Effect of Cu on the Microstructure and Mechanical Properties of a Low-Carbon Martensitic Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
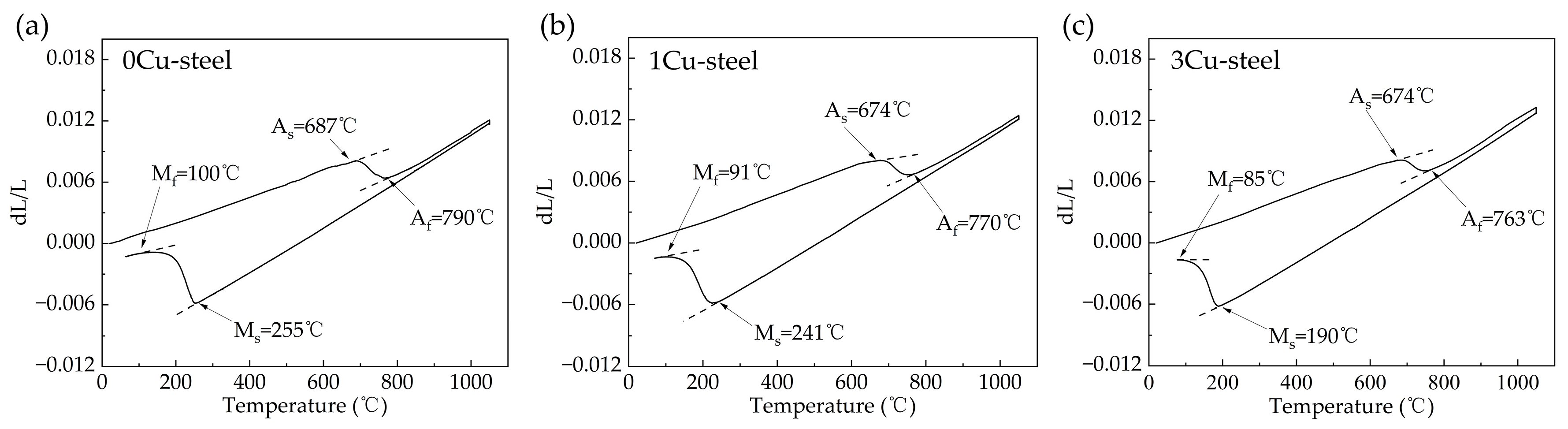
3.1. Phase Transition Temperatures
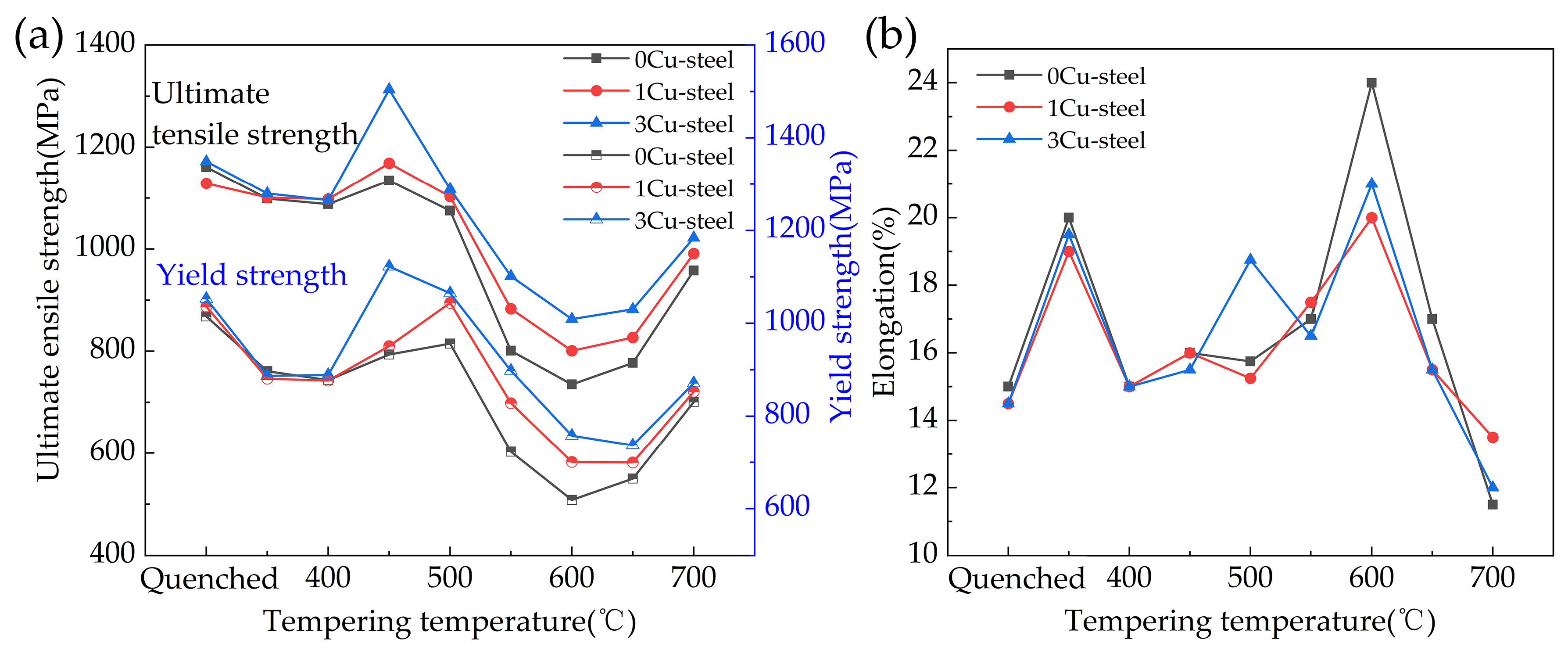
3.2. Mechanical Properties
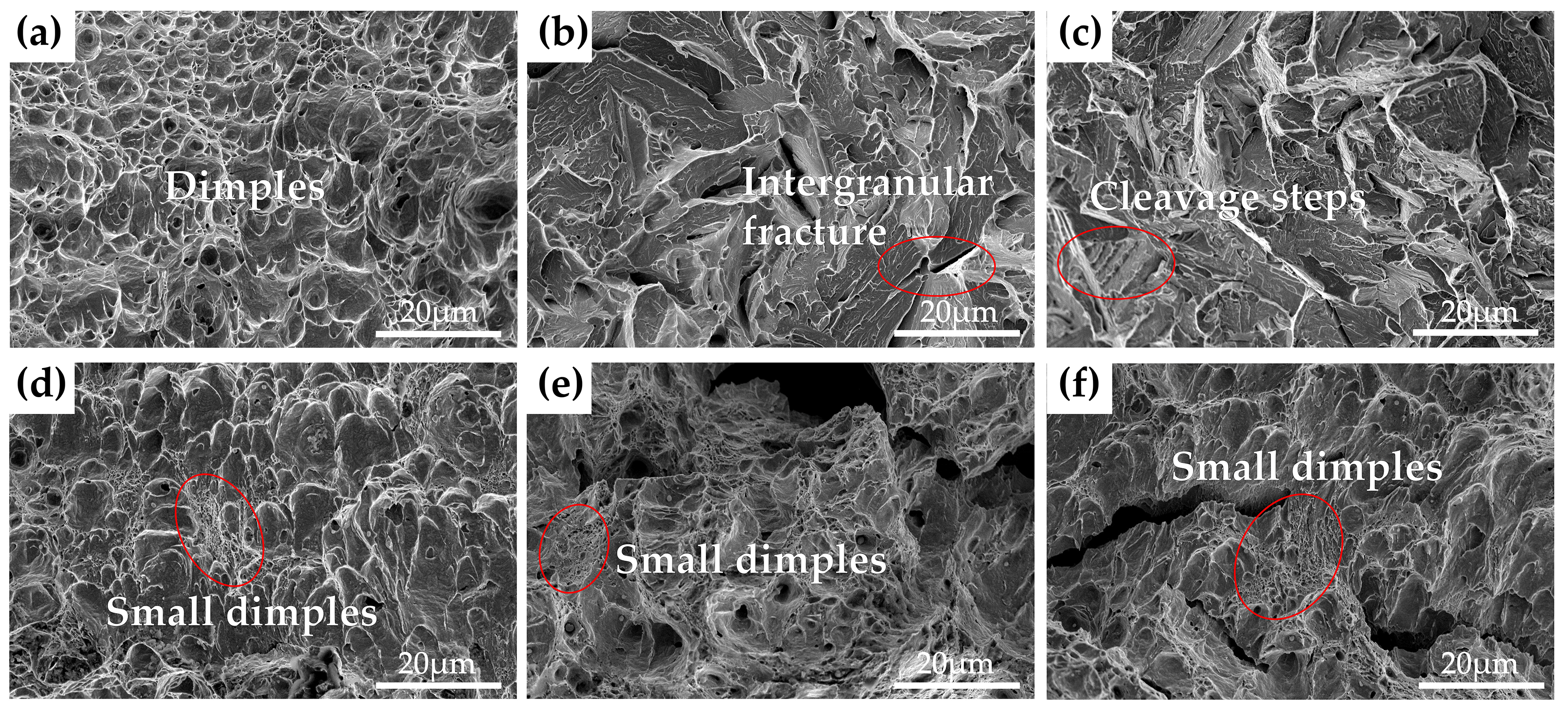
3.3. Microstructures
3.3.1. Microstructures of the Steels Tempered at 450 °C
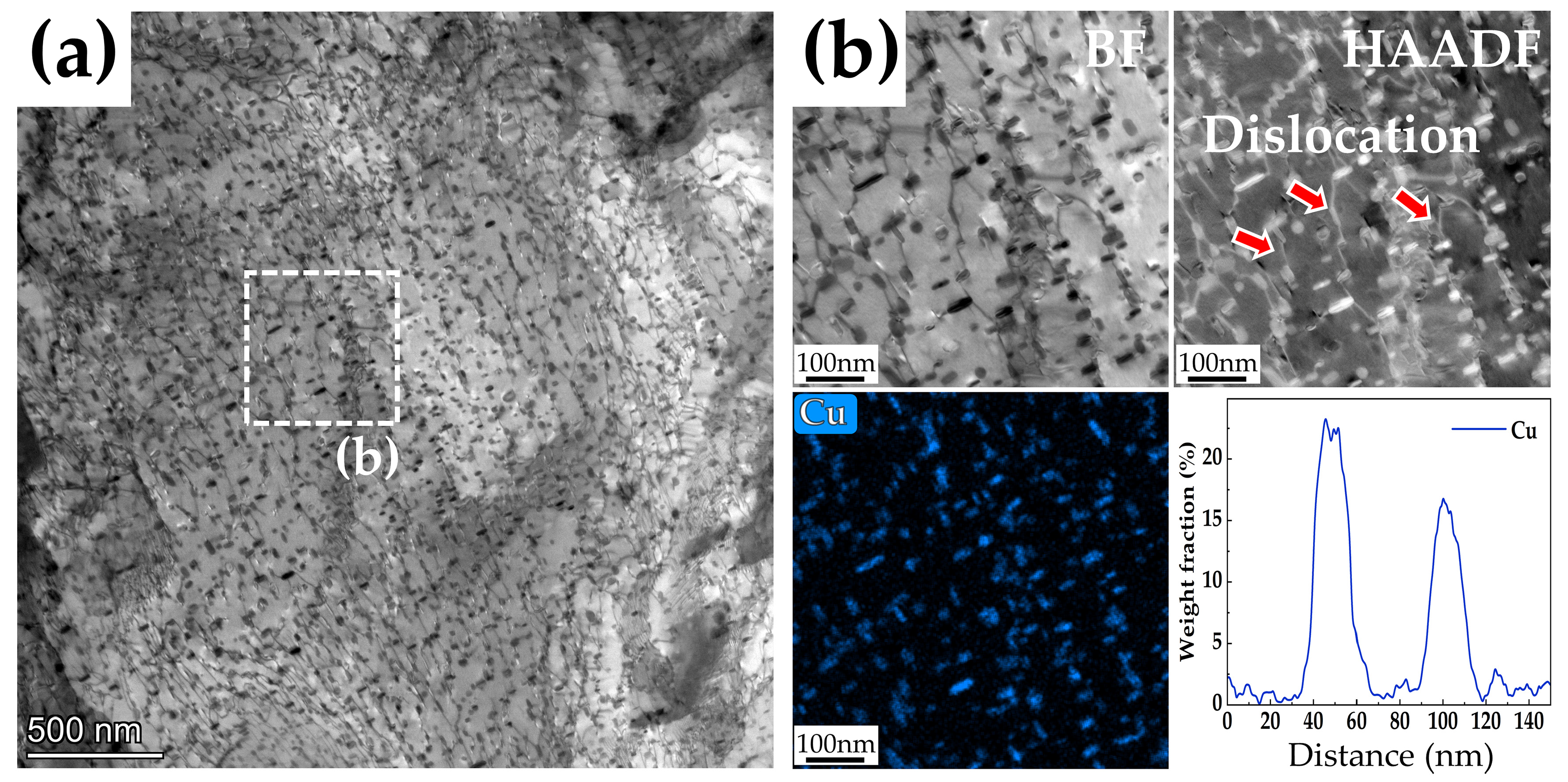
3.3.2. Microstructures of the Steels Tempered at 600 °C
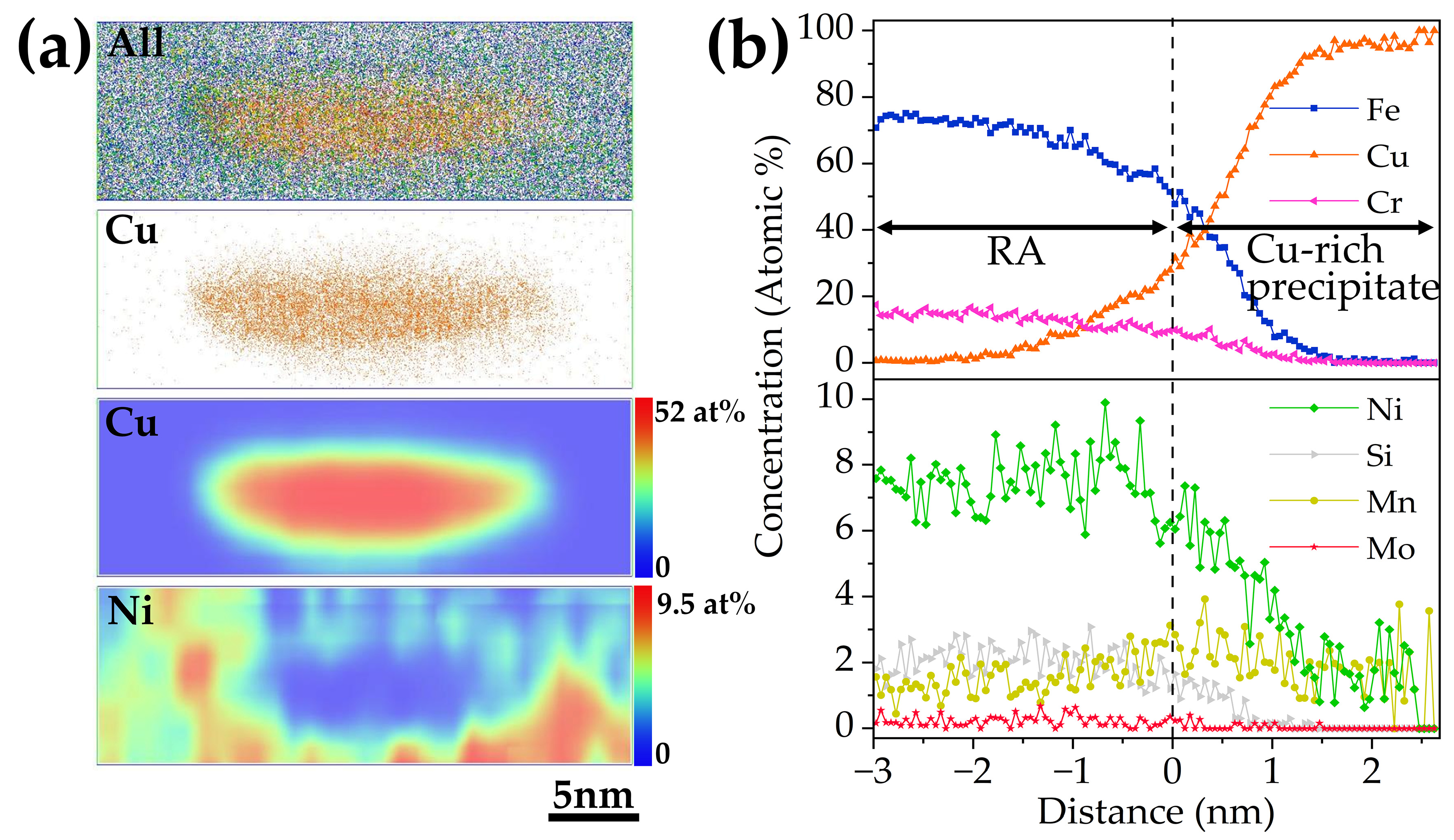
3.4. Effect of Cu on the Formation of Reversed Austenite
3.5. Effect of Cu on Mechanical Properties
4. Conclusions
- (i)
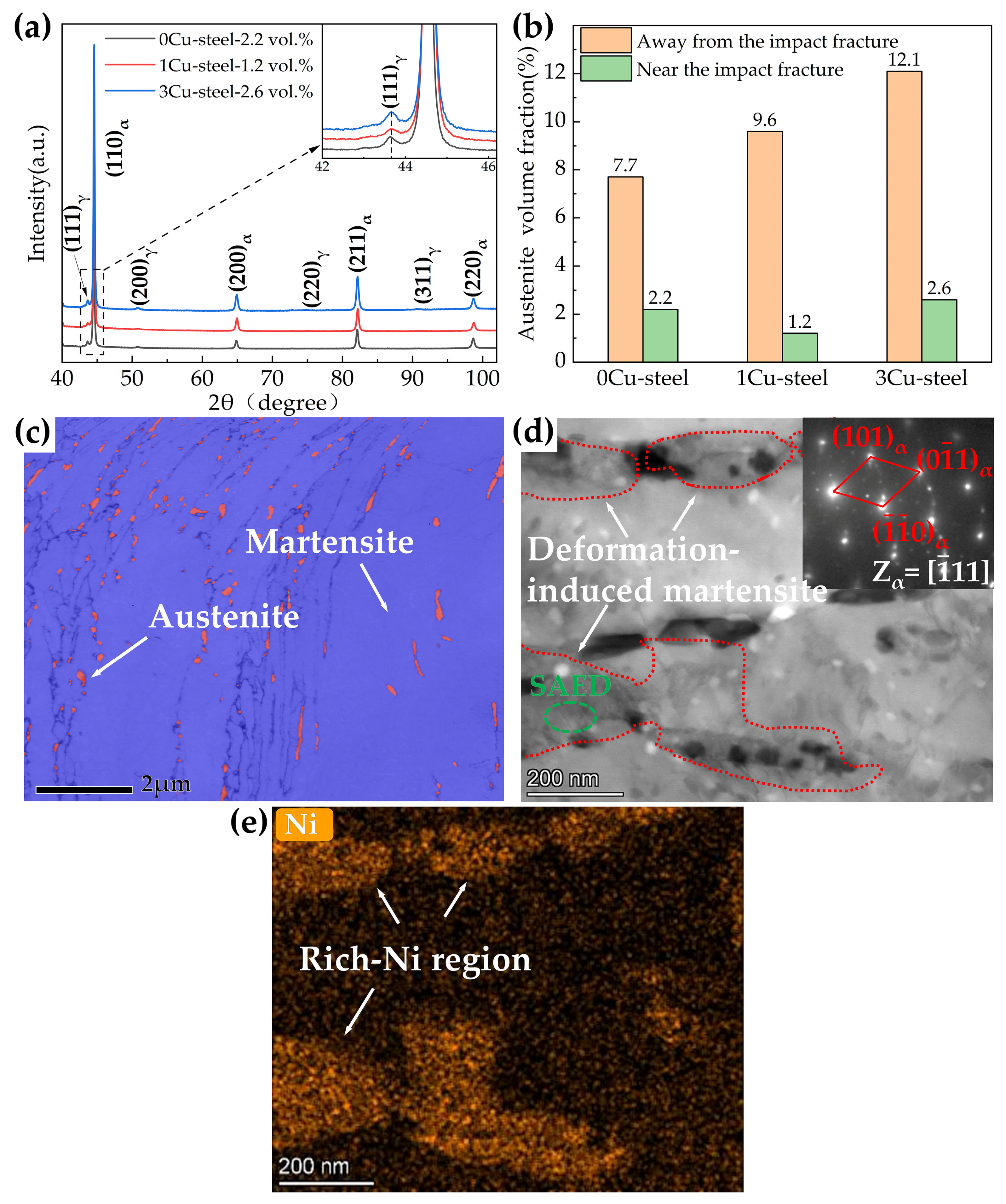
- The addition of Cu decreases martensite and austenite phase transition temperature, especially the starting temperature of martensitic transformation (Ms). The volume fractions of reversed austenite increased with the increase in Cu content and were 7.7 vol.% for 0Cu-steel, 9.6 vol.% for 1Cu-steel, and 12.1 vol.% for 3Cu-steel tempered at 600 °C.
- (ii)
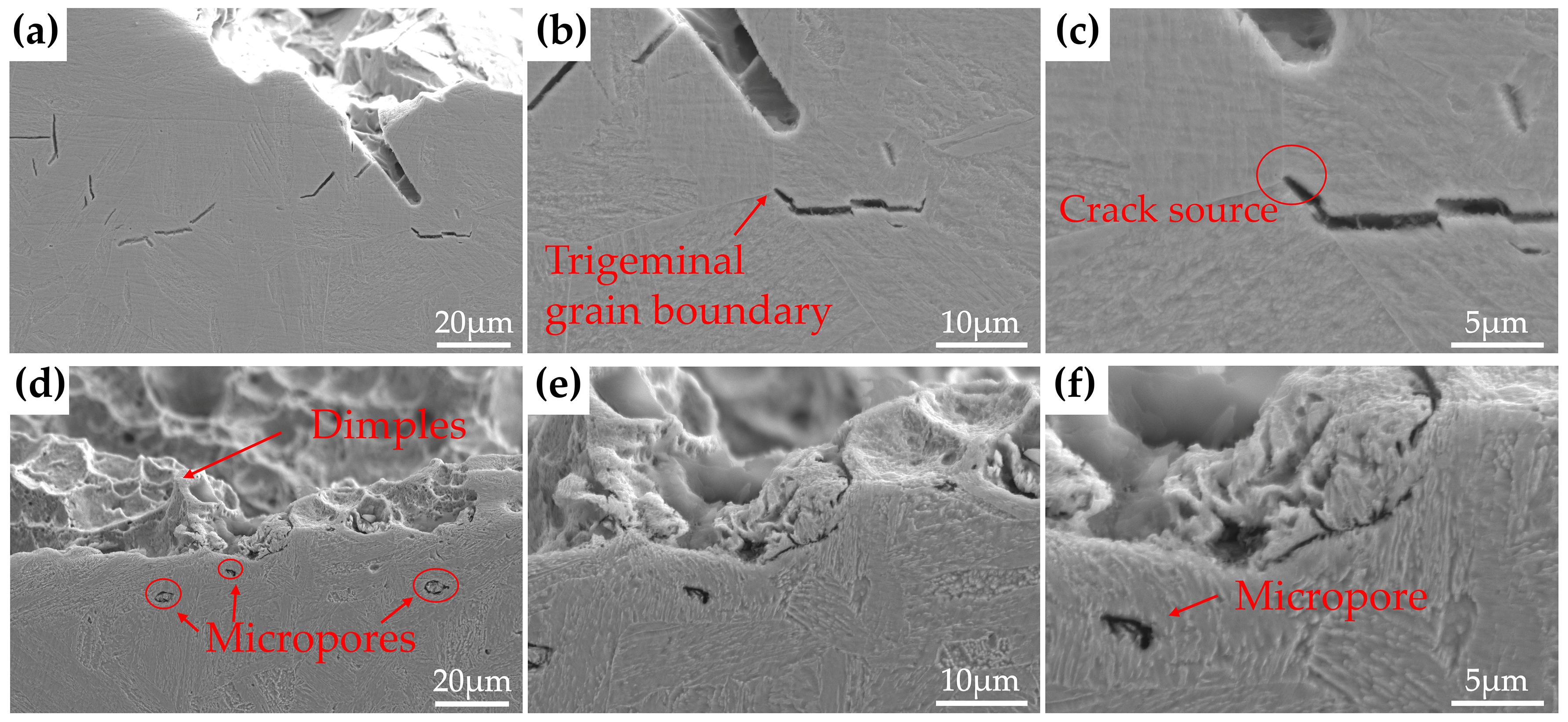
- The samples tempered at 600 °C had a TRIP effect during impact deformation, and the content of reversed austenite in the three steels decreased significantly to only about 2 vol.%. In addition, deformation-induced martensite was observed in the microstructure near the impact fracture surface of 3Cu-steel.
- (iii)
- APT results indicated that Cu-rich clusters initially formed with other alloying elements such as Fe and Ni and then grew to become precipitates through rejection of the alloying elements. The segregation of Ni atoms at the interface between the Cu-rich precipitates and the martensite matrix provided composition conditions for the formation of reversed austenite.
- (iv)
- Cu precipitations increase tensile strength during tempering. However, the Cu precipitations also generate temper brittleness at a tempering temperature of 450 °C, resulting in the impact energy of the 3Cu-steel being only 7 J. The 3Cu-steel tempered at 600 °C for 2 h has an ultimate tensile strength of 863 Mpa, good ductility of 21%, and excellent impact energy of 192 J, achieving suitable strength–ductility–toughness matching.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, T.; Neding, B.; Lin, S.; Tseng, J.-C.; Hedström, P. Cu precipitation-mediated formation of reverted austenite during ageing of a 15–5 PH stainless steel. Scr. Mater. 2021, 202, 114007. [Google Scholar] [CrossRef]
- Zhou, T.; Faleskog, J.; Babu, R.P.; Odqvist, J.; Yu, H.; Hedström, P. Exploring the relationship between the microstructure and strength of fresh and tempered martensite in a maraging stainless steel Fe–15Cr–5Ni. Mater. Sci. Eng. A 2019, 745, 420–428. [Google Scholar] [CrossRef]
- Niu, M.C.; Yang, K.; Luan, J.H.; Wang, W.; Jiao, Z.B. Cu-assisted austenite reversion and enhanced TRIP effect in maraging stainless steels. J. Mater. Sci. Technol. 2022, 104, 52–58. [Google Scholar] [CrossRef]
- He, B.B.; Hu, B.; Yen, H.W.; Cheng, G.J.; Wang, Z.K.; Luo, H.W.; Huang, M.X. High dislocation density-induced large ductility in deformed and partitioned steels. Science 2017, 357, 1029–1032. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Rivera-Díaz-del-Castillo, P.E.J.; Yan, W.; Yang, K.; San Martín, D.; Kestens, L.A.I.; van der Zwaag, S. A new ultrahigh-strength stainless steel strengthened by various coexisting nanoprecipitates. Acta Mater. 2010, 58, 4067–4075. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Prasath Babu, R.; Odqvist, J.; Yu, H.; Hedström, P. Quantitative electron microscopy and physically based modelling of Cu precipitation in precipitation-hardening martensitic stainless steel 15-5 PH. Mater. Des. 2018, 143, 141–149. [Google Scholar] [CrossRef]
- Wang, J.; Zou, H.; Li, C.; Qiu, S.-y.; Shen, B.-l. The effect of microstructural evolution on hardening behavior of type 17-4PH stainless steel in long-term aging at 350 °C. Mater. Charact. 2006, 57, 274–280. [Google Scholar] [CrossRef]
- Govindaraj, V.; Hodgson, P.; Singh, R.P.; Beladi, H. The effect of austenite reversion on the microstructure and mechanical properties of a 12Cr–3Ni–3Mn–3Cu-0.15Nb–0.05C maraging stainless steel. Mater. Sci. Eng. A 2021, 828, 142097. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, D.; Xiong, J. The effect of reversed austenite on mechanical properties of 13Cr4NiMo steel: A CPFEM study. J. Mater. Res. Technol. 2022, 18, 2963–2976. [Google Scholar] [CrossRef]
- Slamani, F.; Thibault, D.; Verreman, Y.; Bocher, P. A new morphology of retained austenite obtained by intercritical tempering of 13Cr-4Ni martensitic stainless steel. Mater. Charact. 2022, 193, 112255. [Google Scholar] [CrossRef]
- Chen, W.; Luan, J.H.; Xu, L.; Han, Y.; Zhao, L.; Kai, J.-J.; Xiao, B.; Jing, H. Reversed austenite in additively manufactured martensitic stainless steel. Mater. Sci. Eng. A 2022, 834, 142597. [Google Scholar] [CrossRef]
- Markfeld, A.; Rosen, A. The effect of reverted austenite on the plastic-deformation of maraging-steel. Mater. Sci. Eng. 1980, 46, 151–157. [Google Scholar] [CrossRef]
- Galindo-Nava, E.I.; Rainforth, W.M.; Rivera-Díaz-del-Castillo, P.E.J. Predicting microstructure and strength of maraging steels: Elemental optimisation. Acta Mater. 2016, 117, 270–285. [Google Scholar] [CrossRef]
- Wang, M.M.; Tasan, C.C.; Ponge, D.; Raabe, D. Spectral TRIP enables ductile 1.1 GPa martensite. Acta Mater. 2016, 111, 262–272. [Google Scholar] [CrossRef]
- Song, Y.Y.; Li, X.Y.; Rong, L.; Li, Y.Y. Anomalous Phase Transformation from Martensite to Austenite in Fe-13%Cr-4%Ni-Mo Martensitic Stainless Steel. J. Mater. Sci. Technol. 2010, 26, 823–826. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Rong, L.; Li, Y. The influence of tempering temperature on the reversed austenite formation and tensile properties in Fe–13%Cr–4%Ni–Mo low carbon martensite stainless steels. Mater. Sci. Eng. A 2011, 528, 4075–4079. [Google Scholar] [CrossRef]
- Song, Y.Y.; Li, X.Y.; Yin, F.X.; Ping, D.H.; Rong, L.J.; Li, Y.Y. Variation of the Reversed Austenite Amount with the Tempering Temperature in a Fe-13%Cr-4%Ni-Mo Martensitic Stainless Steel. Mater. Sci. Forum 2010, 650, 193–198. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Luan, J.H.; Miller, M.K.; Liu, C.T. Precipitation mechanism and mechanical properties of an ultra-high strength steel hardened by nanoscale NiAl and Cu particles. Acta Mater. 2015, 97, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Z.B.; Luan, J.H.; Zhang, Z.W.; Miller, M.K.; Ma, W.B.; Liu, C.T. Synergistic effects of Cu and Ni on nanoscale precipitation and mechanical properties of high-strength steels. Acta Mater. 2013, 61, 5996–6005. [Google Scholar] [CrossRef]
- Shen, Q.; Huang, D.; Liu, W.; Li, F.; Lu, Q. Effect of Cu content on the precipitation behavior of Cu-rich and NiAl phases in steel. Mater. Charact. 2022, 187, 111849. [Google Scholar] [CrossRef]
- Li, Z.; Chai, F.; Zhang, Z.; Luo, X. Effect of intercritical heat treatment on microstructure, nano-size precipitation and mechanical properties of Fe–Ni–Cu–Al low carbon steel. Mater. Sci. Eng. A 2022, 857, 144033. [Google Scholar] [CrossRef]
- Cho, H.-J.; Cho, Y.; Gwon, H.; Lee, S.; Sohn, S.S.; Kim, S.-J. Effects of Ni/Cu replacement on improvement of tensile and hydrogen-embrittlement properties in austenitic stainless steels. Acta Mater. 2022, 235, 118093. [Google Scholar] [CrossRef]
- Kan, L.; Ye, Q.; Wang, Z.; Zhao, T. Improvement of strength and toughness of 1 GPa Cu-bearing HSLA steel by direct quenching. Mater. Sci. Eng. A 2022, 855, 143875. [Google Scholar] [CrossRef]
- Sun, W.W.; Wu, Y.X.; Yang, S.C.; Hutchinson, C.R. Advanced high strength steel (AHSS) development through chemical patterning of austenite. Scr. Mater. 2018, 146, 60–63. [Google Scholar] [CrossRef]
- Wu, X.L.; Yang, M.X.; Yuan, F.P.; Chen, L.; Zhu, Y.T. Combining gradient structure and TRIP effect to produce austenite stainless steel with high strength and ductility. Acta Mater. 2016, 112, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Dhua, S.K.; Mukerjee, D.; Sarma, D.S. Influence of tempering on the microstructure and mechanical properties of HSLA-100 steel plates. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2001, 32, 2259–2270. [Google Scholar]
- Zhang, H.; Sun, M.; Liu, Y.; Ma, D.; Xu, B.; Huang, M.; Li, D.; Li, Y. Ultrafine-grained dual-phase maraging steel with high strength and excellent cryogenic toughness. Acta Mater. 2021, 211, 116878. [Google Scholar] [CrossRef]
- Du, Y.B.; Hu, X.F.; Song, Y.Y.; Zhang, Y.P.; Rong, L.J. Effect of Nanoscale Cu-Riched Clusters on Strength and Impact Toughness in a Tempered Cu-Bearing HSLA Steel. Acta Metall. Sin. 2022, 35, 537–550. [Google Scholar] [CrossRef]
- Lim, N.S.; Bang, C.W.; Das, S.; Jin, H.W.; Ayer, R.; Park, C.G. Influence of tempering temperature on both the microstructural evolution and elemental distribution in AISI 4340 steels. Met. Mater. Int. 2012, 18, 87–94. [Google Scholar] [CrossRef]
- Wen, Y.R.; Hirata, A.; Zhang, Z.W.; Fujita, T.; Liu, C.T.; Jiang, J.H.; Chen, M.W. Microstructure characterization of Cu-rich nanoprecipitates in a Fe–2.5 Cu–1.5 Mn–4.0 Ni–1.0 Al multicomponent ferritic alloy. Acta Mater. 2013, 61, 2133–2147. [Google Scholar] [CrossRef]
- Seko, A.; Nishitani, S.R.; Tanaka, I.; Adachi, H.; Fujita, E.F. First-principles calculation on free energy of precipitate nucleation. Calphad 2004, 28, 173–176. [Google Scholar] [CrossRef]
- Zhang, C.; Enomoto, M. Study of the influence of alloying elements on Cu precipitation in steel by non-classical nucleation theory. Acta Mater. 2006, 54, 4183–4191. [Google Scholar] [CrossRef]
- Koyama, T.; Hashimoto, K.; Onodera, H. Phase-Field Simulation of Phase Transformation in Fe-Cu-Mn-Ni Quaternary Alloy. Mater. Trans. 2006, 47, 2765–2772. [Google Scholar] [CrossRef] [Green Version]
- Heo, Y.U.; Kim, Y.K.; Kim, J.S.; Kim, J.K. Phase transformation of Cu precipitates from bcc to fcc in Fe–3Si–2Cu alloy. Acta Mater. 2013, 61, 519–528. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Luan, J.H.; Miller, M.K.; Chung, Y.W.; Liu, C.T. Co-precipitation of nanoscale particles in steels with ultra-high strength for a new era. Mater. Today 2017, 20, 142–154. [Google Scholar] [CrossRef]
- Yan, J.; Xu, H.; Zuo, X.; Jia, T.; Wang, E. Strategies for strengthening-ductility and hierarchical co-precipitation in multicomponent nano-precipitated steels by Cu partitioning. Mater. Sci. Eng. A 2019, 739, 225–234. [Google Scholar] [CrossRef]
- Yuasa, M.; Mabuchi, M. Effects of segregated Cu on an Fe grain boundary by first-principles tensile tests. J. Phys. Condens. Matter 2010, 22, 505705. [Google Scholar] [CrossRef]
- Kapoor, M.; Isheim, D.; Vaynman, S.; Fine, M.E.; Chung, Y.W. Effects of increased alloying element content on NiAl-type precipitate formation, loading rate sensitivity, and ductility of Cu- and NiAl-precipitation-strengthened ferritic steels. Acta Mater. 2016, 104, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.K.; Russell, K.F. Embrittlement of RPV steels: An atom probe tomography perspective. J. Nucl. Mater. 2007, 371, 145–160. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Morris, J.W. Cryogenic Steels. In Encyclopedia of Materials: Science and Technology; Elsevier: Oxford, UK, 2001; pp. 1849–1851. [Google Scholar]


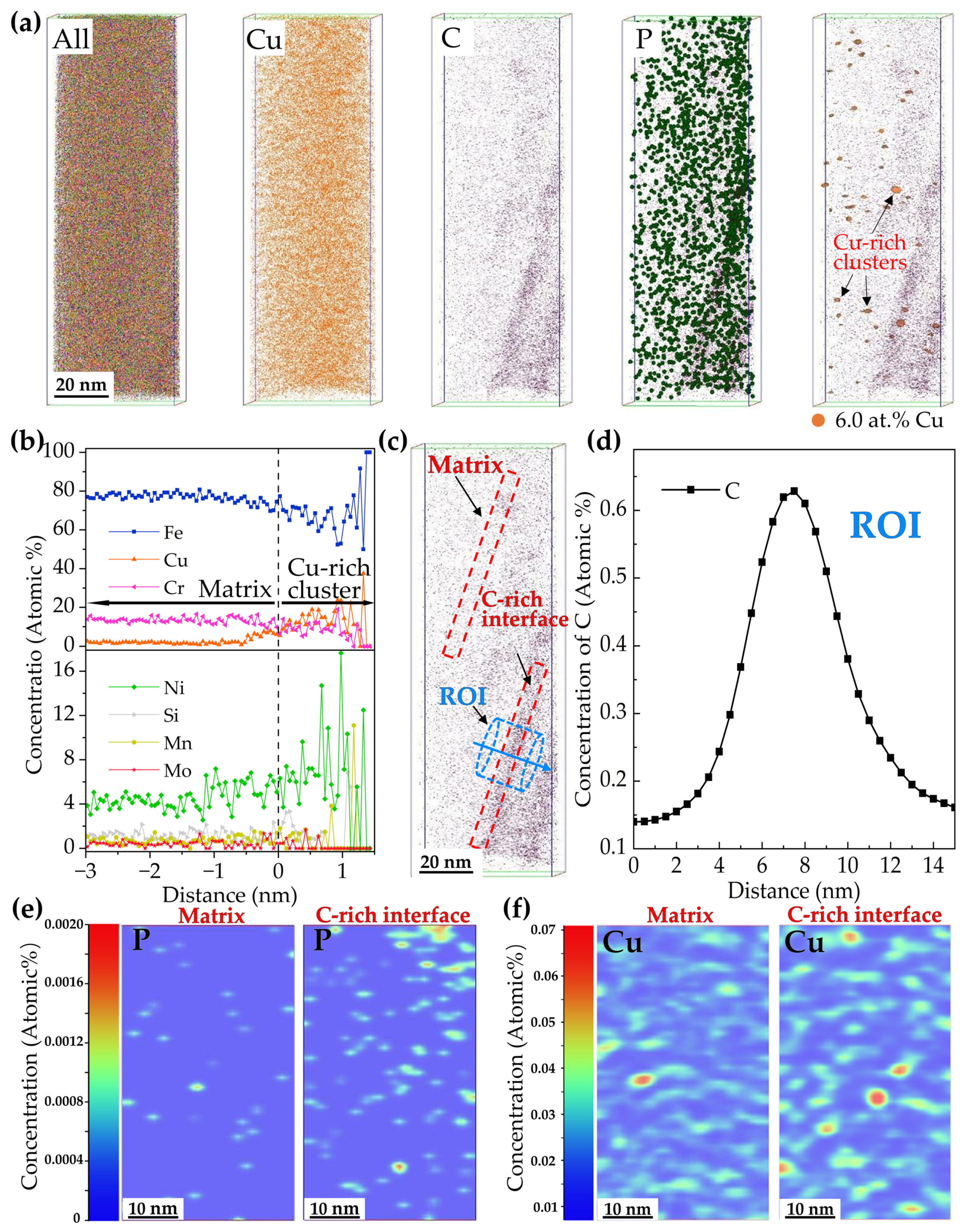
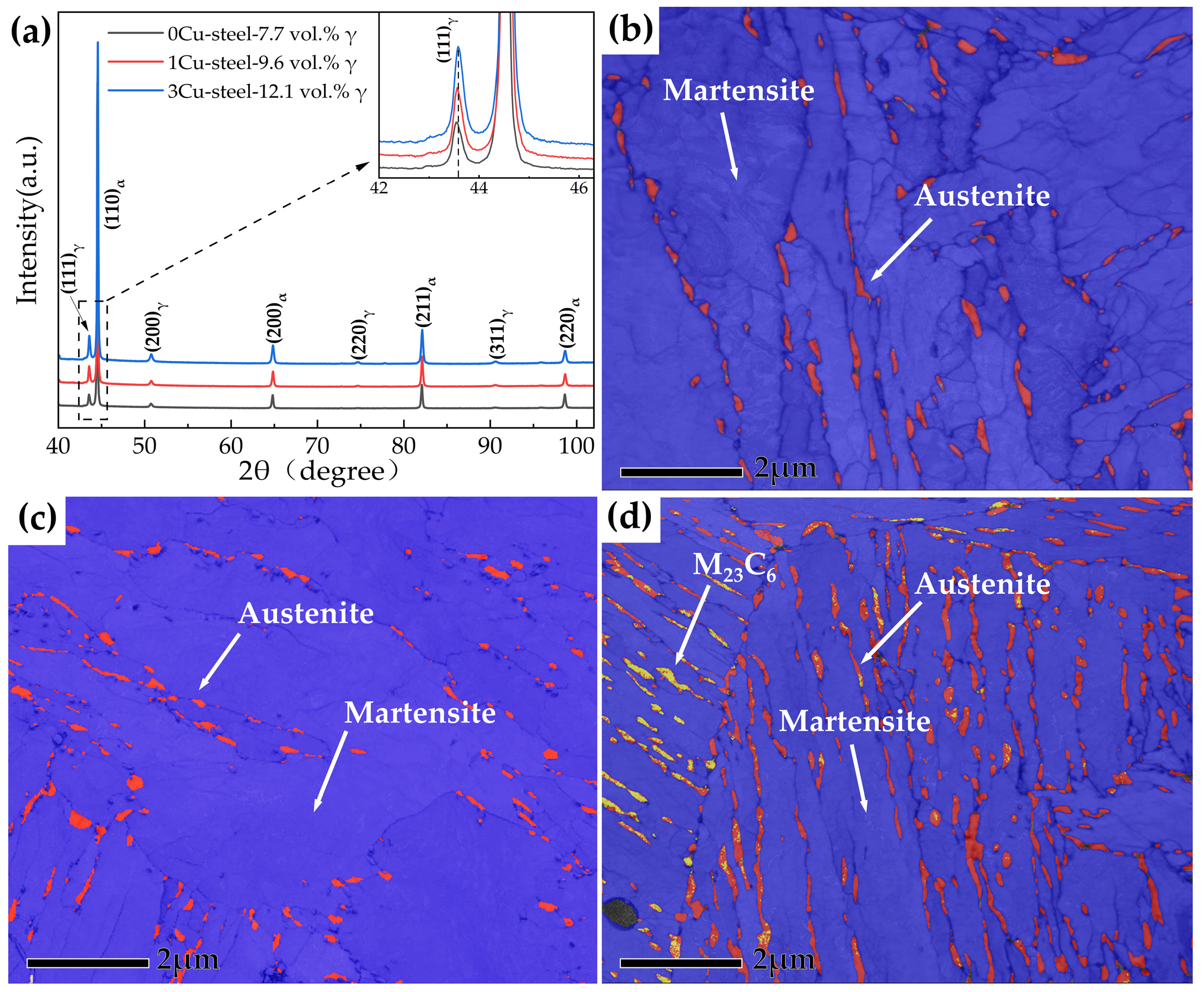
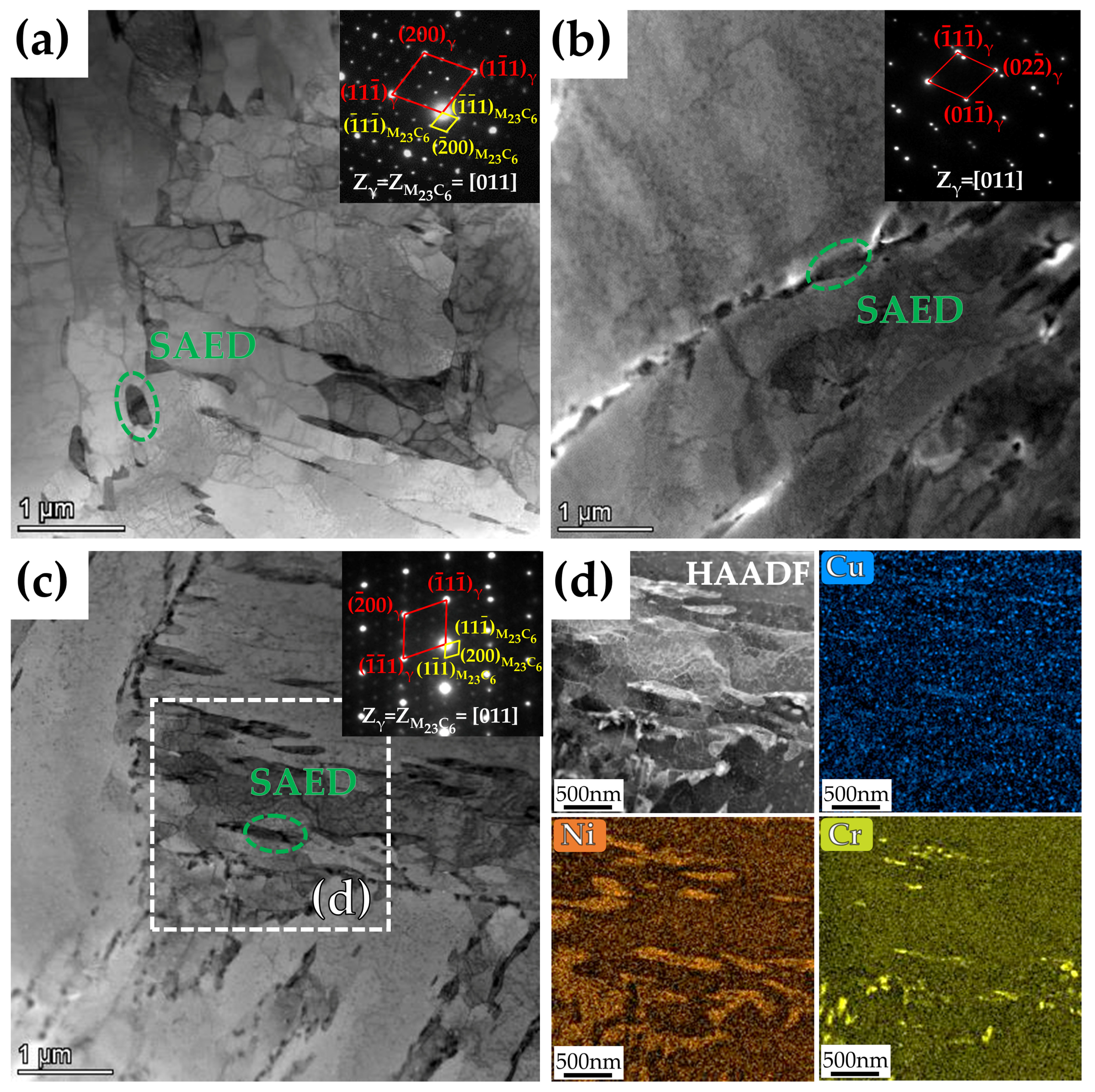
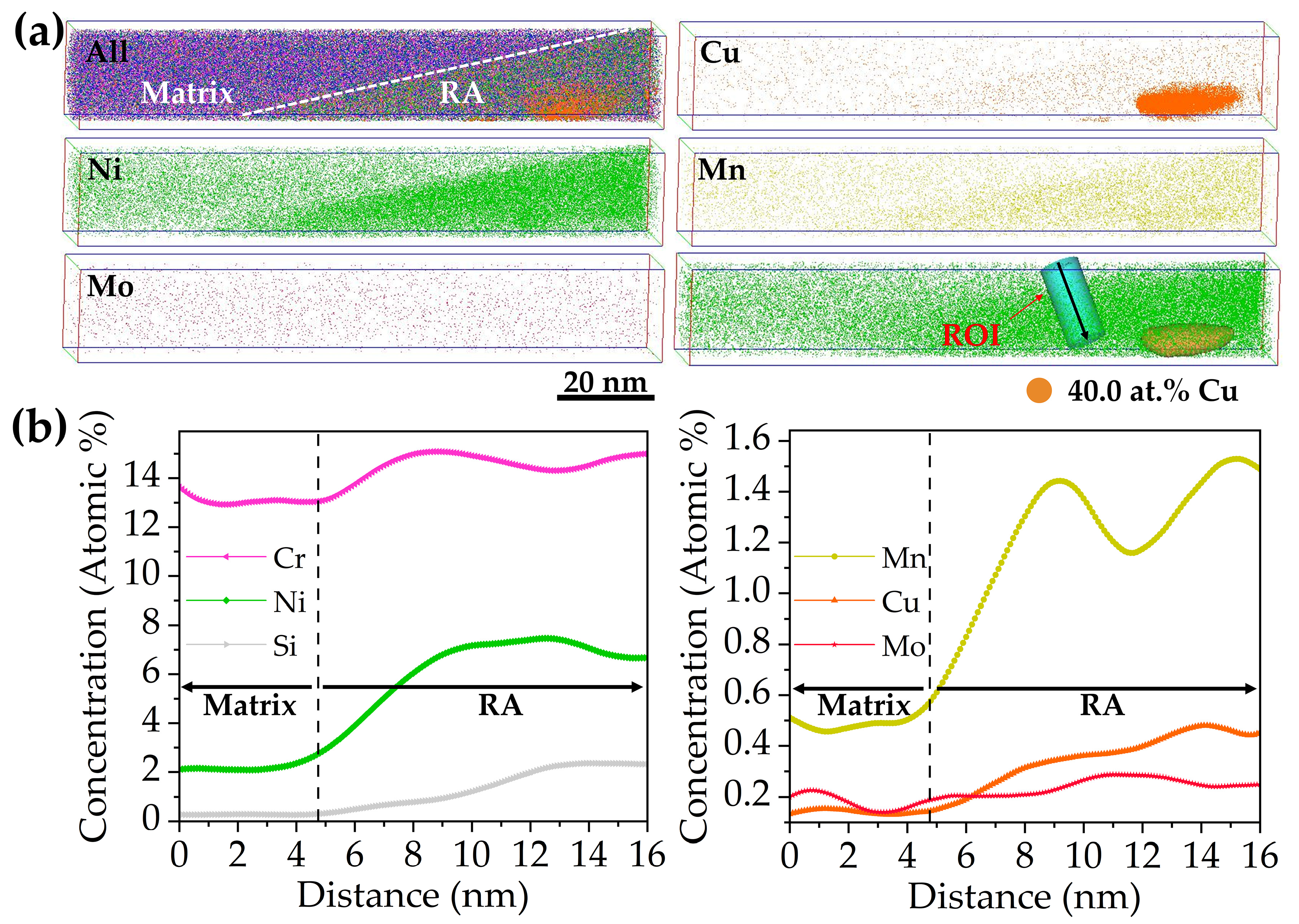
| Steel | Cu | C | Si | Cr | Ni | Mn | Mo | P | S | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 0Cu-steel | 0.01 | 0.051 | 0.43 | 12.87 | 4.06 | 0.68 | 0.47 | 0.005 | 0.0041 | Bal. |
| 1Cu-steel | 1.17 | 0.056 | 0.51 | 13.06 | 4.15 | 0.67 | 0.49 | 0.005 | 0.0047 | Bal. |
| 3Cu-steel | 2.82 | 0.056 | 0.51 | 13.03 | 4.12 | 0.66 | 0.48 | 0.005 | 0.0050 | Bal. |
| Steel | As (°C) | Af (°C) | Ms (°C) | Mf (°C) |
|---|---|---|---|---|
| 0Cu-steel | 687 | 790 | 255 | 100 |
| 1Cu-steel | 674 | 770 | 241 | 91 |
| 3Cu-steel | 674 | 763 | 190 | 85 |
| Microstructure | Fe | Cr | Ni | Cu | Mn | Mo | Si |
|---|---|---|---|---|---|---|---|
| Matrix | 82.2 | 13.0 | 2.0 | 0.1 | 0.5 | 0.2 | 0.9 |
| Reversed austenite | 72.8 | 14.4 | 7.5 | 1.0 | 1.4 | 0.2 | 2.0 |
| Cu-rich cluster | 69.3 | 7.8 | 6.2 | 13.2 | 0.9 | 0.2 | 1.3 |
| Cu-rich precipitate | 13.6 | 2.7 | 3.1 | 78.3 | 1.9 | 3.1 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Song, Y.; Jiang, H.; Rong, L. Effect of Cu on the Microstructure and Mechanical Properties of a Low-Carbon Martensitic Stainless Steel. Materials 2022, 15, 8849. https://doi.org/10.3390/ma15248849
Ma J, Song Y, Jiang H, Rong L. Effect of Cu on the Microstructure and Mechanical Properties of a Low-Carbon Martensitic Stainless Steel. Materials. 2022; 15(24):8849. https://doi.org/10.3390/ma15248849
Chicago/Turabian StyleMa, Jun, Yuanyuan Song, Haichang Jiang, and Lijian Rong. 2022. "Effect of Cu on the Microstructure and Mechanical Properties of a Low-Carbon Martensitic Stainless Steel" Materials 15, no. 24: 8849. https://doi.org/10.3390/ma15248849
APA StyleMa, J., Song, Y., Jiang, H., & Rong, L. (2022). Effect of Cu on the Microstructure and Mechanical Properties of a Low-Carbon Martensitic Stainless Steel. Materials, 15(24), 8849. https://doi.org/10.3390/ma15248849






