Electrical and Gas Sensor Properties of Nb(V) Doped Nanocrystalline β-Ga2O3
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
2.3. Surface Reactivity Assessment
2.4. Gas Sensor Measurements
3. Results and Discussion
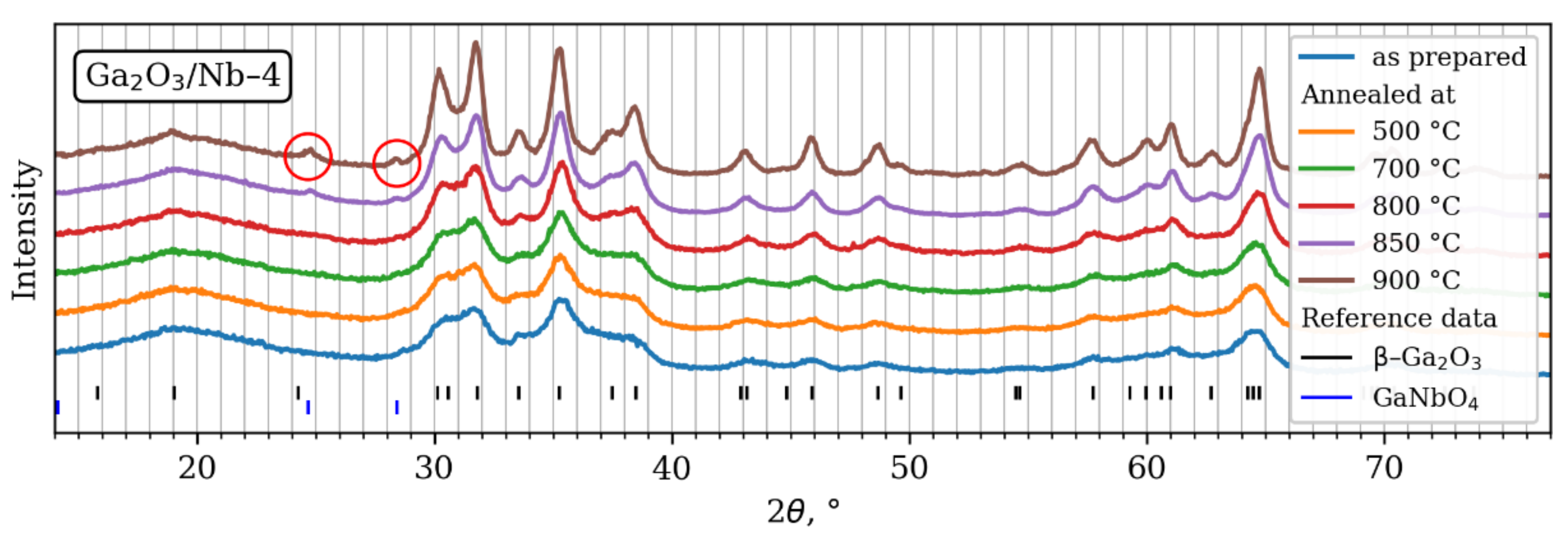
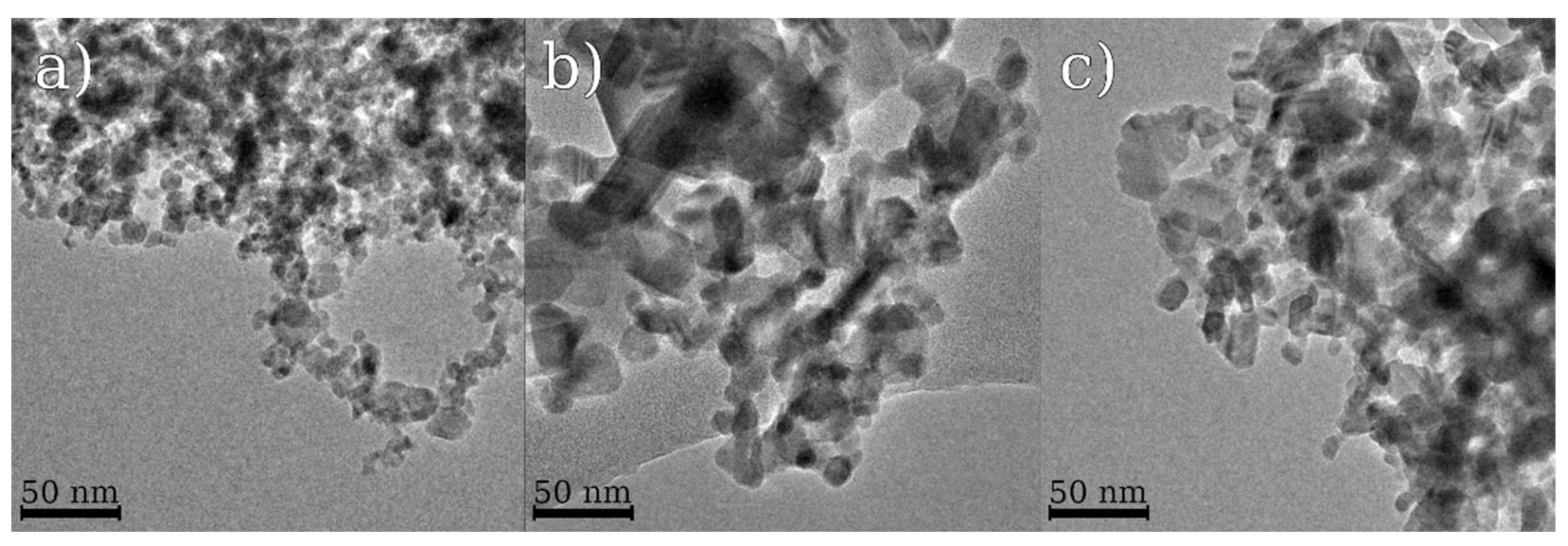
3.1. Materials Structure and Chemical Composition
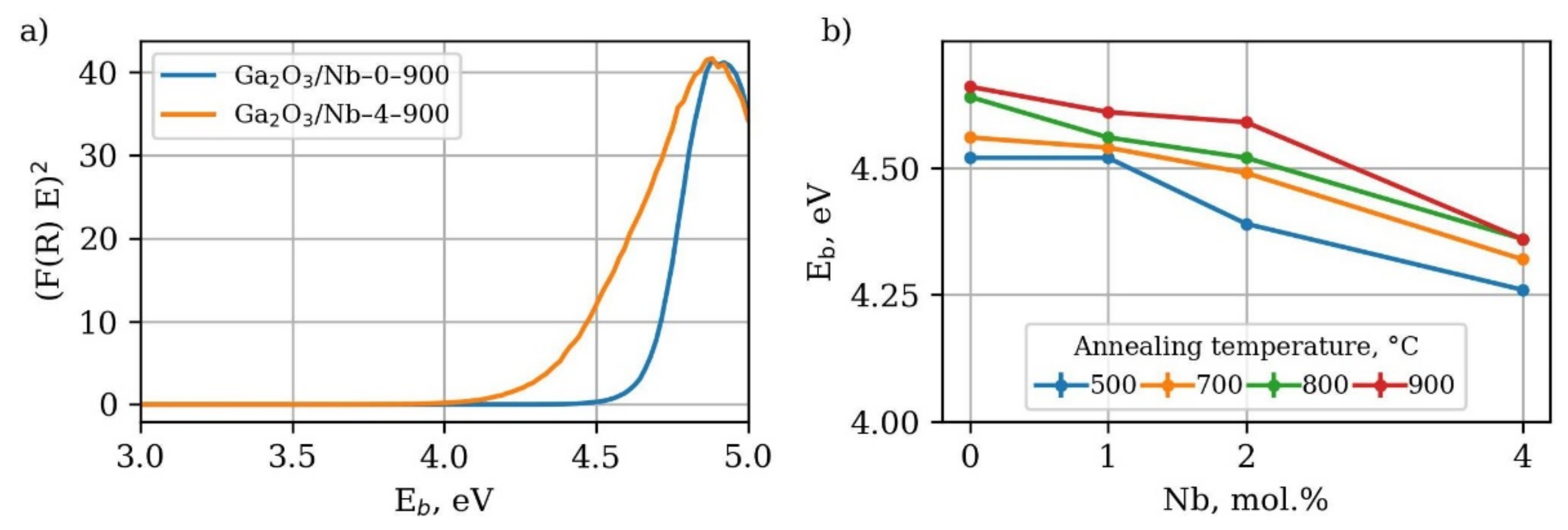
3.2. Optical Band Gap
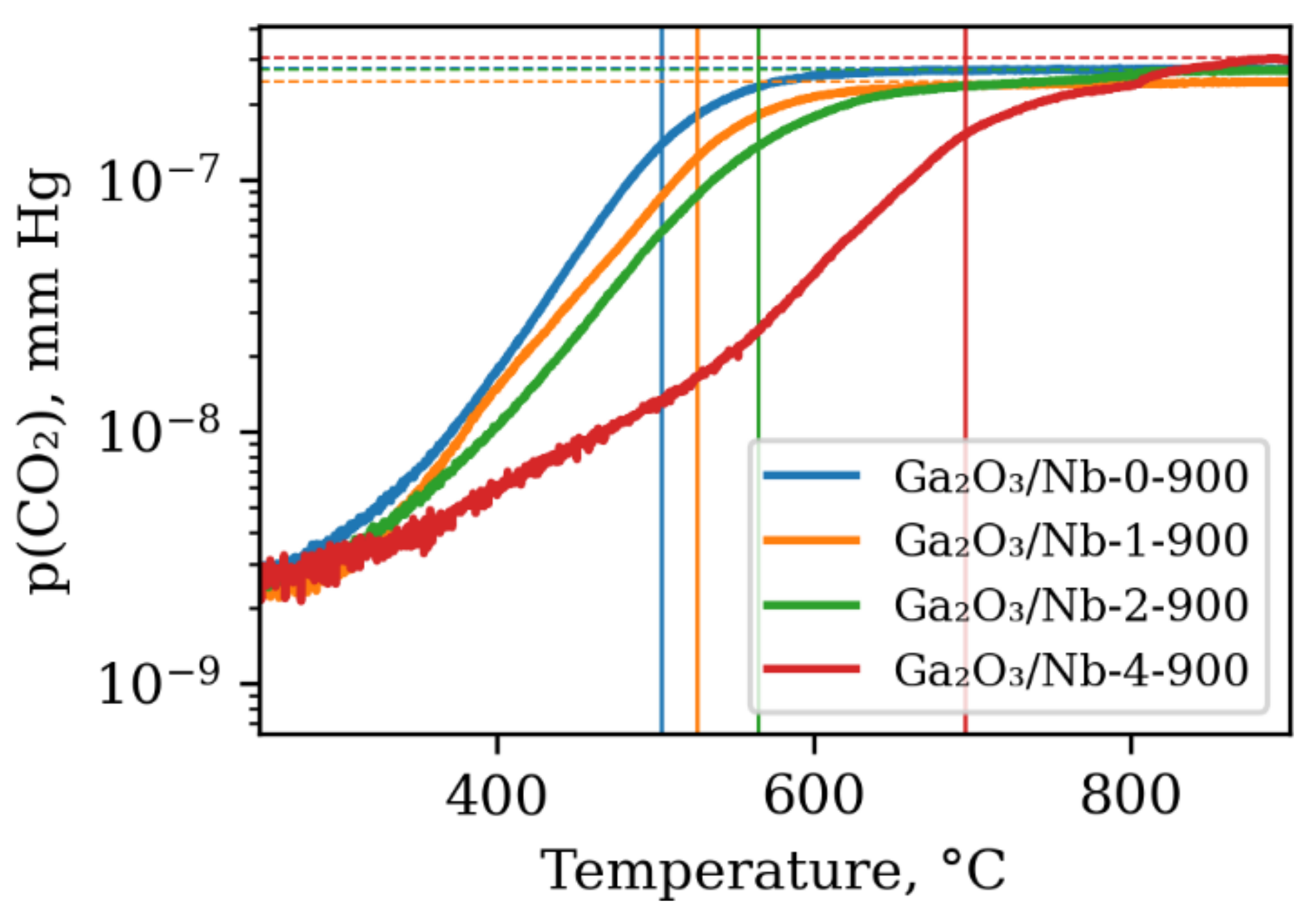
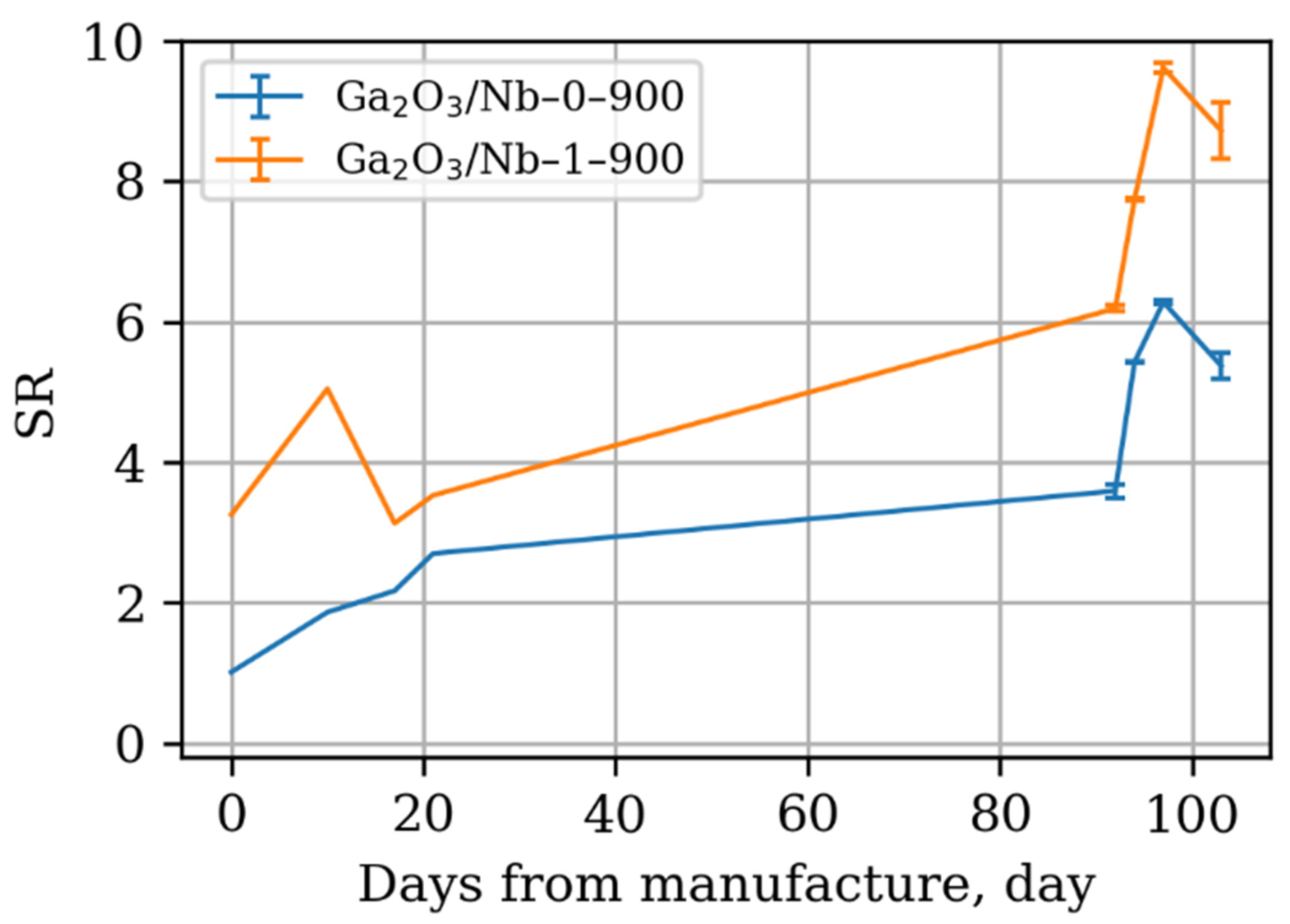
3.3. Surface Reactivity
3.4. Gas Sensor Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tonezzer, M.; Le, D.T.T.; Iannotta, S.; Van Hieu, N. Selective discrimination of hazardous gases using one single metal oxide resistive sensor. Sens. Actuators B Chem. 2018, 277, 121–128. [Google Scholar] [CrossRef]
- Güntner, A.T.; Weber, I.C.; Schon, S.; Pratsinis, S.E.; Gerber, P.A. Monitoring rapid metabolic changes in health and type-1 diabetes with breath acetone sensors. Sens. Actuators B Chem. 2022, 367, 132182. [Google Scholar] [CrossRef]
- Connolly, R.E.; Yu, Q.; Wang, Z.; Chen, Y.-H.; Liu, J.Z.; Collier-Oxandale, A.; Papapostolou, V.; Polidori, A.; Zhu, Y. Long-term evaluation of a low-cost air sensor network for monitoring indoor and outdoor air quality at the community scale. Sci. Total Environ. 2022, 807, 150797. [Google Scholar] [CrossRef] [PubMed]
- Schultealbert, C.; Amann, J.; Baur, T.; Schütze, A. Measuring Hydrogen in Indoor Air with a Selective Metal Oxide Semiconductor Sensor. Atmosphere 2021, 12, 366. [Google Scholar] [CrossRef]
- Pineau, N.J.; Magro, L.; van den Broek, J.; Anderhub, P.; Güntner, A.T.; Pratsinis, S.E. Spirit Distillation: Monitoring Methanol Formation with a Hand-Held Device. ACS Food Sci. Technol. 2021, 1, 839–844. [Google Scholar] [CrossRef]
- Fonollosa, J.; Solórzano, A.; Marco, S. Chemical Sensor Systems and Associated Algorithms for Fire Detection: A Review. Sensors 2018, 18, 553. [Google Scholar] [CrossRef]
- Thai, N.X.; Tonezzer, M.; Masera, L.; Nguyen, H.; Duy, N.V.; Hoa, N.D. Multi gas sensors using one nanomaterial, temperature gradient, and machine learning algorithms for discrimination of gases and their concentration. Anal. Chim. Acta 2020, 1124, 85–93. [Google Scholar] [CrossRef]
- Krivetskiy, V.; Efitorov, A.; Arkhipenko, A.; Vladimirova, S.; Rumyantseva, M.; Dolenko, S.; Gaskov, A. Selective detection of individual gases and CO/H-2 mixture at low concentrations in air by single semiconductor metal oxide sensors working in dynamic temperature mode. Sens. Actuat. B Chem. 2018, 254, 502–513. [Google Scholar] [CrossRef]
- Liu, H.; Meng, G.; Deng, Z.; Nagashima, K.; Wang, S.; Dai, T.; Li, L.; Yanagida, T.; Fang, X. Discriminating BTX Molecules by the Nonselective Metal Oxide Sensor-Based Smart Sensing System. ACS Sens. 2021, 6, 4167–4175. [Google Scholar] [CrossRef]
- Krivetskiy, V.V.; Andreev, M.D.; Efitorov, A.O.; Gaskov, A.M. Statistical shape analysis pre-processing of temperature modulated metal oxide gas sensor response for machine learning improved selectivity of gases detection in real atmospheric conditions. Sens. Actuators B Chem. 2021, 329, 129187. [Google Scholar] [CrossRef]
- Robin, Y.; Amann, J.; Baur, T.; Goodarzi, P.; Schultealbert, C.; Schneider, T.; Schütze, A. High-Performance VOC Quantification for IAQ Monitoring Using Advanced Sensor Systems and Deep Learning. Atmosphere 2021, 12, 1487. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Instability of metal oxide-based conductometric gas sensors and approaches to stability improvement (short survey). Sens. Actuators B Chem. 2011, 156, 527–538. [Google Scholar] [CrossRef]
- Fleischer, M.; Hanrieder, W.; Meixner, H. Stability of semiconducting gallium oxide thin films. Thin Solid Film. 1990, 190, 93–102. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Gallium Oxide Thin-Films—A New Material for High-Temperature Oxygen Sensors. Sens. Actuators B Chem. 1991, 4, 437–441. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Sensing Reducing Gases at High-Temperatures Using Long-Term Stable Ga2o3 Thin-Films. Sens. Actuators B Chem. 1992, 6, 257–261. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Z.; Ha, S.; Li, D.; Zhang, K.; Zhang, H.; Feng, J. Gallium Oxide for Gas Sensor Applications: A Comprehensive Review. Materials 2022, 15, 7339. [Google Scholar] [CrossRef]
- Bagheri, M.; Khodadadi, A.A.; Mahjoub, A.R.; Mortazavi, Y. Strong effects of gallia on structure and selective responses of Ga2O3–In2O3 nanocomposite sensors to either ethanol, CO or CH4. Sens. Actuators B Chem. 2015, 220, 590–599. [Google Scholar] [CrossRef]
- Balasubramani, V.; Ahamed, A.N.; Chandraleka, S.; Kumar, K.K.; Kuppusamy, M.R.; Sridhar, T.M. Highly Sensitive and Selective H2S Gas Sensor Fabricated with β-Ga2O3/rGO. ECS J. Solid State Sci. Technol. 2020, 9, 055009. [Google Scholar] [CrossRef]
- Almaev, A.V.; Chernikov, E.V.; Novikov, V.V.; Kushnarev, B.O.; Yakovlev, N.N.; Chuprakova, E.V.; Oleinik, V.L.; Lozinskaya, A.D.; Gogova, D.S. Impact of Cr2O3 additives on the gas-sensitive properties of β-Ga2O3 thin films to oxygen, hydrogen, carbon monoxide, and toluene vapors. J. Vac. Sci. Technol. A 2021, 39, 023405. [Google Scholar] [CrossRef]
- Schwebel, T.; Fleischer, M.; Meixner, H.; Kohl, C.D. CO-sensor for domestic use based on high temperature stable Ga2O3 thin films. Sens. Actuators B Chem. 1998, 49, 46–51. [Google Scholar] [CrossRef]
- Tsai, J.-H.; Niu, J.-S.; Shao, W.-C.; Liu, W.-C. Characteristics of chemiresistive-type ammonia sensor based on Ga2O3 thin film functionalized with platinum nanoparticles. Sens. Actuators B Chem. 2022, 371, 132589. [Google Scholar] [CrossRef]
- Afzal, A. β-Ga2O3 nanowires and thin films for metal oxide semiconductor gas sensors: Sensing mechanisms and performance enhancement strategies. J. Mater. 2019, 5, 542–557. [Google Scholar] [CrossRef]
- Weng, T.-F.; Ho, M.-S.; Sivakumar, C.; Balraj, B.; Chung, P.-F. VLS growth of pure and Au decorated β-Ga2O3 nanowires for room temperature CO gas sensor and resistive memory applications. Appl. Surf. Sci. 2020, 533, 147476. [Google Scholar] [CrossRef]
- Dela Torre, H.M.B.; Santos, G.N.C. Synthesis and Characterization of Monoclinic Gallium Oxide Nanomaterials for High-Concentration Ethanol Vapor Detection. IOP Conf. Ser. Mater. Sci. Eng. 2020, 739, 012031. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. Conductometric gas sensors based on metal oxides modified with gold nanoparticles: A review. Microchim. Acta 2016, 183, 1033–1054. [Google Scholar] [CrossRef]
- Frank, J.; Fleischer, M.; Meixner, H.; Feltz, A. Enhancement of sensitivity and conductivity of semiconducting Ga2O3 gas sensors by doping with SnO2. Sens. Actuators B Chem. 1998, 49, 110–114. [Google Scholar] [CrossRef]
- Vorobyeva, N.; Rumyantseva, M.; Platonov, V.; Filatova, D.; Chizhov, A.; Marikutsa, A.; Bozhev, I.; Gaskov, A. Ga2O3(Sn) Oxides for High-Temperature Gas Sensors. Nanomaterials 2021, 11, 2938. [Google Scholar] [CrossRef]
- Manandhar, S.; Battu, A.K.; Devaraj, A.; Shutthanandan, V.; Thevuthasan, S.; Ramana, C.V. Rapid Response High Temperature Oxygen Sensor Based on Titanium Doped Gallium Oxide. Sci. Rep. 2020, 10, 178. [Google Scholar] [CrossRef]
- Almaev, A.V.; Chernikov, E.V.; Kushnarev, B.O.; Yakovlev, N.N. Effect of oxygen on the properties of Ga2O3:Si thin films. J. Phys. Conf. Ser. 2019, 1410, 012201. [Google Scholar] [CrossRef]
- Zhou, W.; Xia, C.; Sai, Q.; Zhang, H. Controlling n-type conductivity of β-Ga2O3 by Nb doping. Appl. Phys. Lett. 2017, 111, 242103. [Google Scholar] [CrossRef]
- Saleh, M.; Bhattacharyya, A.; Varley, J.B.; Swain, S.; Jesenovec, J.; Krishnamoorthy, S.; Lynn, K. Electrical and optical properties of Zr doped β-Ga2O3 single crystals. Appl. Phys. Express 2019, 12, 085502. [Google Scholar] [CrossRef]
- Alema, F.; Seryogin, G.; Osinsky, A.; Osinsky, A. Ge doping of β-Ga2O3 by MOCVD. APL Mater. 2021, 9, 091102. [Google Scholar] [CrossRef]
- Peelaers, H.; Van de Walle, C.G. Doping of Ga2O3 with transition metals. Phys. Rev. B 2016, 94, 195203. [Google Scholar] [CrossRef]
- Chen, J.-X.; Li, X.-X.; Tao, J.-J.; Cui, H.-Y.; Huang, W.; Ji, Z.-G.; Sai, Q.-L.; Xia, C.-T.; Lu, H.-L.; Zhang, D.W. Fabrication of a Nb-Doped β-Ga2O3 Nanobelt Field-Effect Transistor and Its Low-Temperature Behavior. ACS Appl. Mater. Inter. 2020, 12, 8437–8445. [Google Scholar] [CrossRef]
- Li, R.; Deng, J.; Kong, L.; Meng, J.; Luo, J.; Zhang, Q.; Gao, H.; Yang, Q.; Wang, G.; Wang, X. Influence of Substrate Temperature on Structure and Properties of Nb-Doped β-Ga2O3 Films. J. Electron. Mater. 2022, 51, 2390–2395. [Google Scholar] [CrossRef]
- Krivetskiy, V.; Zamanskiy, K.; Beltyukov, A.; Asachenko, A.; Topchiy, M.; Nechaev, M.; Garshev, A.; Krotova, A.; Filatova, D.; Maslakov, K.; et al. Effect of AuPd Bimetal Sensitization on Gas Sensing Performance of Nanocrystalline SnO2 Obtained by Single Step Flame Spray Pyrolysis. Nanomaterials 2019, 9, 728. [Google Scholar] [CrossRef]
- Schimmoeller, B.; Pratsinis, S.E.; Baiker, A. Flame Aerosol Synthesis of Metal Oxide Catalysts with Unprecedented Structural and Catalytic Properties. ChemCatChem 2011, 3, 1234–1256. [Google Scholar] [CrossRef]
- Perfler, L.; Kahlenberg, V.; Többens, D.; Schaur, A.; Tribus, M.; Orlova, M.; Kaindl, R. Mechanical Properties, Quantum Mechanical Calculations, and Crystallographic/Spectroscopic Characterization of GaNbO4, Ga(Ta,Nb)O4, and GaTaO4. Inorg. Chem. 2016, 55, 5384–5397. [Google Scholar] [CrossRef]
- Yoshida, K.; Kumagai, H.; Yamane, T.; Hayashi, A.; Koyama, C.; Oda, H.; Ito, T.; Ishikawa, T. Thermophysical properties of molten Ga2O3 by using the electrostatic levitation furnace in the International Space Station. Appl. Phys. Express 2022, 15, 085503. [Google Scholar] [CrossRef]
- Pittman, R.M.; Bell, A.T. Raman studies of the structure of niobium oxide/titanium oxide (Nb2O5.TiO2). J. Phys. Chem. 1993, 97, 12178–12185. [Google Scholar] [CrossRef]
- Carli, R.; Bianchi, C.L. XPS analysis of gallium oxides. Appl. Surf. Sci. 1994, 74, 99–102. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.A.; Gaskov, A. The Key Role of Active Sites in the Development of Selective Metal Oxide Sensor Materials. Sensors 2021, 21, 2554. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, D.; Shah, V. A comparative study of optical properties of site specific doping in GaNbO4. AIP Conf. Proc. 2019, 2115, 030617. [Google Scholar] [CrossRef]
- Delgado, M.R.; Areán, C.O. Infrared Spectroscopic Studies on the Surface Chemistry of High-Surface-Area Gallia Polymorphs. Z. Für Anorg. Und Allg. Chem. 2005, 631, 2115–2120. [Google Scholar] [CrossRef]
- Devi, S.; Kelkar, S.; Kashid, V.; Salunke, H.G.; Gupta, N.M. Preparation-method-dependent morphological, band structural, microstructural, and photocatalytic properties of noble metal–GaNbO4 nanocomposites. Rsc. Adv. 2013, 3, 16817–16828. [Google Scholar] [CrossRef]
- Kohl, D.; Ochs, T.; Geyer, W.; Fleischer, M.; Meixner, H. Adsorption and decomposition of methane on gallium oxide films. Sens. Actuators B Chem. 1999, 59, 140–145. [Google Scholar] [CrossRef]
- Zimmermann, C.; Rønning, V.; Kalmann Frodason, Y.; Bobal, V.; Vines, L.; Varley, J.B. Primary intrinsic defects and their charge transition levels in Ga2O3. Phys. Rev. Mater. 2020, 4, 074605. [Google Scholar] [CrossRef]
- Krawczyk, M.; Suchorska-Woźniak, P.; Szukiewicz, R.; Kuchowicz, M.; Korbutowicz, R.; Teterycz, H. Morphology of Ga2O3 Nanowires and Their Sensitivity to Volatile Organic Compounds. Nanomaterials 2021, 11, 456. [Google Scholar] [CrossRef]
- Pohle, R.; Fleischer, M.; Meixner, H. In situ infrared emission spectroscopic study of the adsorption of H2O and hydrogen-containing gases on Ga2O3 gas sensors. Sens. Actuators B Chem. 2000, 68, 151–156. [Google Scholar] [CrossRef]
- Bene, R.; Pintér, Z.; Perczel, I.V.; Fleischer, M.; Réti, F. High-temperature semiconductor gas sensors. Vacuum 2001, 61, 275–278. [Google Scholar] [CrossRef]
- Trinchi, A.; Wlodarski, W.; Li, Y.X. Study of Ga2O3 thin film gas sensors. In Proceedings of the SENSORS 2003, Toronto, ON, Canada, 22–24 October 2003; Volume 131, pp. 133–136. [Google Scholar] [CrossRef]
- Fleischer, M.; Kornely, S.; Weh, T.; Frank, J.; Meixner, H. Selective gas detection with high-temperature operated metal oxides using catalytic filters. Sens. Actuators B Chem. 2000, 69, 205–210. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Sensitive, Selective and Stable Ch4 Detection Using Semiconducting Ga2o3 Thin-Films. Sens. Actuators B Chem. 1995, 26, 81–84. [Google Scholar] [CrossRef]
- Frank, J.; Fleischer, M.; Meixner, H. Gas-sensitive electrical properties of pure and doped semiconducting Ga2O3 thick films. Sens. Actuators B Chem. 1998, 48, 318–321. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Theory of power laws for semiconductor gas sensors. Sens. Actuators B Chem. 2008, 128, 566–573. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Go, S.; Sexton, D.; Li, X.; Alkadi, N.; Kolmakov, A.; Amm, B.; St-Pierre, R.; Scherer, B.; Nayeri, M.; et al. Extraordinary performance of semiconducting metal oxide gas sensors using dielectric excitation. Nat. Electron. 2020, 3, 280–289. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Chwieroth, B.; Patton, B.R.; Wang, Y. Conduction and Gas–Surface Reaction Modeling in Metal Oxide Gas Sensors. J. Electroceram. 2001, 6, 27–41. [Google Scholar] [CrossRef]


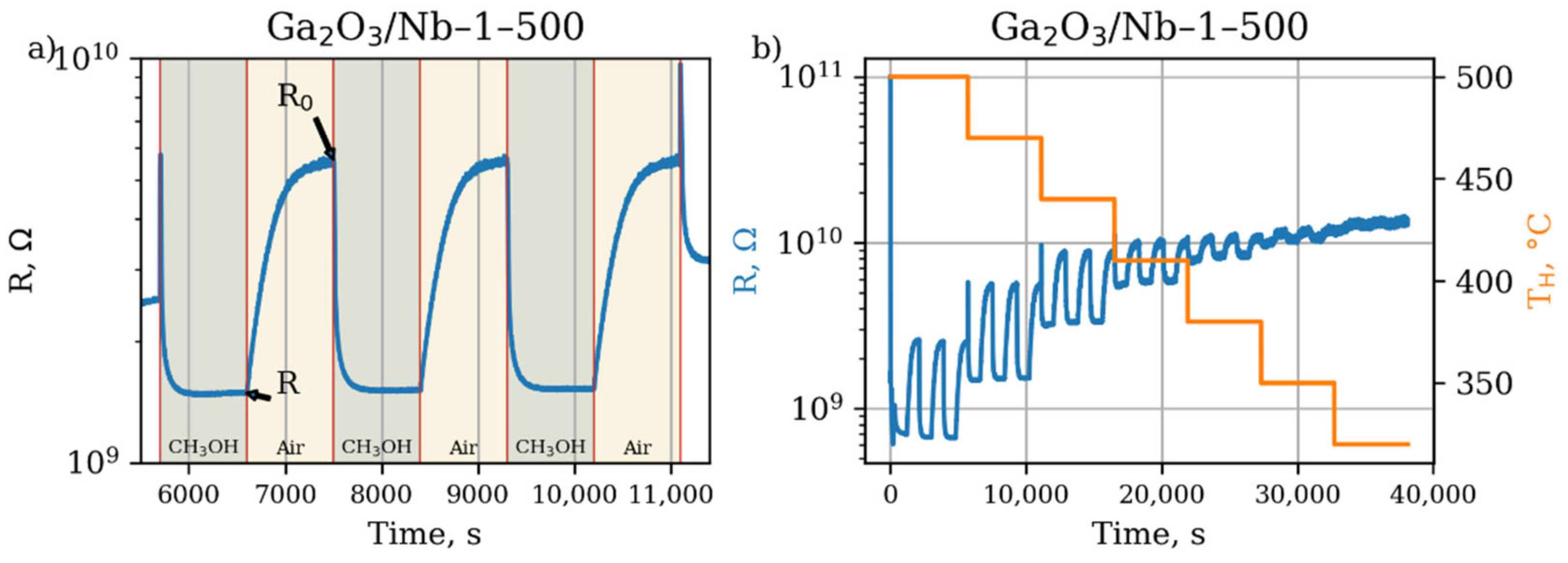
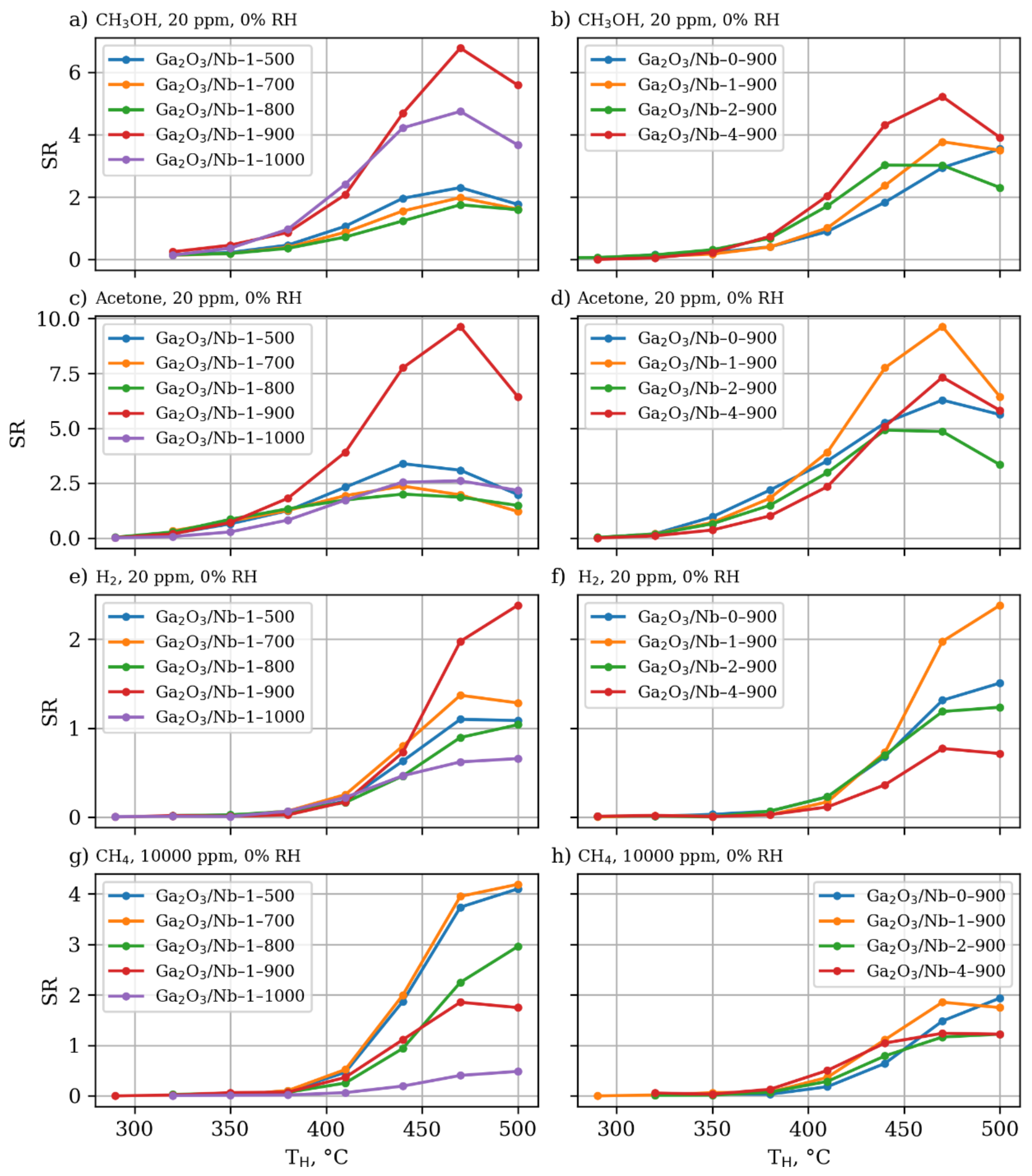
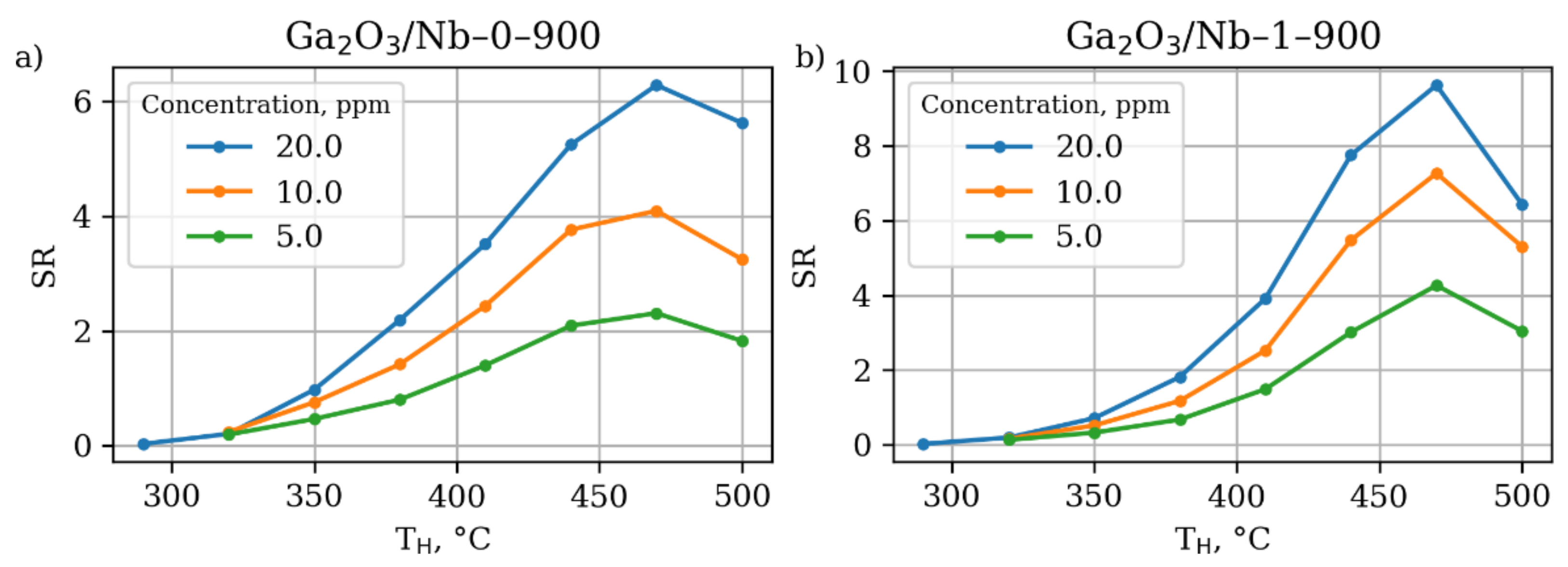

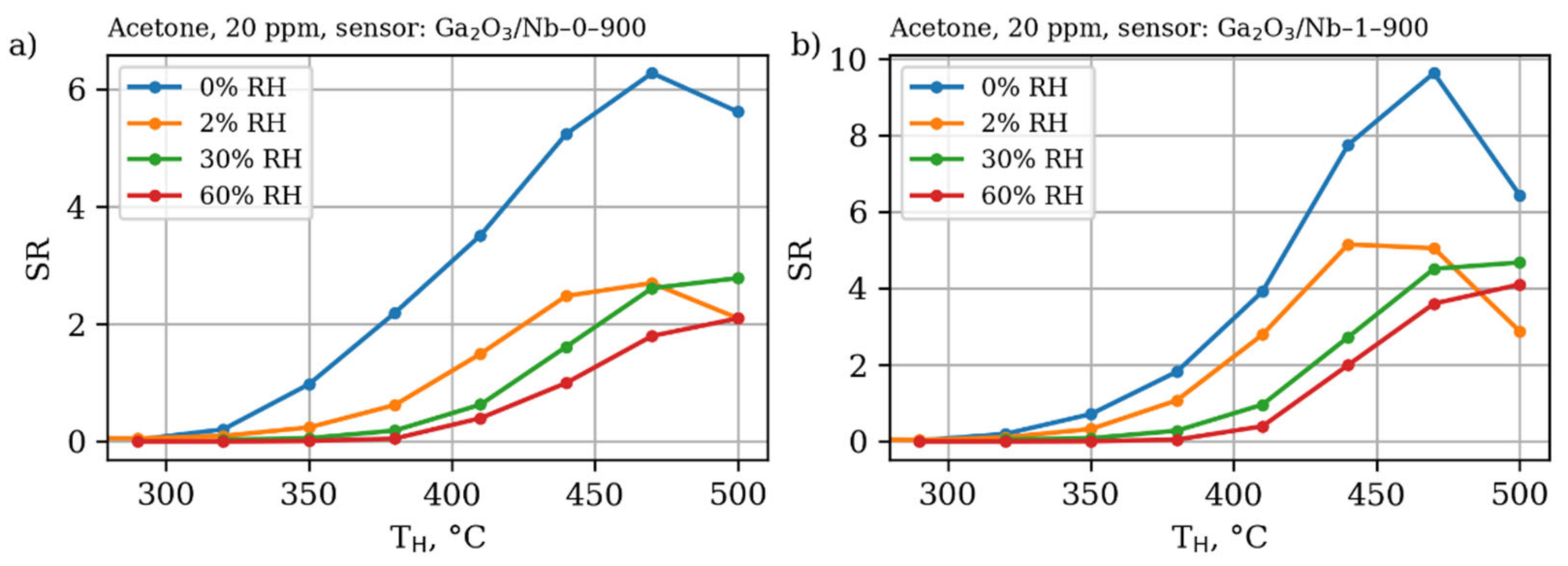

| Annealing Temperature, °C (y) | Nb Content, mol.% (x) | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 4 | |
| - | 6/97 | 6/111 | 6/92 | 6/109 |
| 500 | 6/104 | 6/127 | 6/102 | 6/103 |
| 700 | 7/93 | 7/98 | 7/95 | 6/88 |
| 800 | 9/63 | 8/73 | 7/65 | 7/67 |
| 900 | 12/24 | 11/49 | 10/41 | 11/34 |
| 1000 | 15/11 | 19/1 | - | - |
| Sample | Nb Content, mol.% (x) |
|---|---|
| Ga2O3/Nb-0-500 | 0 |
| Ga2O3/Nb-1-500 | 1.6 |
| Ga2O3/Nb-2-500 | 2.6 |
| Ga2O3/Nb-4 | 4.2 |
| Ga2O3/Nb-4-500 | 3.9 |
| Ga2O3/Nb-4-800 | 5.4 |
| Ga2O3/Nb-4-900 | 8.7 |
| Material | Gas | Tw, °C | S, a.u. | Reference |
|---|---|---|---|---|
| Ga2O3/Nb-1-900 | Acetone, 20 ppm | 470 | 9.8 | This work |
| Ga2O3 NWs | Acetone, 100 ppm | 600 | 40 | [49] |
| Ga2O3 ThF | Acetone, 200 ppm | 480 | 35 | [50] |
| Ga2O3 TF | Acetone, 1600 ppm | 530 | 100 | [51] |
| Ga2O3/Nb-1-900 | H2, 20 ppm | 500 | 2.5 | This work |
| Ga2O3-Cr2O3 TF | H2, 2500 ppm | 500 | 60 | [19] |
| Ga2O3 ThF | H2, 5000 ppm | 500 | 1 | [52] |
| H2, 20 ppm | H2, 20 ppm | 700 | 2 | [53] ** |
| Ga2O3/Nb-1-500 | CH4, 10,000 ppm | 500 | 4 | This work |
| Ga2O3 TF | CH4, 5000 ppm | 840 | 100 | [54] ** |
| Ga2O3 ThF | CH4, 10,000 ppm | 570 | 10 | [55] ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreev, M.; Topchiy, M.; Asachenko, A.; Beltiukov, A.; Amelichev, V.; Sagitova, A.; Maksimov, S.; Smirnov, A.; Rumyantseva, M.; Krivetskiy, V. Electrical and Gas Sensor Properties of Nb(V) Doped Nanocrystalline β-Ga2O3. Materials 2022, 15, 8916. https://doi.org/10.3390/ma15248916
Andreev M, Topchiy M, Asachenko A, Beltiukov A, Amelichev V, Sagitova A, Maksimov S, Smirnov A, Rumyantseva M, Krivetskiy V. Electrical and Gas Sensor Properties of Nb(V) Doped Nanocrystalline β-Ga2O3. Materials. 2022; 15(24):8916. https://doi.org/10.3390/ma15248916
Chicago/Turabian StyleAndreev, Matvei, Maxim Topchiy, Andrey Asachenko, Artemii Beltiukov, Vladimir Amelichev, Alina Sagitova, Sergey Maksimov, Andrei Smirnov, Marina Rumyantseva, and Valeriy Krivetskiy. 2022. "Electrical and Gas Sensor Properties of Nb(V) Doped Nanocrystalline β-Ga2O3" Materials 15, no. 24: 8916. https://doi.org/10.3390/ma15248916
APA StyleAndreev, M., Topchiy, M., Asachenko, A., Beltiukov, A., Amelichev, V., Sagitova, A., Maksimov, S., Smirnov, A., Rumyantseva, M., & Krivetskiy, V. (2022). Electrical and Gas Sensor Properties of Nb(V) Doped Nanocrystalline β-Ga2O3. Materials, 15(24), 8916. https://doi.org/10.3390/ma15248916










