Effect of Ordinary Portland Cement on Mechanical Properties and Microstructures of Metakaolin-Based Geopolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
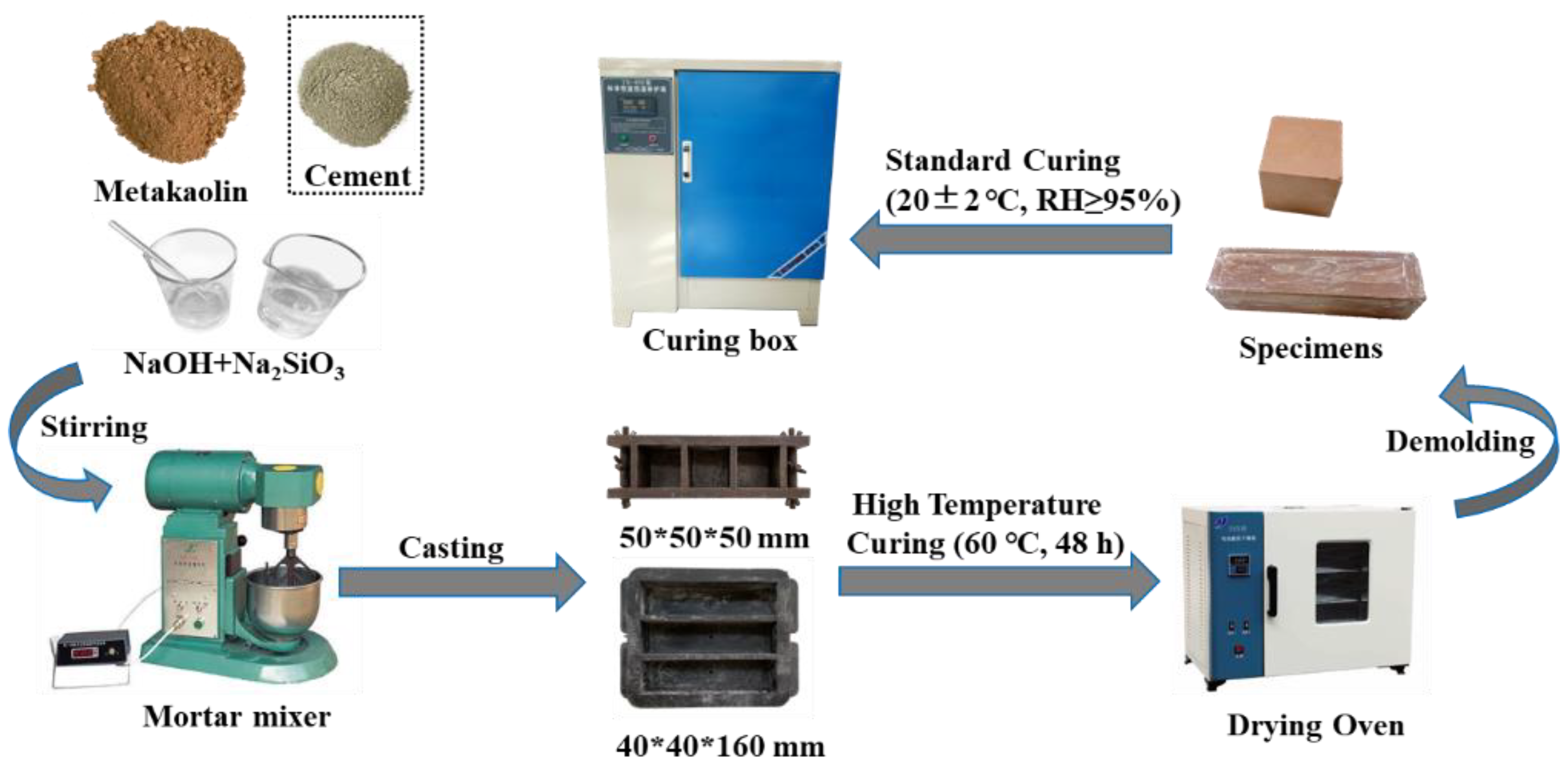
2.2. Mix Proportions and Preparation of Specimens
2.3. Testing Methods
2.3.1. Mechanical Properties Test
2.3.2. Microscopic Performance Test
3. Results and Discussion
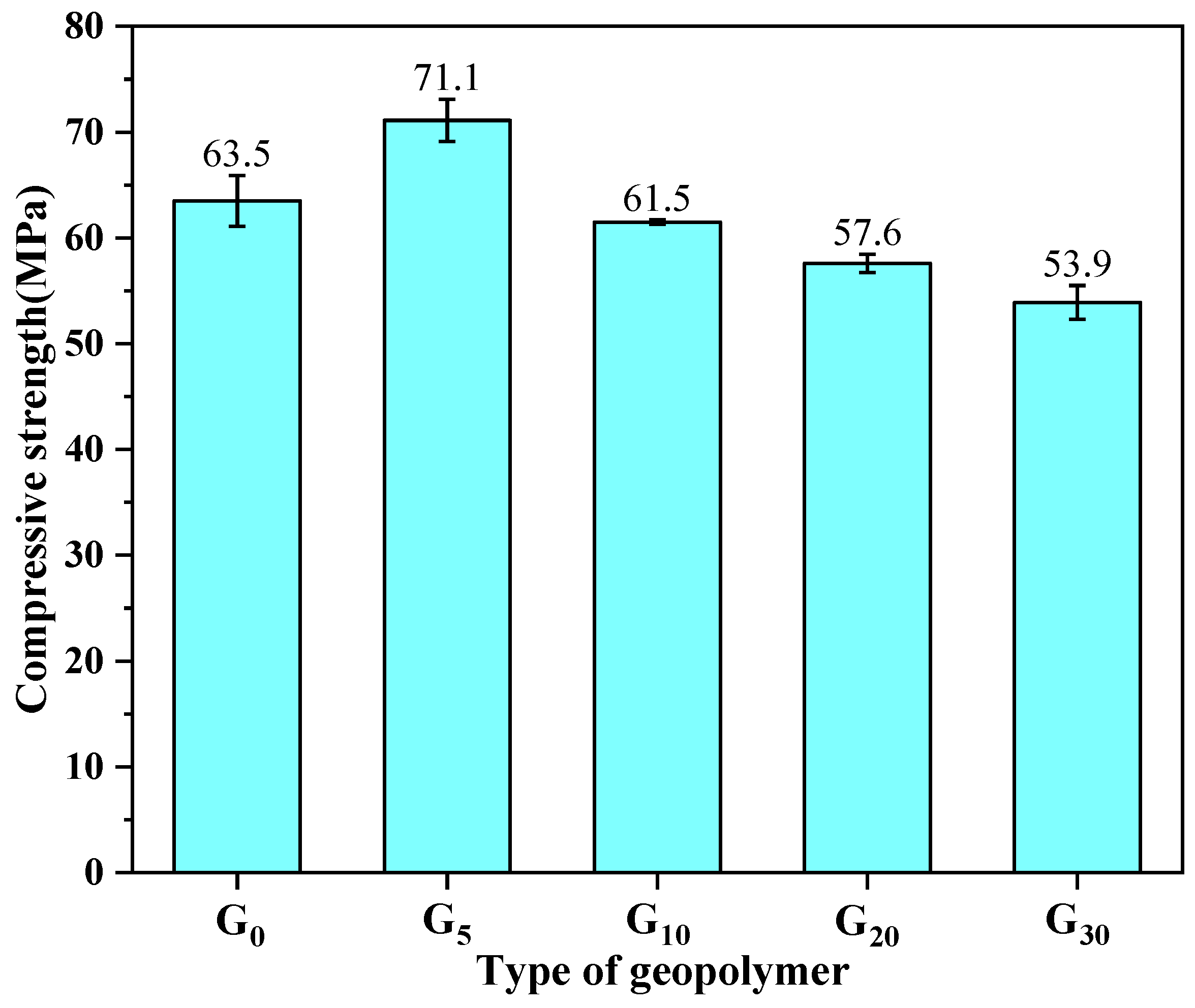
3.1. Compressive Strength of Geopolymer
3.2. Flexural Strength of Geopolymers
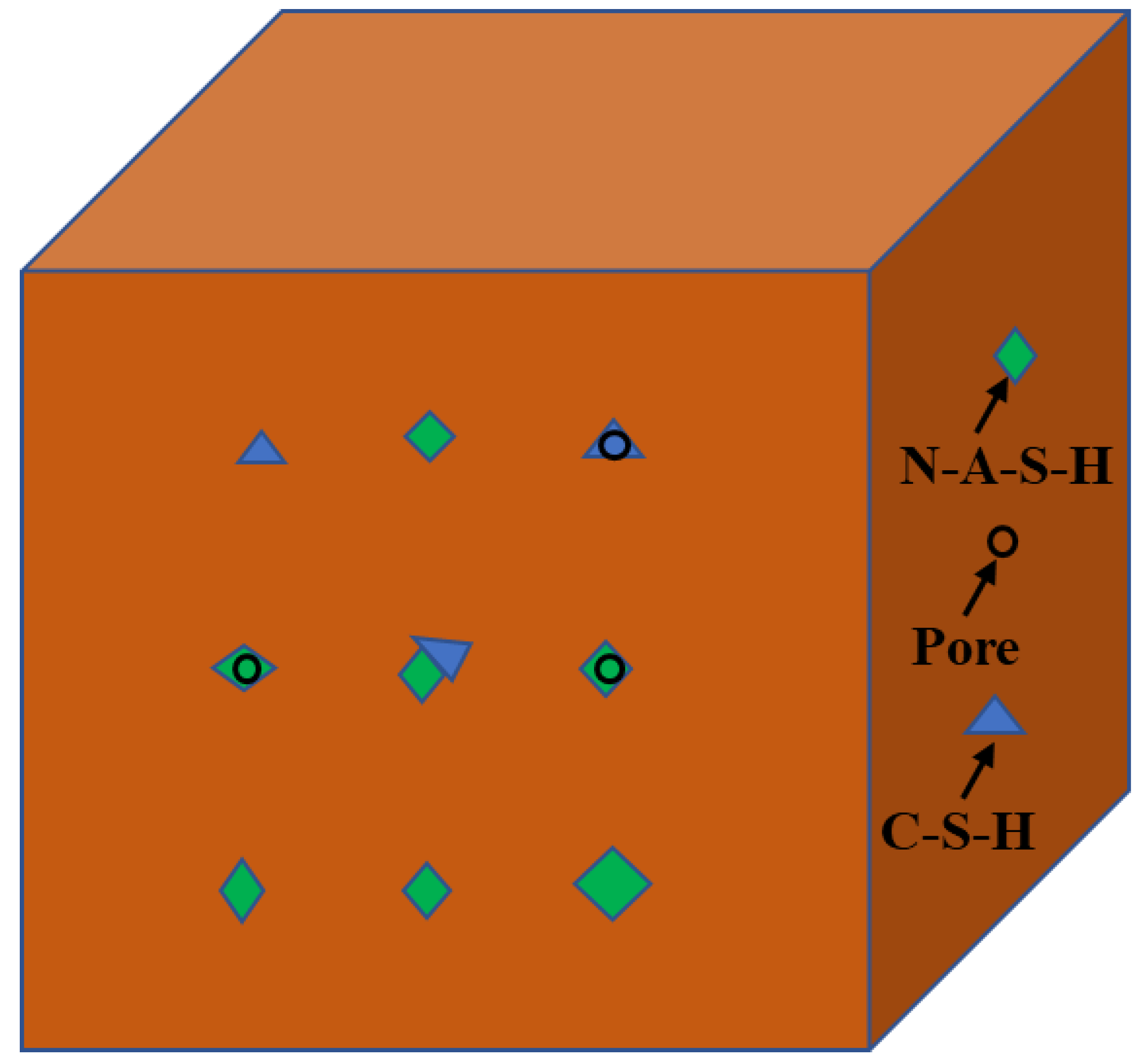
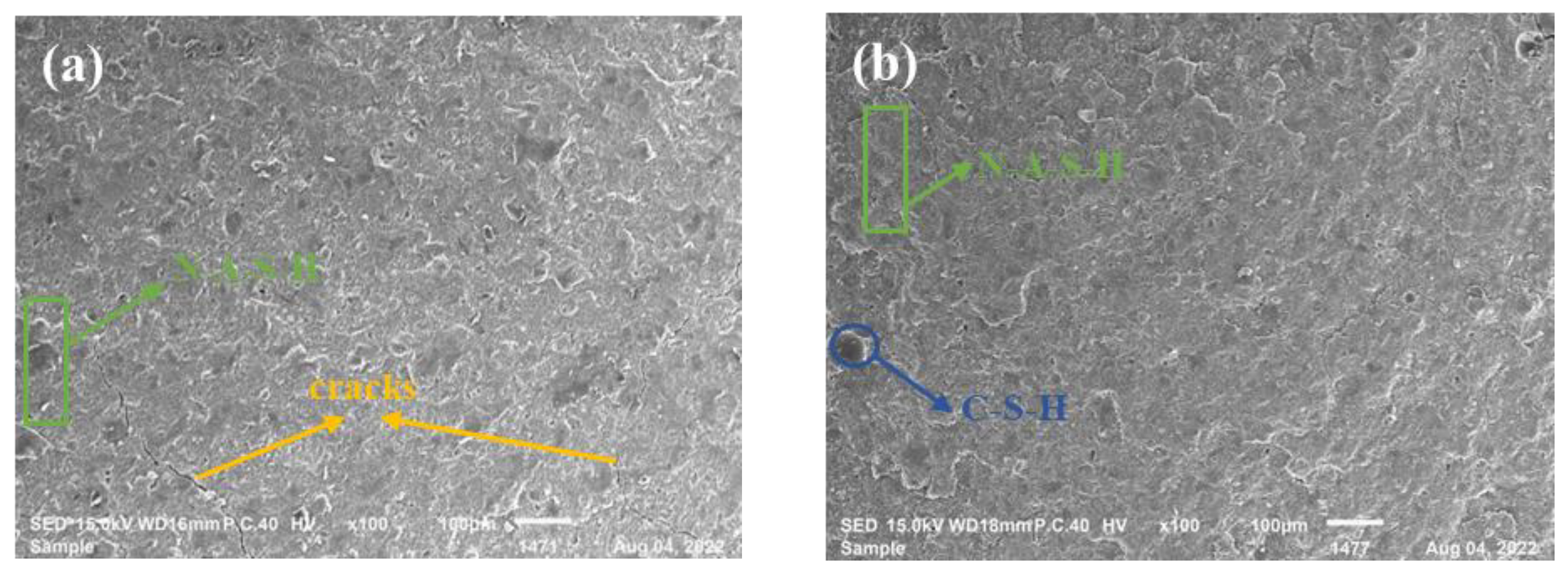
3.3. SEM Analysis
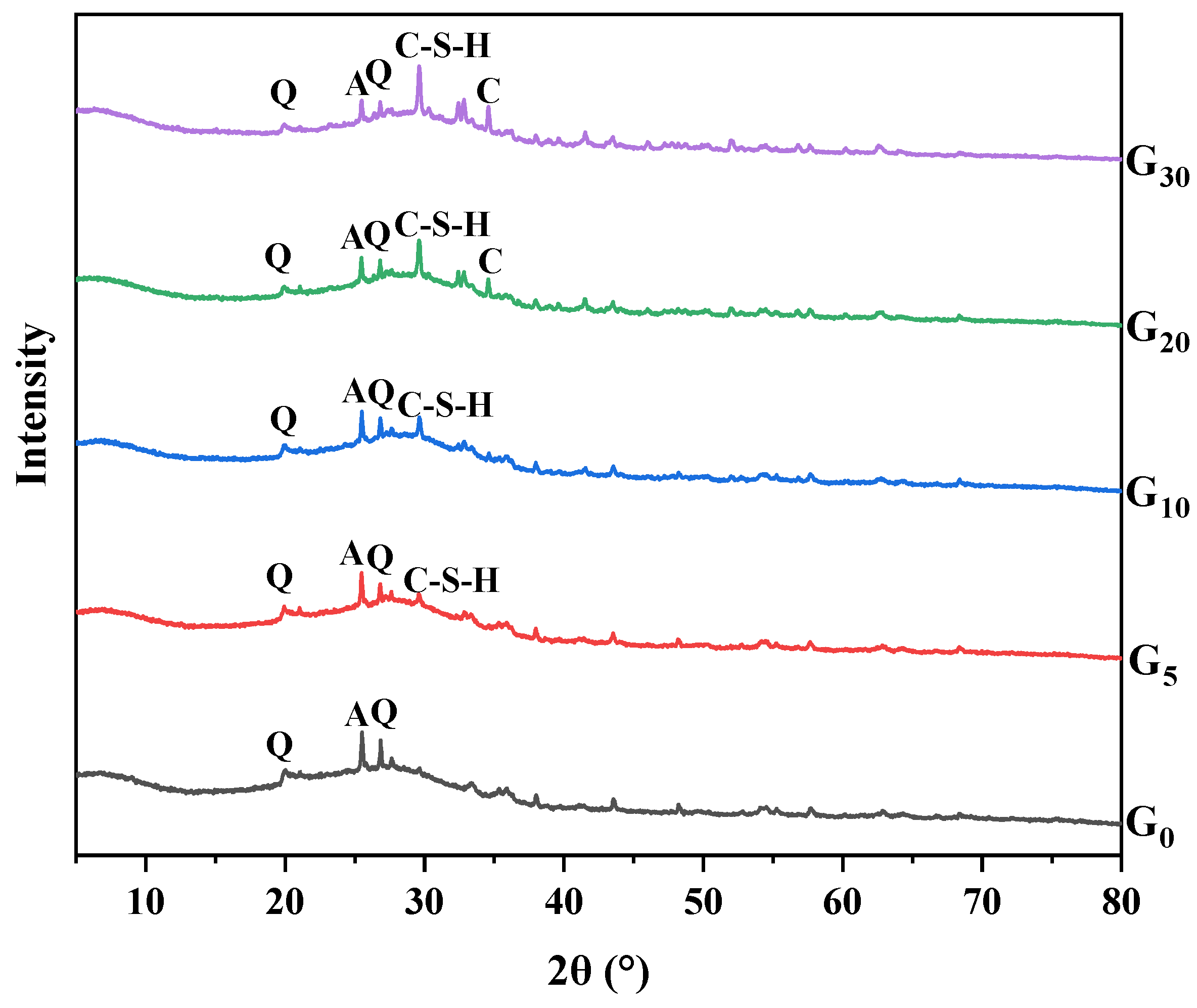
3.4. XRD Analysis
3.5. FT-IR Analysis
4. Conclusions
- (1)
- The optimal incorporation of CEM I was 5%, which would endow geopolymer (G5) with better mechanical properties and a denser internal structure than the pure metakaolin-based geopolymer (G0). The compressive strength and flexural strength of G5 can reach 71.1 MPa and 6.75 MPa, respectively.
- (2)
- The heat released by cement hydration can accelerate the process of geopolymerization. Additionally, the co-existence of the hydration reaction product (C-S-H gel) and the geolpolymerization reaction product (N-A-S-H gel) was beneficial to the development of the geopolymers’ strength. This can be explained by the XRD study, which showed that the peak strength gradually dropped after CEM I was added, showing that CEM I can facilitate the geopolymerization process between silica-alumina materials and alkaline solutions.
- (3)
- With the increase in the content of CEM I, the geopolymer matrix was unable to supply more water for the hydration reaction of CEM I, which prevented excessive CEM I from producing hydration products, weakened the workability of the matrix, and eventually hindered the growth of geopolymer strength. In light of the spectra of FT-IR, as more content of CEM I was added into the geopolymers, the spectral bands of the geopolymers moved to lower wavenumbers, indicating the lower degree of cross-linking of gels in the geopolymers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guerrero, L.A.; Maas, G.; Hogland, W. Solid waste management challenges for cities in developing countries. Waste Manag. 2013, 33, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Zhao, Y.; Bai, H.; Kang, S.; Zhang, T.; Song, S. Eco-friendly geopolymer prepared from solid wastes: A critical review. Chemosphere 2021, 267, 128900. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.S.; Abdel-Gawwad, H.A.; Vásquez-García, S.R.; Israde-Alcántara, I.; Flores-Ramirez, N.; Rico, J.L.; Mohammed, M.S. Cleaner production of one-part white geopolymer cement using pre-treated wood biomass ash and diatomite. J. Clean. Prod. 2019, 209, 1420–1428. [Google Scholar] [CrossRef]
- Tomatis, M.; Jeswani, H.K.; Stamford, L.; Azapagic, A. Assessing the environmental sustainability of an emerging energy technology: Solar thermal calcination for cement production. Sci. Total Environ. 2020, 742, 140510. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Jin, F.; Al-Tabbaa, A.; Shi, B.; Liu, J. Mechanical and hydration properties of ground granulated blastfurnace slag pastes activated with MgO–CaO mixtures. Constr. Build. Mater. 2014, 69, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Meyer, C. The greening of the concrete industry. Cem. Concr. Compos. 2009, 31, 601–605. [Google Scholar] [CrossRef]
- Xinjie, W.; Wei, Y.; Hui, L.I.U.; Pinghua, Z.H.U.; Ningwen, Z.; Jincai, F. Strength and Microstructural Analysis of Geopolymer Prepared with Recycled Geopolymer Powder. J. Wuhan Univ. Technol. Sci. 2021, 36, 439–445. [Google Scholar]
- Akbarnezhad, A.; Huan, M.; Mesgari, S.; Castel, A. Recycling of geopolymer concrete. Constr. Build. Mater. 2015, 101, 152–158. [Google Scholar] [CrossRef]
- Lee, C.-C.; Hussain, J. Carbon neutral sustainability and green development during energy consumption. Innov. Green Dev. 2022, 1, 100002. [Google Scholar] [CrossRef]
- Rao, F.; Liu, Q. Geopolymerization and Its Potential Application in Mine Tailings Consolidation: A Review. Miner. Process. Extr. Metall. Rev. 2015, 36, 399–409. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential applications of geopolymer concrete in construction: A review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Hu, S.; Zhong, L.; Yang, X.; Bai, H.; Ren, B.; Zhao, Y.; Zhang, W.; Ju, X.; Wen, H.; Mao, S.; et al. Synthesis of rare earth tailing-based geopolymer for efficiently immobilizing heavy metals. Constr. Build. Mater. 2020, 254, 119273. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patiño, C.L.; Zhang, Y. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Zaidi, F.H.A.; Ahmad, R.; Abdullah, M.M.a.; Rahim, S.Z.A.; Yahya, Z.; Li, L.Y. Ediati, Geopolymer as underwater concreting material: A review. Constr. Build. Mater. 2021, 291, 123276. [Google Scholar] [CrossRef]
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Heath, A.; Paine, K.; McManus, M. Minimising the global warming potential of clay based geopolymers. J. Clean. Prod. 2014, 78, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Zhu, P.; Liu, H.; Wang, X.; Ge, W.; Hua, M. Resistance to Sulfuric Acid Corrosion of Geopolymer Concrete Based on Different Binding Materials and Alkali Concentrations. Materials 2021, 14, 7109. [Google Scholar] [CrossRef]
- Pangdaeng, S.; Phoo-ngernkham, T.; Sata, V.; Chindaprasirt, P. Influence of curing conditions on properties of high calcium fly ash geopolymer containing Portland cement as additive. Mater. Des. 2014, 53, 269–274. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-K.; Yoo, S.-W.; Jung, S.-H.; Lee, K.-M.; Kwon, S.-J. Effect of Na2O content, SiO2/Na2O molar ratio, and curing conditions on the compressive strength of FA-based geopolymer. Constr. Build. Mater. 2017, 145, 253–260. [Google Scholar] [CrossRef]
- Izadifar, M.; Thissen, P.; Steudel, A.; Kleeberg, R.; Kaufhold, S.; Kaltenbach, J.; Schuhmann, R.; Dehn, F.; Emmerich, K. Comprehensive Examination of Dehydroxylation of Kaolinite, Disordered Kaolinite, and Dickite: Experimental Studies and Density Functional Theory. Clays Clay Miner. 2020, 68, 319–333. [Google Scholar] [CrossRef]
- Noushini, A.; Castel, A. The effect of heat-curing on transport properties of low-calcium fly ash-based geopolymer concrete. Constr. Build. Mater. 2016, 112, 464–477. [Google Scholar] [CrossRef]
- Mesgari, S.; Akbarnezhad, A.; Xiao, J.Z. Recycled geopolymer aggregates as coarse aggregates for Portland cement concrete and geopolymer concrete: Effects on mechanical properties. Constr. Build. Mater. 2020, 236, 117571. [Google Scholar] [CrossRef]
- GB/T 50081-2019; Standard Test Methods for Physical and Mechanical Properties of Concrete. China Building Industry Press: Beijing, China, 2019.
- Revathi, V. Durability studies in alkaline activated systems (metakaolin–bottom ash): A prospective study. Boletín Soc. Española Cerámica Vidr. 2021, 305, 16. [Google Scholar] [CrossRef]
- Tailby, J.; MacKenzie, K.J.D. Structure and mechanical properties of aluminosilicate geopolymer composites with Portland cement and its constituent minerals. Cem. Concr. Res. 2010, 40, 787–794. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Donatello, S.; Fernández-Jiménez, A.; Palomo, Á. Hydration of hybrid alkaline cement containing a very large proportion of fly ash: A descriptive model. Materials 2016, 9, 605. [Google Scholar] [CrossRef] [Green Version]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 38, 3851–3860. [Google Scholar] [CrossRef]
- Rahier, H.; Simons, W.; van Mele, B. Biesemans, Low-temperature synthesized aluminosilicate glasses: Part III Influence of the composition of the silicate solution on production, structure and properties. J. Mater. Sci. 1997, 32, 2237–2247. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Zuo, Y.; Chen, W.; Ye, G. Chemical deformation of metakaolin based geopolymer. Cem. Concr. Res. 2019, 120, 108–118. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. The effect of ionic contaminants on the early-age properties of alkali-activated fly ash-based cements. Cem. Concr. Res. 2002, 32, 577–584. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P.; Lukey, G.C. Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. J. Hazard. Mater. 2007, 139, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Zhu, Z.; Zhang, J.; Kang, Z.; Pu, S.; Wan, Y. Utilization of OPC and FA to enhance reclaimed lime-fly ash macadam based geopolymers cured at ambient temperature. Constr. Build. Mater. 2021, 303, 124378. [Google Scholar] [CrossRef]
- Papa, E.; Medri, V.; Kpogbemabou, D.; Morinière, V.; Laumonier, J.; Vaccari, A.; Rossignol, S. Porosity and insulating properties of silica-fume based foams. Energy Build. 2016, 131, 223–232. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Influence of the slag content on the chloride and sulfuric acid resistances of alkali-activated fly ash/slag paste. Cem. Concr. Compos. 2016, 72, 168–179. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, X.; Yang, T.; Zhang, Z. The degradation mechanisms of alkali-activated fly ash/slag blend cements exposed to sulphuric acid. Constr. Build. Mater. 2018, 186, 1177–1187. [Google Scholar] [CrossRef]
- Ding, Y.-C.; Cheng, T.-W.; Dai, Y.-S. Application of geopolymer paste for concrete repair. Struct. Concr. 2017, 18, 561–570. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, W.; Zhao, X.; Bu, M.; Yuan, Q.; Niu, D. The microstructure and durability of fly ash-based geopolymer concrete: A review. Ceram. Int. 2021, 47, 29550–29566. [Google Scholar] [CrossRef]



| Properties | Metakaolin | CEM I |
|---|---|---|
| Apparent density (kg/m3) | 2480 | 2963 |
| Specific surface area (m2/kg) | 15,600 | 350 |
| Chemical Compositions | Metakaolin | CEM I |
|---|---|---|
| SiO2 | 47.69 | 20.79 |
| Al2O3 | 45.10 | 5.79 |
| Fe2O3 | 3.60 | 2.59 |
| SO3 | 0.19 | 4.56 |
| TiO2 | 1.95 | 0.37 |
| CaO | 0.58 | 60.67 |
| K2O | 0.34 | 0.72 |
| MgO | 0.17 | 3.86 |
| Na2O | 0.05 | 0.20 |
| LOIa | 0.33 | 0.45 |
| Geopolymers | Metakaolin | CEM I | Na2SiO3 | NaOH |
|---|---|---|---|---|
| G0 | 888 | - | 500 | 250 |
| G5 | 843.6 | 44.4 | 500 | 250 |
| G10 | 799.2 | 88.8 | 500 | 250 |
| G20 | 710.4 | 177.6 | 500 | 250 |
| G30 | 621.6 | 266.4 | 500 | 250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Yang, W.; Duan, Z.; Liu, H.; Deng, Q.; Hua, M. Effect of Ordinary Portland Cement on Mechanical Properties and Microstructures of Metakaolin-Based Geopolymers. Materials 2022, 15, 9007. https://doi.org/10.3390/ma15249007
Gao R, Yang W, Duan Z, Liu H, Deng Q, Hua M. Effect of Ordinary Portland Cement on Mechanical Properties and Microstructures of Metakaolin-Based Geopolymers. Materials. 2022; 15(24):9007. https://doi.org/10.3390/ma15249007
Chicago/Turabian StyleGao, Renhui, Wei Yang, Zhenhua Duan, Hui Liu, Qi Deng, and Minqi Hua. 2022. "Effect of Ordinary Portland Cement on Mechanical Properties and Microstructures of Metakaolin-Based Geopolymers" Materials 15, no. 24: 9007. https://doi.org/10.3390/ma15249007







