The Mutual Incorporation of Mg2+ and CO32− into Hydroxyapatite: A DFT Study
Abstract
:1. Introduction
1.1. Hydroxyapatite’s Applications
1.2. Carbonate Substitutions
1.3. Magnesium Substitutions
1.4. Magnesium and Carbonate Co-Substitutions
2. Methods
3. Results and Discussion
3.1. Mg2+ Incorporation into HA
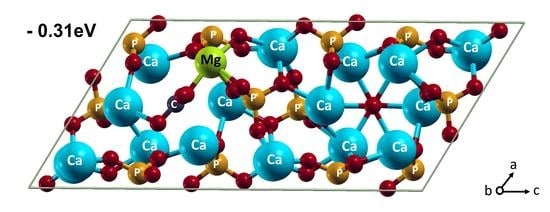
3.2. Mg2+ and CO32− Co-Incorporation into HA
3.2.1. Carbonate in the OH− (A) position
3.2.2. Carbonate− in the PO43− (B) position
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barinov, S.M.; Komlev, V.S. Approaches to the Fabrication of Calcium Phosphate-Based Porous Materials for Bone Tissue Regeneration. Inorg. Mater. 2016, 52, 339–346. [Google Scholar] [CrossRef]
- Komlev, V.S.; Barinov, S.M.; Koplik, E.V. A Method to Fabricate Porous Spherical Hydroxyapatite Granules Intended for Time-Controlled Drug Release. Biomaterials 2002, 23, 3449–3454. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.A.; Smirnov, V.V.; Kutsev, S.V.; Shibaeva, T.V.; Shvorneva, L.I.; Sergeeva, N.S.; Sviridova, I.K.; Barinov, S.M. Hydroxyapatite-Calcium Carbonate Ceramic Composite Materials. Inorg. Mater. 2010, 46, 1269–1273. [Google Scholar] [CrossRef]
- Rajendran, A.; Barik, R.C.; Natarajan, D.; Kiran, M.S.; Pattanayak, D.K. Synthesis, Phase Stability of Hydroxyapatite-Silver Composite with Antimicrobial Activity and Cytocompatability. Ceram. Int. 2014, 40, 10831–10838. [Google Scholar] [CrossRef]
- Kaygili, O.; Tatar, C.; Yakuphanoglu, F.; Keser, S. Nano-Crystalline Aluminum-Containing Hydroxyapatite Based Bioceramics: Synthesis and Characterization. J. Solgel Sci. Technol. 2013, 65, 105–111. [Google Scholar] [CrossRef]
- Shamray, V.F.; Sirotinkin, V.P.; Smirnov, I.V.; Kalita, V.I.; Fedotov, A.Y.; Barinov, S.M.; Komlev, V.S. Structure of the Hydroxyapatite Plasma-Sprayed Coatings Deposited on Pre-Heated Titanium Substrates. Ceram. Int. 2017, 43, 9105–9109. [Google Scholar] [CrossRef]
- Othmani, M.; Aissa, A.; Grelard, A.; Das, R.K.; Oda, R.; Debbabi, M. Synthesis and Characterization of Hydroxyapatite-Based Nanocomposites by the Functionalization of Hydroxyapatite Nanoparticles with Phosphonic Acids. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 336–344. [Google Scholar] [CrossRef]
- Kaygili, O.; Keser, S.; Kom, M.; Eroksuz, Y.; Dorozhkin, S.V.; Ates, T.; Ozercan, I.H.; Tatar, C.; Yakuphanoglu, F. Strontium Substituted Hydroxyapatites: Synthesis and Determination of Their Structural Properties, in Vitro and in Vivo Performance. Mater. Sci. Eng. C 2015, 55, 538–546. [Google Scholar] [CrossRef]
- Gruselle, M.; Tonsuaadu, K. Tunable calcium-apatites as solid catalysts for classical organic reactions. Curr. Org. Chem. 2017, 21, 688–697. [Google Scholar] [CrossRef] [Green Version]
- Kaneda, K.; Mizugaki, T. Design of High-Performance Heterogeneous Catalysts Using Apatite Compounds for Liquid-Phase Organic Syntheses. ACS Catal. 2017, 7, 920–935. [Google Scholar] [CrossRef]
- Gruselle, M. Apatites: A New Family of Catalysts in Organic Synthesis. J. Organomet. Chem. 2015, 793, 93–101. [Google Scholar] [CrossRef]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A Review of Syntheses, Structure and Applications in Heterogeneous Catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Gruselle, M.; Tõnsuaadu, K.; Gredin, P.; Len, C. Apatites Based Catalysts: A Tentative Classification. Mol. Catal. 2022, 519, 112146. [Google Scholar] [CrossRef]
- Šupová, M. Substituted Hydroxyapatites for Biomedical Applications: A Review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates: Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef]
- İnce, T.; Kaygili, O.; Tatar, C.; Bulut, N.; Koytepe, S.; Ates, T. The Effects of Ni-Addition on the Crystal Structure, Thermal Properties and Morphology of Mg-Based Hydroxyapatites Synthesized by a Wet Chemical Method. Ceram. Int. 2018, 44, 14036–14043. [Google Scholar] [CrossRef]
- Iriarte-Velasco, U.; Ayastuy, J.L.; Boukha, Z.; Bravo, R.; Gutierrez-Ortiz, M.Á. Transition Metals Supported on Bone-Derived Hydroxyapatite as Potential Catalysts for the Water-Gas Shift Reaction. Renew. Energy 2018, 115, 641–648. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Shurtakova, D.V.; Vakhin, A.V.; Grishin, P.O.; Gafurov, M.R. Incorporation of Iron(II) and (III) in Hydroxyapatite—A Theoretical Study. Crystals 2021, 11, 1219. [Google Scholar] [CrossRef]
- Shurtakova, D.V.; Grishin, P.O.; Gafurov, M.R.; Mamin, G.V. Using DFT to Calculate the Parameters of the Crystal Field in Mn2+ Doped Hydroxyapatite Crystals. Crystals 2021, 11, 1050. [Google Scholar] [CrossRef]
- Pathak, D.K.; Pandey, P.M. Evaluation of in Vitro Corrosion Behavior of Zinc–Hydroxyapatite and Zinc–Hydroxyapatite–Iron as Biodegradable Composites. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 436–450. [Google Scholar] [CrossRef]
- Barinov, S.M.; Fadeeva, I.V.; Ferro, D.; Rau, J.V.; Cesaro, S.N.; Komlev, V.S.; Fomin, A.S. Stabilization of Carbonate Hydroxyapatite by Isomorphic Substitutions of Sodium for Calcium. Russ. J. Inorg. Chem. 2014, 53, 164–168. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Wang, X.; Jiang, G.; Yang, M. A Comparative Study of the Dissolubility of Pure and Silicon Substituted Hydroxyapatite from Density Functional Theory Calculations. J. Mol. Model. 2018, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Palard, M.; Champion, E.; Foucaud, S. Synthesis of Silicated Hydroxyapatite Ca10(PO4)6 − x(SiO4)x(OH)2 − x. J. Solid State Chem. 2008, 181, 1950–1960. [Google Scholar] [CrossRef]
- Camaioni, A.; Cacciotti, I.; Campagnolo, L.; Bianco, A. Silicon-Substituted Hydroxyapatite for Biomedical Applications. In Hydroxyapatite (Hap) for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2015; pp. 343–373. [Google Scholar] [CrossRef]
- Barralet, J.; Best, S.; Bonfield, W. Carbonate Substitution in Precipitated Hydroxyapatite: An Investigation into the Effects of Reaction Temperature and Bicarbonate Ion Concentration. J. Biomed. Mater. Res. 1998, 41, 79–86. [Google Scholar] [CrossRef]
- Penel, G.; Leroy, G.; Rey, C.; Bres, E. MicroRaman Spectral Study of the PO4 and CO3 Vibrational Modes in Synthetic and Biological Apatites. Calcif. Tissue Int. 1998, 63, 475–481. [Google Scholar] [CrossRef]
- Wilson, R.M.; Elliott, J.C.; Dowker, S.E.P. Rietveld Refinement of the Crystallographic Structure of Human Dental Enamel Apatites. Am. Mineral. 1999, 84, 1406–1414. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Studies in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 1994; Volume 18, pp. 1–389. [Google Scholar]
- Fleet Michael, E.; Liu, X.; King Penelope, L. Accommodation of the Carbonate Ion in Apatite: An FTIR and X-ray Structure Study of Crystals Synthesized at 2–4 GPa. Am. Mineral. 2004, 89, 1422–1432. [Google Scholar] [CrossRef]
- Wilson, R.M.; Elliott, J.C.; Dowker, S.E.P.; Smith, R.I. Rietveld Structure Refinement of Precipitated Carbonate Apatite Using Neutron Diffraction Data. Biomaterials 2004, 25, 2205–2213. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR Studies of Synthetic and Natural Apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef]
- Peeters, A.; de Maeyer, E.A.P.; van Alsenoy, C.; Verbeeck, R.M.H. Solids Modeled by Ab Initio Crystal-Field Methods. 12. Structure, Orientation, and Position of A-Type Carbonate in a Hydroxyapatite Lattice. J. Phys. Chem. B 1997, 101, 3995–3998. [Google Scholar] [CrossRef]
- Peroos, S.; Du, Z.; de Leeuw, N.H. A Computer Modelling Study of the Uptake, Structure and Distribution of Carbonate Defects in Hydroxy-Apatite. Biomaterials 2006, 27, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Lu, X.; Leng, Y. Ab Initio Simulation on the Crystal Structure and Elastic Properties of Carbonated Apatite. J. Mech. Behav. Biomed. Mater. 2013, 26, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ulian, G.; Valdrè, G.; Corno, M.; Ugliengo, P. DFT Investigation of Structural and Vibrational Properties of Type B and Mixed AB Carbonated Hydroxylapatite. Am. Mineral. 2014, 99, 117–127. [Google Scholar] [CrossRef]
- Yang, W.H.; Xi, X.F.; Li, J.F.; Cai, K.Y. Comparison of Crystal Structure between Carbonated Hydroxyapatite and Natural Bone Apatite with Theoretical Calculation. Asian J. Chem. 2013, 25, 3673–3678. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Fomin, A.S.; Murzakhanov, F.F.; Makshakova, O.N.; Donskaya, N.O.; Antonova, O.S.; Gnezdilov, O.I.; Mikheev, I.V.; Knotko, A.V.; Kudryavtsev, E.A.; et al. The Improved Textural Properties, Thermal Stability, and Cytocompatibility of Mesoporous Hydroxyapatite by Mg2+ Doping. Mater. Chem. Phys. 2022, 289, 126461. [Google Scholar] [CrossRef]
- Miake, Y.; Aoba, T.; Shimazu, Y.; Taya, Y.; Soeno, Y.; Sato, K. Fluoride and Apatite Formation in Vivo and in Vitro. J. Electron Microsc. 2003, 52, 615–625. [Google Scholar] [CrossRef]
- Astala, R.; Stott, M.J. First Principles Investigation of Mineral Component of Bone: CO3 Substitutions in Hydroxyapatite. Chem. Mater. 2005, 17, 4125–4133. [Google Scholar] [CrossRef]
- Lamonier, C.; Lamonier, J.-F.O.; Aellach, B.; Ezzamarty, A.; Leglise, J. Specific Tuning of Acid/Base Sites in Apatite Materials to Enhance Their Methanol Thiolation Catalytic Performances. Catal. Today 2010, 164, 124–130. [Google Scholar] [CrossRef]
- Laurencin, D.; Almora-Barrios, N.; de Leeuw, N.H.; Gervais, C.; Bonhomme, C.; Mauri, F.; Chrzanowski, W.; Knowles, J.C.; Newport, R.J.; Wong, A.; et al. Magnesium Incorporation into Hydroxyapatite. Biomaterials 2011, 32, 1826–1837. [Google Scholar] [CrossRef] [Green Version]
- Cacciotti, I.; Bianco, A.; Lombardi, M.; Montanaro, L. Mg-Substituted Hydroxyapatite Nanopowders: Synthesis, Thermal Stability and Sintering Behaviour. J. Eur. Ceram. Soc. 2009, 29, 2969–2978. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Mattioli-Belmonte, M.; Celotti, G.; Sandri, M.; Gigante, A.; Fava, P.; Biagini, G. Biomimetic Mg- and Mg,CO3-Substituted Hydroxyapatites: Synthesis Characterization and in Vitro Behaviour. J. Eur. Ceram. Soc. 2006, 26, 2593–2601. [Google Scholar] [CrossRef]
- Rude, R.K. Magnesium Deficiency: A Cause of Heterogenous Disease in Humans. J. Bone Miner. Res. 1998, 13, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Leng, Y.; Xin, R.; Ge, X. Synthesis, Characterization and Ab Initio Simulation of Magnesium-Substituted Hydroxyapatite. Acta Biomater. 2010, 6, 2787–2796. [Google Scholar] [CrossRef]
- Karunakaran, G.; Cho, E.B.; Kumar, G.S.; Kolesnikov, E.; Janarthanan, G.; Pillai, M.M.; Rajendran, S.; Boobalan, S.; Sudha, K.G.; Rajeshkumar, M.P. Mesoporous Mg-Doped Hydroxyapatite Nanorods Prepared from Bio-Waste Blue Mussel Shells for Implant Applications. Ceram. Int. 2020, 46, 28514–28527. [Google Scholar] [CrossRef]
- Sader, M.S.; Lewis, K.; Soares, G.A.; LeGeros, R.Z. Simultaneous Incorporation of Magnesium and Carbonate in Apatite: Effect on Physico-Chemical Properties. Mater. Res. 2013, 16, 779–784. [Google Scholar] [CrossRef]
- Sprio, S.; Pezzotti, G.; Celotti, G.; Landi, E.; Tampieri, A. Raman and Cathodoluminescence Spectroscopies of Magnesium-Substituted Hydroxyapatite Powders. J. Mater. Res. 2005, 20, 1009–1016. [Google Scholar] [CrossRef]
- Kaygili, O.; Keser, S.; Bulut, N.; Ates, T. Characterization of Mg-Containing Hydroxyapatites Synthesized by Combustion Method. Phys. B Condens. Matter 2018, 537, 63–67. [Google Scholar] [CrossRef]
- Stipniece, L.; Stepanova, V.; Narkevica, I.; Salma-Ancane, K.; Boyd, A.R. Comparative Study of Surface Properties of Mg-Substituted Hydroxyapatite Bioceramic Microspheres. J. Eur. Ceram. Soc. 2018, 38, 761–768. [Google Scholar] [CrossRef]
- Lala, S.; Ghosh, M.; Das, P.K.; Das, D.; Kar, T.; Pradhan, S.K. Magnesium Substitution in Carbonated Hydroxyapatite: Structural and Microstructural Characterization by Rietveld’s Refinement. Mater. Chem. Phys. 2016, 170, 319–329. [Google Scholar] [CrossRef]
- Cüneyt Tas, A. Synthesis of Biomimetic Ca-Hydroxyapatite Powders at 37 °C in Synthetic Body Fluids. Biomaterials 2000, 21, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K. First-Principles Study of Substitutional Magnesium and Zinc in Hydroxyapatite and Octacalcium Phosphate. J. Chem. Phys. 2008, 128, 06B618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gafurov, M.; Biktagirov, T.; Mamin, G.; Klimashina, E.; Putlayev, V.; Kuznetsova, L.; Orlinskii, S. The Interplay of manganese and nitrate in hydroxyapatite nanoparticles as revealed by pulsed EPR and DFT. Phys. Chem. Chem. Phys. 2015, 17, 20331–20337. [Google Scholar] [CrossRef] [PubMed]
- Yashima, M.; Yonehara, Y.; Fujimori, H. Experimental Visualization of Chemical Bonding and Structural Disorder in Hydroxyapatite through Charge and Nuclear-Density Analysis. J. Phys. Chem. C 2011, 115, 25077–25087. [Google Scholar] [CrossRef]
- Biktagirov, T.; Gafurov, M.; Mamin, G.; Klimashina, E.; Putlayev, V.; Orlinskii, S. Combination of EPR Measurements and DFT Calculations to Study Nitrate Impurities in the Carbonated Nanohydroxyapatite. J. Phys. Chem. A 2014, 118, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.A.; Akopyan, A.V.; Gafurov, M.R.; Makshakova, O.N.; Donskaya, N.O.; Fomin, A.S.; Polikarpova, P.P.; Anisimov, A.V.; Murzakhanov, F.F.; Leonov, A.V.; et al. Iron-Doped Mesoporous Powders of Hydroxyapatite as Molybdenum-Impregnated Catalysts for Deep Oxidative Desulfurization of Model Fuel: Synthesis and Experimental and Theoretical Studies. J. Phys. Chem. C 2021, 125, 11604–11619. [Google Scholar] [CrossRef]
- Biktagirov, T.; Gafurov, M.; Iskhakova, K.; Mamin, G.; Orlinskii, S. Phonon Spectrum in Hydroxyapatite: Calculations and EPR Study at Low Temperatures. J. Low Temp. Phys. 2016, 185, 627–632. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, M.; Gafurov, M.; Makshakova, O.; Smirnov, V.; Komlev, V.; Barinov, S.; Kudryavtsev, E.; Sergeeva, N.; Achmedova, S.; Mamin, G.; et al. Influence of Al on the Structure and in Vitro Behavior of Hydroxyapatite Nanopowders. J. Phys. Chem. B 2019, 123, 9143–9154. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, M.A.; Gafurov, M.R.; Murzakhanov, F.F.; Fomin, A.S.; Antonova, O.S.; Khairutdinova, D.R.; Pyataev, A.V.; Makshakova, O.N.; Konovalov, A.A.; Leonov, A.V.; et al. Mesoporous Iron(Iii)-Doped Hydroxyapatite Nanopowders Obtained via Iron Oxalate. Nanomaterials 2021, 11, 811. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft Self-Consistent Pseudopotentials in a Generalized Eigenvalue Formalism. Phys. Rev. B 1990, 41, 7892. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Pair-Distribution Function and Its Coupling-Constant Average for the Spin-Polarized Electron Gas. Phys. Rev. B 1992, 46, 12947–12954. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Andrés, N.C.; D’Elía, N.L.; Ruso, J.M.; Campelo, A.E.; Massheimer, V.L.; Messina, P.V. Manipulation of Mg2+-Ca2+ Switch on the Development of Bone Mimetic Hydroxyapatite. ACS Appl. Mater. Interfaces 2017, 9, 15698–15710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Yokoi, T.; Nakamura, A.; Matsunaga, K. Formation Energies and Site Preference of Substitutional Divalent Cations in Carbonated Apatite. J. Am. Ceram. Soc. 2020, 103, 5354–5364. [Google Scholar] [CrossRef]
- Boivin, G.; Meunier, A.P.J. The mineralization of bone tissue: A forgotten dimension in osteoporosis research. Osteoporos. Int. 2003, 14, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, F.; Mutlu, M.; Mermerkaya, M.U.; Karaoǧlu, S.; Saçmaci, Ş.; Kartal, Ş. Comparison of Bone Tissue Trace-Element Concentrations and Mineral Density in Osteoporotic Femoral Neck Fractures and Osteoarthritis. Clin. Interv. Aging 2014, 9, 1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnell, J.M.; Teubner, E.J.; Miller, A.G. Normal Maturational Changes in Bone Matrix, Mineral, and Crystal Size in the Rat. Calcif. Tissue Int. 1980, 31, 13–19. [Google Scholar] [CrossRef]
- Rey, C.; Renugopalakrishman, V.; Collins, B.; Glimcher, M.J. Fourier Transform Infrared Spectroscopic Study of the Carbonate Ions in Bone Mineral during Aging. Calcif. Tissue Int. 1991, 49, 251–258. [Google Scholar] [CrossRef]
- Rey, C.; Collins, B.; Goehl, T.; Dickson, I.R.; Glimcher, M.J. The Carbonate Environment in Bone Mineral: A Resolution-Enhanced Fourier Transform Infrared Spectroscopy Study. Calcif. Tissue Int. 1989, 45, 157–164. [Google Scholar] [CrossRef]
- Tampieri, A.; Celotti, G.; Landi, E. From Biomimetic Apatites to Biologically Inspired Composites. Anal. Bioanal. Chem. 2005, 381, 568–576. [Google Scholar] [CrossRef] [PubMed]




| Incorporation | ΔE, eV | Cell Volume, Å3 | Mg–O, Å | Ca–O, Å |
|---|---|---|---|---|
| HA | 1068.2 | |||
| Mg in Ca(1) | 0.042 | 1056.9 | 2.19 | 2.48 |
| 2.19 | 2.42 | |||
| 2.16 | 2.39 | |||
| 2.22 | 2.45 | |||
| 2.23 | 2.61 | |||
| 3.02 | 2.52 | |||
| Mg in Ca(2) | 0 | 1059.2 | 2.09 | 2.38 |
| 2.12 | 2.41 | |||
| 2.21 | 2.55 | |||
| 2.1 | 2.36 | |||
| 2.52 | 2.37 | |||
| 2.97 | 2.73 | |||
| 3.75 | 3.49 |
| Incorporation | ΔE, eV | Cell Volume, Å3 | Mg–CO32−, Å | Na–CO32−, Å |
|---|---|---|---|---|
| CO32− in A | - | 1082.2 | - | - |
| Mg in Ca(1) | −0.005 | 1072.0 | 5.07 | - |
| Mg in Ca(2) | −0.308 | 1082.6 | 1.98 | - |
| CO32− in B, Na in Ca(1) | - | 1062.2 | - | 4.08 |
| Mg in Ca(1) | −0.076 | 1052.6 | 2.15 | 4.06 |
| Mg in Ca(2) | −0.037 | 1056.2 | 2.25 | 3.87 |
| CO32− in B, Na in Ca(2) | - | 1060.2 | - | 2.86 |
| Mg in Ca(1) | −0.055 | 1049.4 | 2.31 | 2.74 |
| Mg in Ca(2) | −0.045 | 1053.1 | 2.12 | 2.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makshakova, O.N.; Gafurov, M.R.; Goldberg, M.A. The Mutual Incorporation of Mg2+ and CO32− into Hydroxyapatite: A DFT Study. Materials 2022, 15, 9046. https://doi.org/10.3390/ma15249046
Makshakova ON, Gafurov MR, Goldberg MA. The Mutual Incorporation of Mg2+ and CO32− into Hydroxyapatite: A DFT Study. Materials. 2022; 15(24):9046. https://doi.org/10.3390/ma15249046
Chicago/Turabian StyleMakshakova, Olga N., Marat R. Gafurov, and Margarita A. Goldberg. 2022. "The Mutual Incorporation of Mg2+ and CO32− into Hydroxyapatite: A DFT Study" Materials 15, no. 24: 9046. https://doi.org/10.3390/ma15249046