Effect of Modified Magnesium Oxide on the Properties of Magnesium Phosphate Cement under a Negative Temperature Environment
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Materials
2.2. Mixture Design and Specimen Preparation
2.3. Analysis and Test Methods
3. Results and Analysis
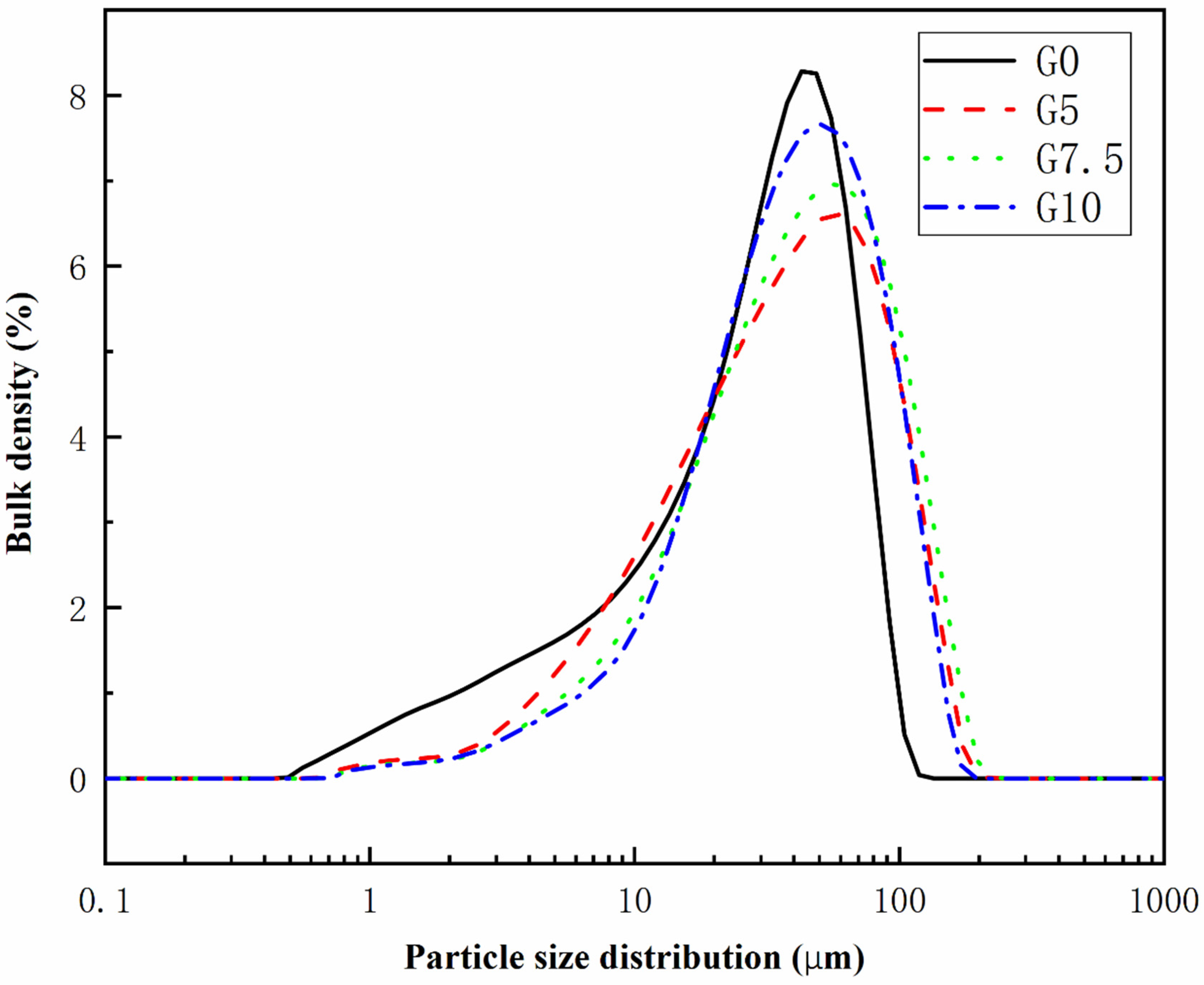
3.1. Modification of MgO
3.2. Working Performance
3.3. Mechanical Properties
3.4. Effect of Modified MgO on the Hydration Characteristics of MPC at Negative Temperature
3.5. Effect of Modified MgO on the Microstructure of MPC at Negative Temperature
3.6. Effect of Modified MgO on the Pore Structure of MPC at Negative Temperature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chau, C.; Qiao, F.; Li, Z. Microstructure of magnesium potassium phosphate cement. Constr. Build. Mater. 2011, 25, 2911–2917. [Google Scholar] [CrossRef]
- Torras, J.; Buj, I.; Rovira, M.; de Pablo, J. Semi-dynamic leaching tests of nickel containing wastes stabilized/solidified with magnesium potassium phosphate cements. J. Hazard. Mater. 2011, 186, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Qiao, F.; Chau, C.; Li, Z. Property evaluation of magnesium phosphate cement mortar as patch repair material. Constr. Build. Mater. 2010, 24, 695–700. [Google Scholar] [CrossRef]
- Hall, D.A.; Stevens, R.; El-Jazairi, B. The effect of retarders on the microstructure and mechanical properties of magnesia–phosphate cement mortar. Cem. Concr. Res. 2001, 31, 455–465. [Google Scholar] [CrossRef]
- Whiting, D.; Nagi, M.; Okamoto, P.; Yu, T.; Peshkin, D.; Smith, K.; Darter, M.; Clifton, J.; Kaetzel, L. Optimization of Highway Concrete Technology; National Academy of Sciences: Washington, DC, USA, 1994. [Google Scholar]
- Walling, S.A.; Provis, J.L. Magnesia-based cements: A journey of 150 years, and cements for the future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, M.A.; Chen, B. Research progresses on magnesium phosphate cement: A review. Constr. Build. Mater. 2019, 211, 885–898. [Google Scholar] [CrossRef]
- Ma, H.; Xu, B.; Li, Z. Magnesium potassium phosphate cement paste: Degree of reaction, porosity and pore structure. Cem. Concr. Res. 2014, 65, 96–104. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Hou, D.; Wang, Z. Nanoscale insight on the initial hydration mechanism of magnesium phosphate cement. Constr. Build. Mater. 2021, 276, 122213. [Google Scholar] [CrossRef]
- Qi, T.; Yan, W. Mechanical properties and frost resistance of magnesium phosphate cement concrete under negative temperature. J. Glaciol. Geocryol. 2018, 40, 1181–1186. [Google Scholar]
- Buj, I.; Torras, J.; Rovira, M.; de Pablo, J. Leaching behaviour of magnesium phosphate cements containing high quantities of heavy metals. J. Hazard. Mater. 2010, 175, 789–794. [Google Scholar] [CrossRef]
- Yu, B.; Zhou, J.; Cheng, B.; Yang, W. Compressive strength development and microstructure of magnesium phosphate cement concrete. Constr. Build. Mater. 2021, 283, 122585. [Google Scholar] [CrossRef]
- Mahjoory, M.; Shahgholi, M.; Karimipour, A. The effects of initial temperature and pressure on the mechanical properties of reinforced calcium phosphate cement with magnesium nanoparticles: A molecular dynamics approach. Int. Commun. Heat Mass Transf. 2022, 135, 106067. [Google Scholar] [CrossRef]
- Fada, R.; Shahgholi, M.; Karimian, M. Improving the mechanical properties of strontium nitrate doped dicalcium phosphate cement nanoparticles for bone repair application. Ceram. Int. 2021, 47, 14151–14159. [Google Scholar] [CrossRef]
- Chen, B.; Oderji, S.Y.; Chandan, S.; Fan, S. Feasibility of Magnesium Phosphate Cement (MPC) as a repair material for ballastless track slab. Constr. Build. Mater. 2017, 154, 270–274. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, J.; Li, T.; Wang, Y.; Liu, Y.; De Backer, H. Feasibility analysis of magnesium phosphate cement as a repair material for base slab of China railway track system II ballastless track. Constr. Build. Mater. 2022, 326, 126821. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Tian, C.; Li, X.; Tan, Y. Effect of Aging on the Viscoelastic Mechanical Properties of Cement/Emulsified Asphalt Composite Repair Material. J. Test. Eval. 2022, 50, 5. [Google Scholar] [CrossRef]
- Ghahari, S.; Ramezanianpour, A.; Ramezanianpour, A.; Esmaeili, M. An accelerated test method of simultaneous carbonation and chloride ion ingress: Durability of silica fume concrete in severe environments. Adv. Mater. Sci. Eng. 2016, 2016, 1650979. [Google Scholar] [CrossRef] [Green Version]
- Arora, A.; Singh, B.; Kaur, P. Novel material ie magnesium phosphate cement (MPC) as repairing material in roads and buildings. Mater. Today Proc. 2019, 17, 70–76. [Google Scholar] [CrossRef]
- Liu, F.; Pan, B.; Cao, P.; Zhou, C. Multi-scale characterization on behaviors of the interface between magnesium phosphate cement and portland cement. Constr. Build. Mater. 2021, 275, 122139. [Google Scholar] [CrossRef]
- Yi, H.; Yi, Z.; Lu, J.; Li, D. Failure Mechanism of Magnesia-phosphate Cement under Low Temperature Curing Condition. Bull. Chin. Ceram. Soc 2014, 33, 197–201. [Google Scholar]
- Jia, X.; Luo, J.; Zhang, W.; Qian, J.; Li, J.; Wang, P.; Tang, M. Preparation and Application of Self-Curing Magnesium Phosphate Cement Concrete with High Early Strength in Severe Cold Environments. Materials 2020, 13, 5587. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Qian, J.; Qin, J.; Wang, H.; Wang, Q.; Ye, Z. Effect of early hydration temperature on hydration product and strength development of magnesium phosphate cement (MPC). Cem. Concr. Res. 2015, 78, 179–189. [Google Scholar] [CrossRef]
- Jia, X.-W.; Luo, J.-Y.; Zhang, W.-X.; Tang, M.-H.; Qian, J.-S.; Wang, P.; Li, J.-M. Reaction characteristics and compressive strength of magnesia-phosphate cement at negative temperatures. Constr. Build. Mater. 2021, 305, 124819. [Google Scholar] [CrossRef]
- Liu, R.; Yang, Y.; Sun, S. Effect of M/P and borax on the hydration properties of magnesium potassium phosphate cement blended with large volume of fly ash. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2018, 33, 1159–1167. [Google Scholar] [CrossRef]
- Shi, C.; Yang, J.; Yang, N.; Chang, Y. Effect of waterglass on water stability of potassium magnesium phosphate cement paste. Cem. Concr. Compos. 2014, 53, 83–87. [Google Scholar] [CrossRef]
- Liu, M.; Lai, Z.; Deng, Q.; Xiao, R.; Liu, Z.; Luo, X.; Lu, Z.; Lv, S. Surface modification of magnesium oxide and its effect on the performance of magnesium phosphate cement. Constr. Build. Mater. 2022, 316, 125880. [Google Scholar] [CrossRef]
- Wang, H.; Darter, M.; Zhang, J.; Qi, T. Study on the influent factors of magnesium phosphate cement hydration heat. J. Glaciol. 2015, 22, 22098–22102. [Google Scholar]
- Zhang, S.; Shi, H.-S.; Huang, S.-W.; Zhang, P. Dehydration characteristics of struvite-K pertaining to magnesium potassium phosphate cement system in non-isothermal condition. J. Therm. Anal. Calorim. 2013, 111, 35–40. [Google Scholar] [CrossRef]
- Chauhan, C.; Vyas, P.; Joshi, M. Growth and characterization of Struvite-K crystals. Cryst. Res. Technol. 2011, 46, 187–194. [Google Scholar] [CrossRef]
- Lahalle, H.; Coumes, C.C.D.; Mesbah, A.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Investigation of magnesium phosphate cement hydration in diluted suspension and its retardation by boric acid. Cem. Concr. Res. 2016, 87, 77–86. [Google Scholar] [CrossRef]













| MgO | SiO2 | CaO | P2O5 | Fe2O3 | Al2O3 | Others |
|---|---|---|---|---|---|---|
| 95.12 | 1.76 | 1.04 | 0.83 | 0.56 | 0.49 | 0.2 |
| Series | W/M Ratio (wt%) | Thermal Treatment Temperature | Thermal Treatment Time | M/P a | B/M b | w/s c |
|---|---|---|---|---|---|---|
| G0 | 0 | — | — | |||
| G5 | 5 | 150 °C | 2 h | 3:1 | 0.1 | 0.2 |
| G7.5 | 7.5 | |||||
| G10 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Lai, Z.; Liu, Z.; Xiao, R.; Chen, J.; Lu, Z.; Lv, S.; Wang, J. Effect of Modified Magnesium Oxide on the Properties of Magnesium Phosphate Cement under a Negative Temperature Environment. Materials 2022, 15, 9047. https://doi.org/10.3390/ma15249047
Luo X, Lai Z, Liu Z, Xiao R, Chen J, Lu Z, Lv S, Wang J. Effect of Modified Magnesium Oxide on the Properties of Magnesium Phosphate Cement under a Negative Temperature Environment. Materials. 2022; 15(24):9047. https://doi.org/10.3390/ma15249047
Chicago/Turabian StyleLuo, Xuanzhang, Zhenyu Lai, Zhi Liu, Rui Xiao, Jiawei Chen, Zhongyuan Lu, Shuzhen Lv, and Jin Wang. 2022. "Effect of Modified Magnesium Oxide on the Properties of Magnesium Phosphate Cement under a Negative Temperature Environment" Materials 15, no. 24: 9047. https://doi.org/10.3390/ma15249047






