys. Materials, 15(24), 8733. https://doi.org/10.3390/ma15248733






