Effect of Poly(vinyl alcohol) Concentration on the Micro/Mesopore Structure of SBA15 Silica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of Mesoporous SBA15 with PVA Addition
2.3. Characterization
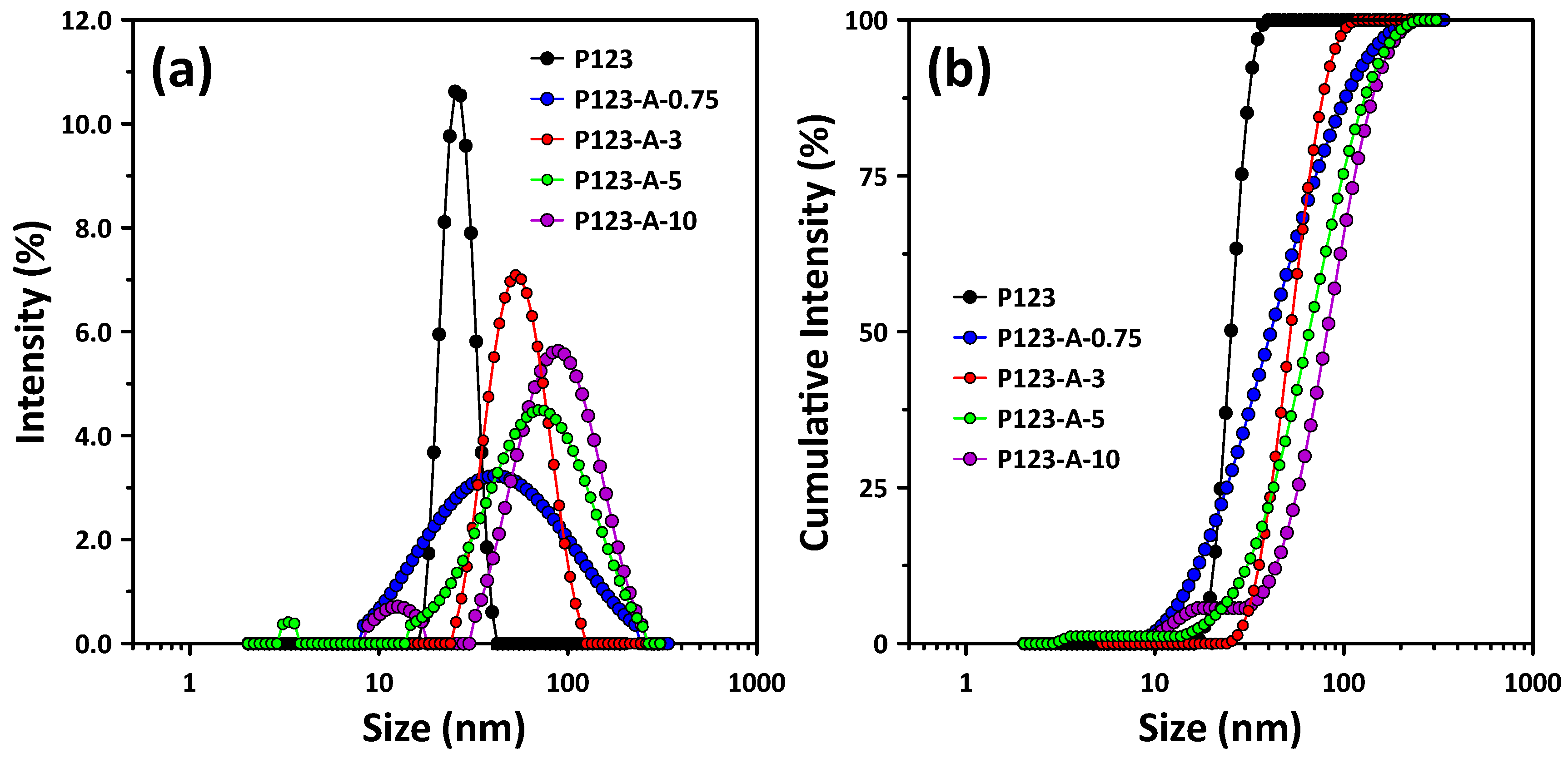
2.4. Variation in P123 Micelle Properties with PVA Concentration
3. Results and Discussion
3.1. Mesopore Structure of Mesoporous SBA15 with PVA Addition
3.2. Micropore Structure of Mesoporous SBA15 with Addition of PVA
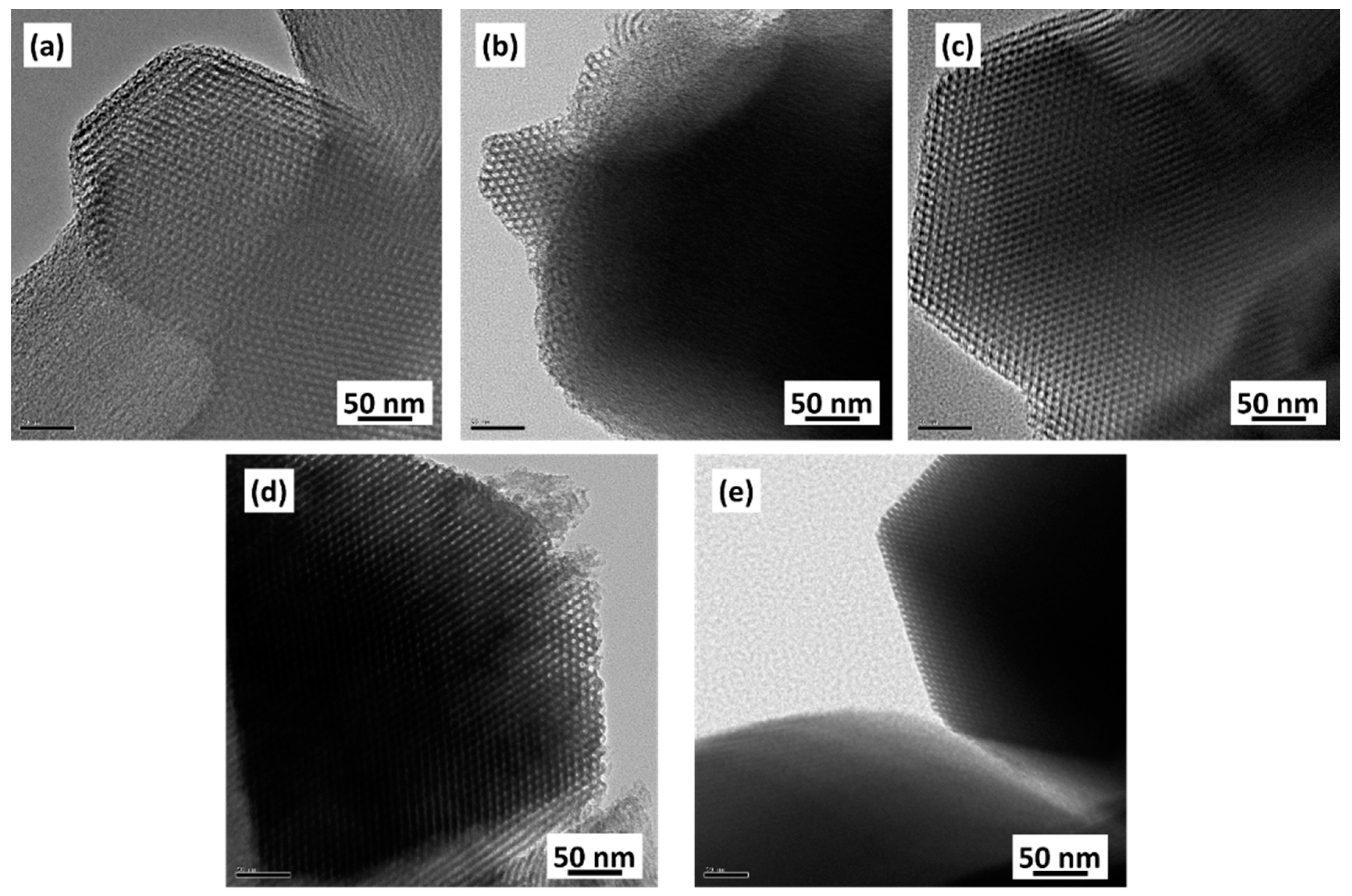
3.3. Microstructure and Morphology of SBA15 Synthesized with PVA
3.4. Self-Assembly Properties of P123/PVA Mixed Micelles According to PVA Concentration
4. Conclusions
- (1)
- The pore structure of SBA15 changed according to the added PVA concentration. In particular, increases in micropore volume and wall thickness were observed with the addition of PVA. SBA15-A-3 was confirmed to have the highest BET surface area and micropore volume, as well as the thickest pore walls. Notably, as the PVA concentration increased, the mesopore volume and size increased, resulting in a significant increase in the total pore volume.
- (2)
- All of the SBA15 samples synthesized with PVA had a cylindrical and hexagonal mesopore structure, which is the typical pore shape of SBA15. The mesopore structure and morphology of the synthesized materials did not change with the PVA addition.
- (3)
- At a certain PVA concentration (below P123-A-3), the PVA was able to interact with the shell PEO chains of P123 micelles, resulting in an improved micropore volume and specific surface area of the synthesized SBA15. However, the core PPO block also became larger owing to the aggregation of P123/PVA mixed micelles at high PVA concentrations.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Caberera, I. Modifying the hierarchical porosity of SBA-15 via mild-detemplation followed by secondary treatments. J. Phys. Chem. 2014, 118, 28689–28698. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Setiabudi, H.D.; Nanda, S.; Vo, D.N. Advanced synthesis strategies of mesoporous SBA-15 supported catalysts for catalytic reforming applications: A state-of-the-art review. Appl. Catal. A 2018, 559, 57–74. [Google Scholar] [CrossRef]
- Kim, S.; Han, Y.; Park, J.; Park, J. Adsorption characteristics of mesoporous silica SBA-15 synthesized from mine tailing. Int. J. Miner. Process. 2015, 140, 88–94. [Google Scholar] [CrossRef]
- Wang, J.; Ge, H.; Bao, W. Synthesis and characteristics of SBA-15 with thick pore wall and high hydrothermal stability. Mater. Lett. 2015, 145, 312–315. [Google Scholar] [CrossRef]
- Ahmadian, M.; Anbia, M. Synthesis of spherical SBA-15 by poly (vinyl alcohol)/hydroxyl-terminated polybutadiene supermicelles as co-surfactant and its application for removal of diazinon from aqueous solution. Environ. Technol. Innov. 2021, 24, 101899. [Google Scholar] [CrossRef]
- Kurdyukov, D.A.; Eurov, D.A.; Kirilenko, D.A.; Kukushkina, J.A.; Sokolov, V.V.; Yagovkina, M.A.; Golubev, V.G. High-surface area spherical micro-mesoporous silica particles. Micropor. Mesopor. Mater. 2016, 223, 225–229. [Google Scholar] [CrossRef]
- Thahir, R.; Wahab, A.W.; Nafie, N.L.; Raya, I. Synthesis of high surface area mesoporous silica SBA-15 by adjusting hydrothermal treatment time and the amount of polyvinyl alcohol. Open Chem. 2019, 17, 963–971. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Chang, W.; Sun, R.; Li, D.; Ru, H. Simple and controllable preparation of SBA-15 microspheres by poly(vinyl alcohol)-assisted P123 templating system. Micropor. Mesopor. Mater. 2020, 302, 110211. [Google Scholar] [CrossRef]
- Erdem, B.; Erdem, S.; Öksüzoğlu, R.M.; Çıtak, A. High-surface-area SBA-15–SO3H with enhanced catalytic activity by the addition of poly(ethylene glycol). J. Porous Mater. 2013, 20, 1041–1049. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, T.; Xu, X.; Xiao, P.; Li, J. Pt nanoparticles supported on SBA-15: Synthesis, characterization and applications in heterogeneous catalysis. Appl. Catal. B 2013, 130, 197–217. [Google Scholar] [CrossRef]
- Ming, W.; Ma, J.; Wang, Y.; Li, R. Preparation and characterisation of MCM-41 materials with intra-wall cross-mesopores. Micro Nano Lett. 2015, 10, 363–366. [Google Scholar] [CrossRef]
- Han, Y.; Hwang, G.; Kim, H.; Haznedaroglu, B.Z.; Lee, B. Amine-impregnated millimeter-sized spherical silica foams with hierarchical mesoporous–macroporous structure for CO2 capture. Chem. Eng. J. 2015, 259, 653–662. [Google Scholar] [CrossRef]
- Wang, J.; Tian, G.; Li, Z.; Ji, X.; Bao, W. A novel and facile strategy for synthesis plugged SBA-15. Mater. Lett. 2016, 162, 110–113. [Google Scholar] [CrossRef]
- Ma, M.; Huang, H.; Chen, C.; Zhu, Q.; Yue, L.; Albilali, R.; He, C. Highly active SBA-15-confined Pd catalyst with short rod-like micro-mesoporous hybrid nanostructure for n-butylamine low-temperature destruction. Mol. Catal. 2018, 455, 192–203. [Google Scholar] [CrossRef]
- Zhong, X.; Barbier, J.; Duprez, D.; Zhang, H.; Royer, S. Modulating the copper oxide morphology and accessibility by using micro-/mesoporous SBA-15 structures as host support: Effect on the activity for the CWPO of phenol reaction. Appl. Catal. B 2012, 121, 123–134. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Han, S.; Han, Y.; Park, J. Silanol-rich ordered mesoporous silica modified thiol group for enhanced recovery performance of Au(III) in acidic leachate solution. Chem. Eng. J. 2018, 351, 1027–1037. [Google Scholar] [CrossRef]
- Wu, Q.L.; Rankin, S.E. Tuning the mesopore size of titania thin films using a polymeric swelling agent. J. Phys. Chem. C 2011, 115, 11925–11933. [Google Scholar] [CrossRef]
- Han, Y.; Choi, J.; Tong, M.; Kim, H. Synthesis and characterization of high-surface-area millimeter-sized silica beads with hierarchical multi-modal pore structure by the addition of agar. Mater. Charact. 2014, 90, 31–39. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Han, Y.; Choi, J.; Park, J. Adsorption of Co(II) and Mn(II) ions on mesoporous silica SBA15 functionalized with amine groups. Mater. Trans. 2014, 55, 1494–1499. [Google Scholar] [CrossRef] [Green Version]
- Colilla, M.; Balas, F.; Manzano, M.; Vallet-Regí, M. Novel method to enlarge the surface area of SBA-15. Chem. Mater. 2007, 19, 3099–3101. [Google Scholar] [CrossRef]
- Pan, Y.; Abazari, R.; Yao, J.; Gao, J. Recent progress in 2D metal-organic framework photocatalysts: Synthesis, photocatalytic mechanism and applications. J. Phys. Energy 2021, 3, 032010. [Google Scholar] [CrossRef]
- Chen, J.; Abazari, R.; Adegoke, K.A.; Maxakato, N.W.; Bello, O.S.; Tahir, M.; Tasleem, S.; Sanati, S.; Kirillov, A.M.; Zhou, Y. Metal–organic frameworks and derived materials as photocatalysts for water splitting and carbon dioxide reduction. Coord. Chem. Rev. 2022, 469, 214664. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous catalysis in zeolites, mesoporous silica, and metal-organic frameworks. Adv. Mat. 2017, 29, 1701139. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, H.; Park, J.; Lee, S.H.; Kim, J.Y. Influence of Ti doping level on hydrogen adsorption of mesoporous Ti-SBA-15 materials prepared by direct synthesis. Int. J. Hydrogen Energy 2012, 37, 14240–14247. [Google Scholar] [CrossRef]
- Zhu, J.; Kailasam, K.; Xie, X.; Schomaecker, R.; Thomas, A. High-surface-area SBA-15 with enhanced mesopore connectivity by the addition of poly(vinyl alcohol). Chem. Mater. 2011, 23, 2062–2067. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Lv, L.; Gao, X.; Meng, H.; Liu, H. Obtaining of mesoporous aluminosilicates with high hydrothermal stability by composite organic templates: Utility and mechanism. Langmuir 2021, 37, 9137–9143. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, R.; Yu, L.; Wang, W. Facile preparation of hierarchical mesoporous silica microspheres with tunable porous structure and particle sizes. Ceram. Int. 2022, 49, 3030–3040. [Google Scholar] [CrossRef]
- Ma, X.; Yu, Y.; Xing, J.; Yang, T.; Lam, K.F.; Xue, Q.; Albela, B.; Bonneviot, L.; Zhang, K. Tailoring porosity and dimensionality of Co3O4 nanophase using channel interconnectivity control by steaming of nanocasting SBA-15. Micropor. Mesopor. Mater. 2014, 200, 182–189. [Google Scholar] [CrossRef]
- Han, Y.; Han, S.; Kim, S.; Jung, M.; Jeon, H.S.; Choi, S.Q.; Kim, K.H.; Kim, Y. Mesoporous silica derived from municipal solid waste incinerator (MSWI) ash slag: Synthesis, characterization and use as supports for Au(III) recovery. Materials 2021, 14, 6894. [Google Scholar] [CrossRef]
- Han, Y.; Kim, S.; Yu, S.; Myung, N.V.; Kim, H. Electrospun hydrogen manganese oxide nanofibers as effective adsorbents for Li+ recovery from seawater. J. Ind. Eng. Chem. 2020, 81, 115–123. [Google Scholar] [CrossRef]
- Han, Y.; Park, S.; Kim, S.; Han, S.; Kim, Y.; Jeon, H. Enhanced photocatalytic activity of ultrahigh-surface-area TiO2@C nanorod aggregates with hierarchical porosities synthesized from natural ilmenite. J. Environ. Chem. Eng. 2021, 9, 104438. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.S.; Kim, S.; Jeon, H.S.; Choi, J.; Han, Y. Correlation between adsorbed methane concentration and pore stucture of organic-rich black shale from the Liard Basin, Canada. J. Nat. Gas Eng. 2021, 95, 104226. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A.V. Characterization of micro- and mesoporosity in SBA-15 materials from absorption data by the NLDFT method. J. Phys. Chem. B 2001, 105, 6817–6823. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Für Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Fraser, S.J.; Mulet, X.; Martin, L.; Praporski, S.; Mechler, A.; Hartley, P.G.; Polyzos, A.; Separovic, F. Surface immobilization of bio-functionalized cubosomes: Sensing of proteins by quartz crystal microbalance. Langmuir 2012, 28, 620–627. [Google Scholar] [CrossRef]
- Johnsson, M.; Bergstrand, N.; Edwards, K.; Stålgren, J.J.R. Adsorption of a PEO-PPO-PEO triblock copolymer on small unilamellar vesicles: Equilibrium and kinetic properties and correlation with membrane permeability. Langmuir 2001, 17, 3902–3911. [Google Scholar] [CrossRef]
- Gao, F.; Hu, J.; Peng, C.; Liu, H.; Hu, Y. Synergic effects of imidazolium ionic liquids on P123 mixed micelles for inducing micro/mesoporous materials. Langmuir 2012, 28, 2950–2959. [Google Scholar] [CrossRef]
- Vradman, L.; Titelman, L.; Herskowitz, M. Size effect on SBA-15 microporosity. Micropor. Mesopor. Mater. 2006, 93, 313–317. [Google Scholar] [CrossRef]
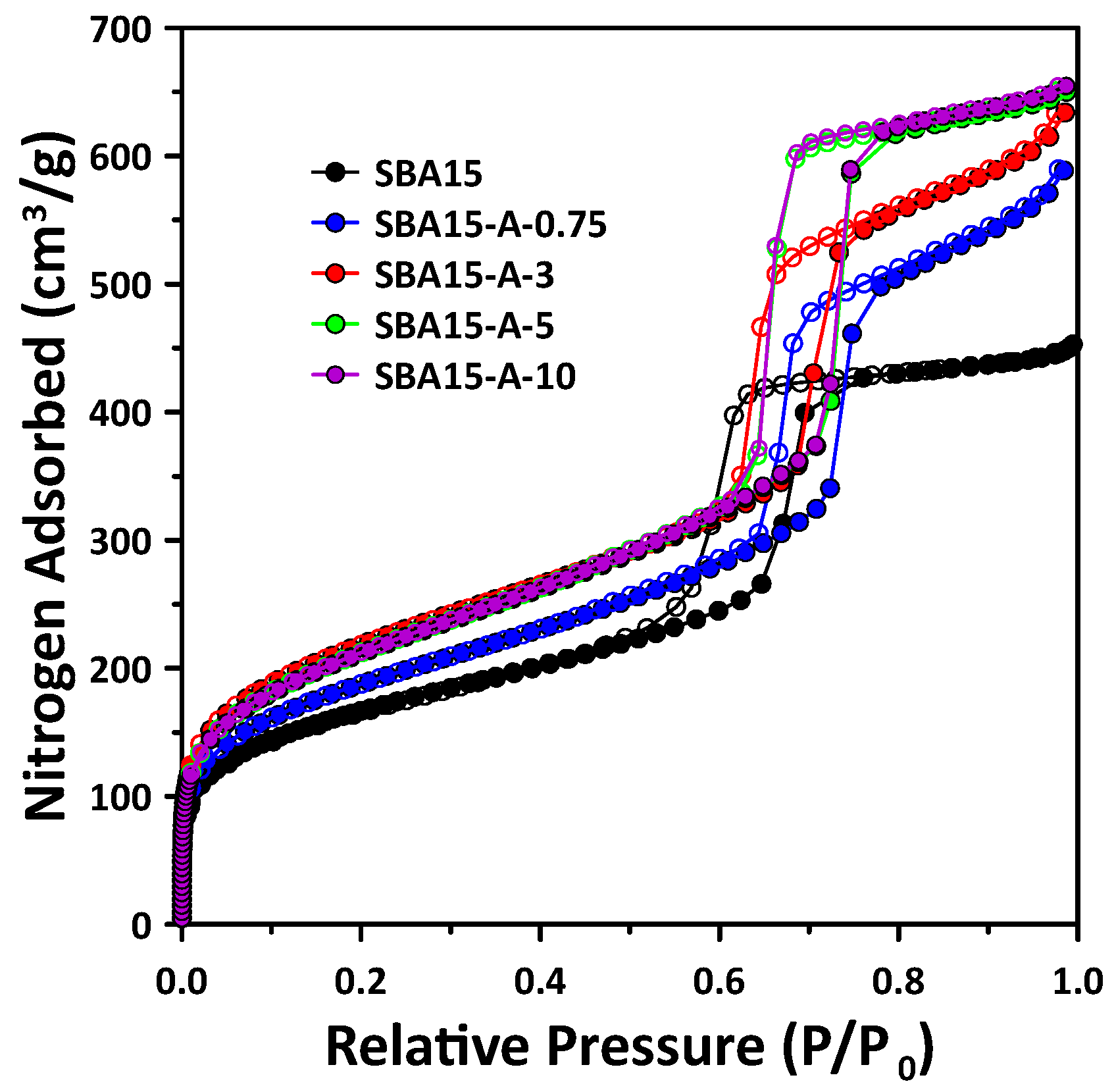
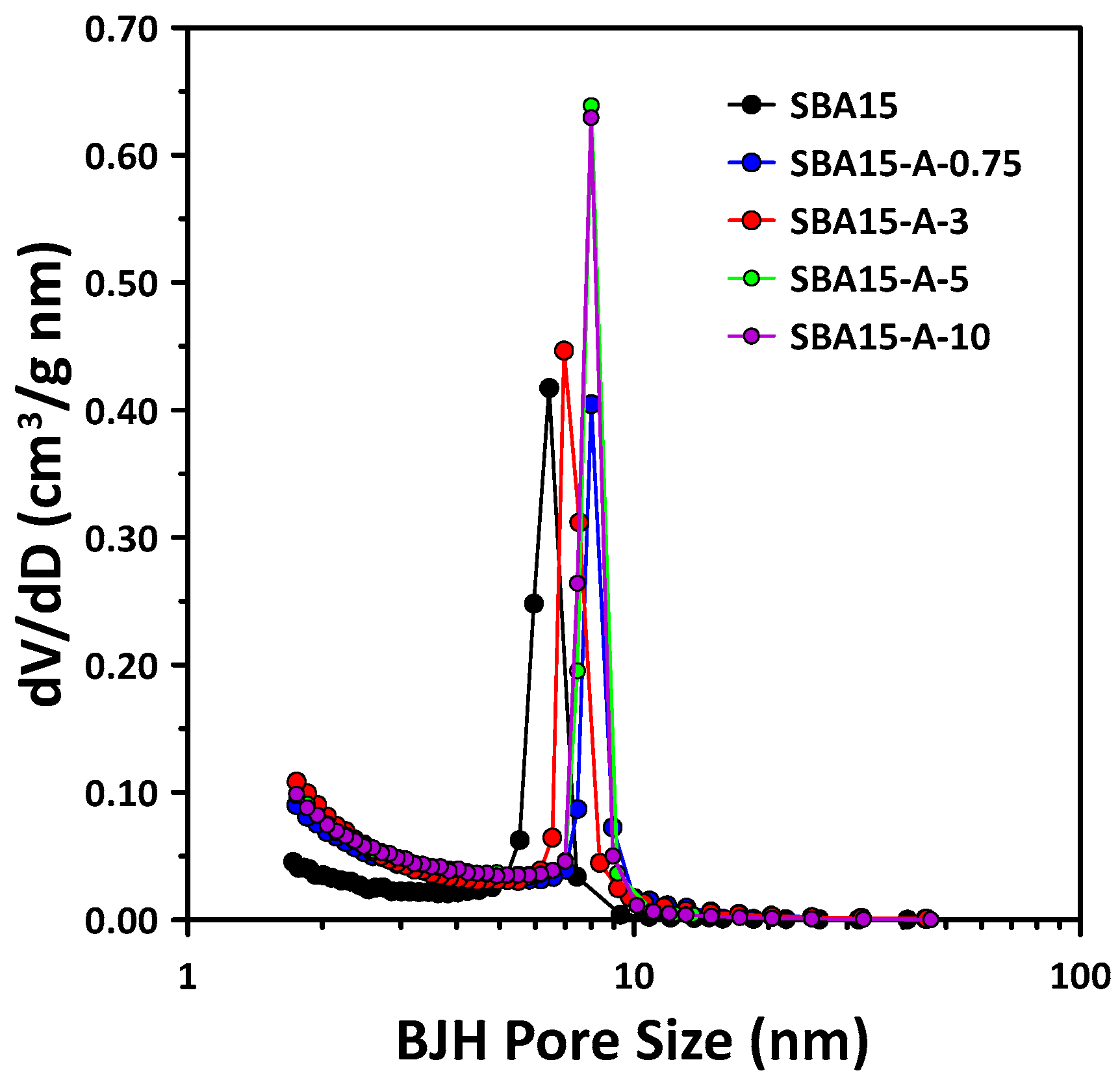
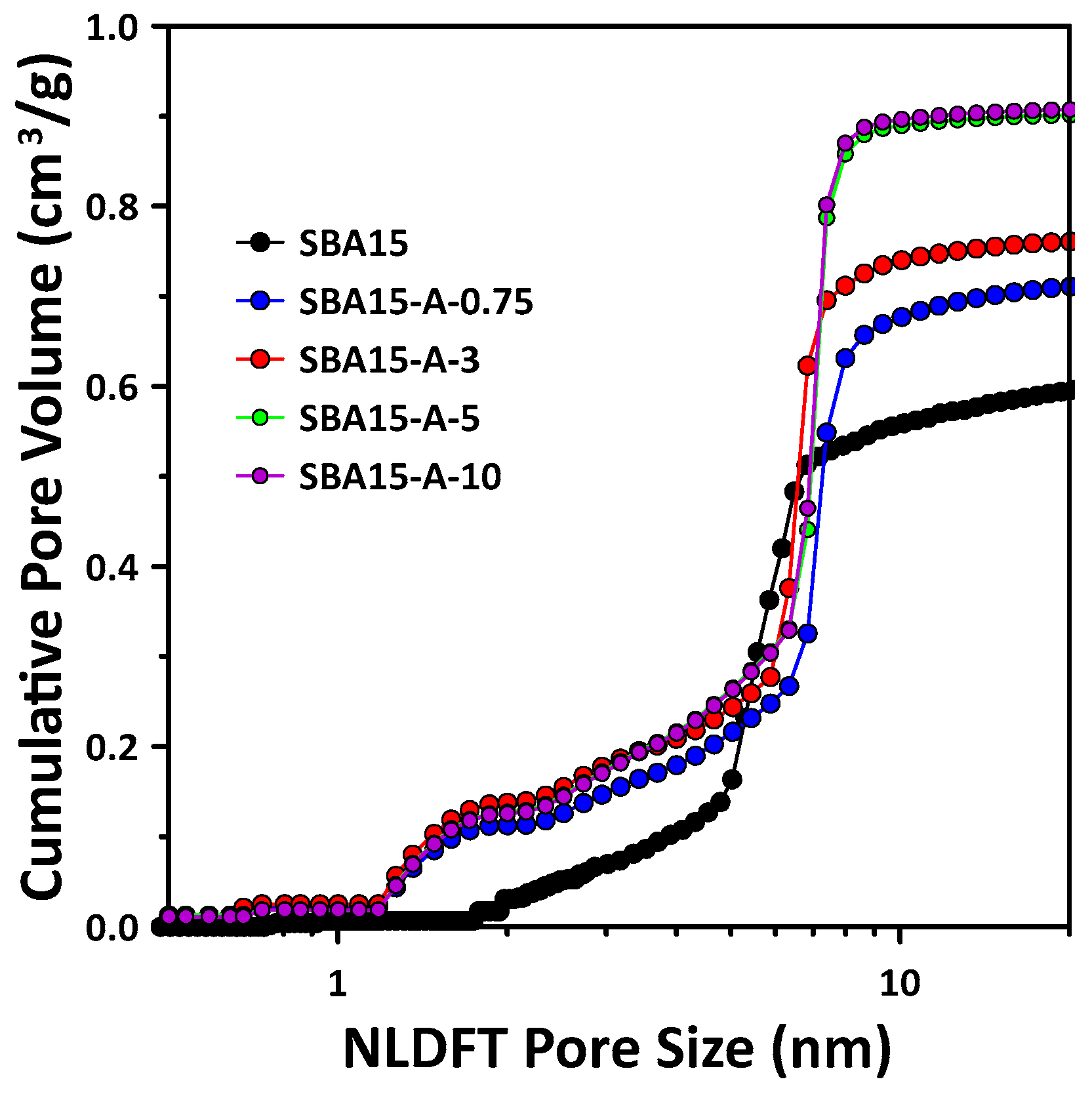

| Sample | d100 (nm) 1 | Surface Area (m2/g) 2 | Micropore Volume (cm3/g) 3 | Mesopore Volume (cm3/g) 4 | Mesopore Size (nm) 4 | Total Pore Volume (cm3/g) | Wall Thickness (nm) 5 |
|---|---|---|---|---|---|---|---|
| SBA15 | 9.25 | 599.8 | 0.031 | 0.541 | 6.65 | 0.596 | 4.03 |
| SBA15-A-0.75 | 10.62 | 683.5 | 0.113 | 0.598 | 7.86 | 0.711 | 4.16 |
| SBA15-A-3 | 10.56 | 807.3 | 0.137 | 0.624 | 7.48 | 0.761 | 4.82 |
| SBA15-A-5 | 10.47 | 775.1 | 0.127 | 0.774 | 7.89 | 0.901 | 4.20 |
| SBA15-A-10 | 10.43 | 772.7 | 0.125 | 0.782 | 7.91 | 0.907 | 4.13 |
| Micelle Conditions | (Hz) | Layer Thickness (nm) | Areal Mass | |
|---|---|---|---|---|
| P123 | –64.68 ± 0.08 | 24.63 ± 0.04 | 38.4 ± 0.11 | 3.88 ± 0.01 |
| P123-A-0.75 | –82.45 ± 0.09 | 25.40 ± 0.05 | 38.7 ± 0.47 | 4.84 ± 0.06 |
| P123-A-3 | –92.16 ± 0.12 | 30.43 ± 0.05 | 39.6 ± 1.01 | 5.13 ± 0.11 |
| P123-A-5 | –98.44 ± 0.11 | 28.84 ± 0.03 | 101.2 ± 1.95 | 13.30 ± 0.24 |
| P123-A-10 | –102.57 ± 0.11 | 33.03 ± 0.06 | 98.6 ± 2.81 | 12.33 ± 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Jung, M.; Han, S.; Jeon, H.-S.; Han, Y. Effect of Poly(vinyl alcohol) Concentration on the Micro/Mesopore Structure of SBA15 Silica. Materials 2022, 15, 8900. https://doi.org/10.3390/ma15248900
Kim S, Jung M, Han S, Jeon H-S, Han Y. Effect of Poly(vinyl alcohol) Concentration on the Micro/Mesopore Structure of SBA15 Silica. Materials. 2022; 15(24):8900. https://doi.org/10.3390/ma15248900
Chicago/Turabian StyleKim, Seongmin, Minuk Jung, Seongsoo Han, Ho-Seok Jeon, and Yosep Han. 2022. "Effect of Poly(vinyl alcohol) Concentration on the Micro/Mesopore Structure of SBA15 Silica" Materials 15, no. 24: 8900. https://doi.org/10.3390/ma15248900





