Application of Ion Exchangers with the N-Methyl-D-Glucamine Groups in the V(V) Ions Adsorption Process
Abstract
:1. Introduction
2. Materials and Methods
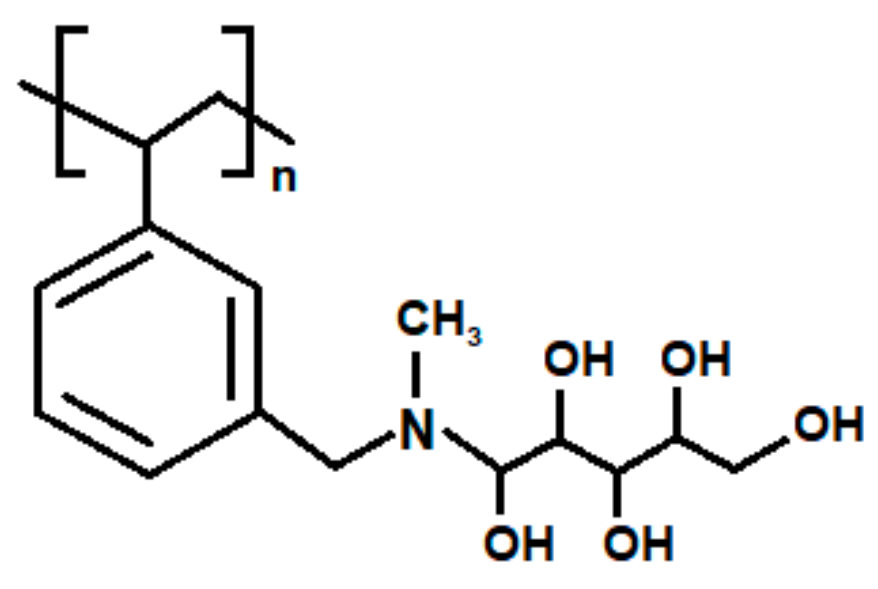
2.1. Ion Exchangers
- Amberlite IRA 743—a polystyrene-divinylbezene macroporous matrix (ST-DVB) with N-methyl-D-glucamine (NMDG) groups, strongly basic, bead size 0.5–0.7 mm, total exchange capacity 0.7 meq/mL, water content 48%–54%, maximum temperature operation 348 K, pH range 0–14,
- Lewatit MK 51—a polystyrene-divinylbezene macroporous matrix (ST-DVB) with N-methyl-D-glucamine (NMDG) groups, strongly basic, bead size 0.3–1.2 mm, total exchange capacity 0.9 meq/mL, water content 40%–50%, maximum temperature operation 333 K, pH range 3–10,
- Purolite S 110—a polystyrene-divinylbezene macroporous matrix (ST-DVB) with N-methyl-D-glucamine (NMDG) groups, strongly basic, bead size 0.5–0.7 mm, total exchange capacity 0.8 meq/mL, water content 40%–50%, maximum temperature operation 333 K, pH range 0–14,
- Purolite S 108—a polystyrene-divinylbezene macroporous matrix (ST-DVB) with N-methyl-D-glucamine (NMDG) groups, strongly basic, bead size 0.4–0.6 mm, total exchange capacity 0.6 meq/mL, water content 48%–55%, maximum temperature operation 333 K, pH range 0–14.
2.2. Experimental Conditions
2.3. Column Studies
2.4. Instruments
3. Results and Discussion
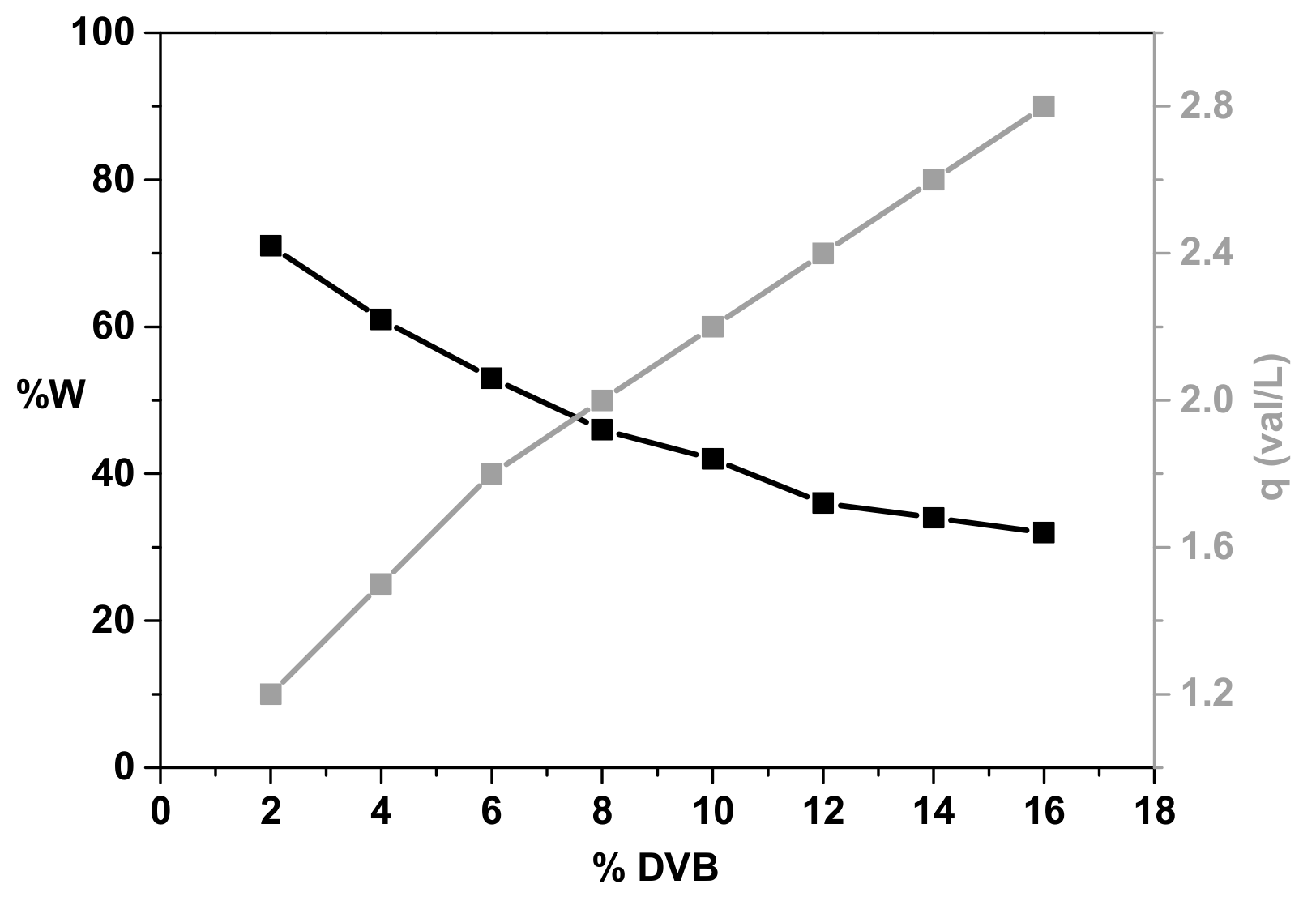
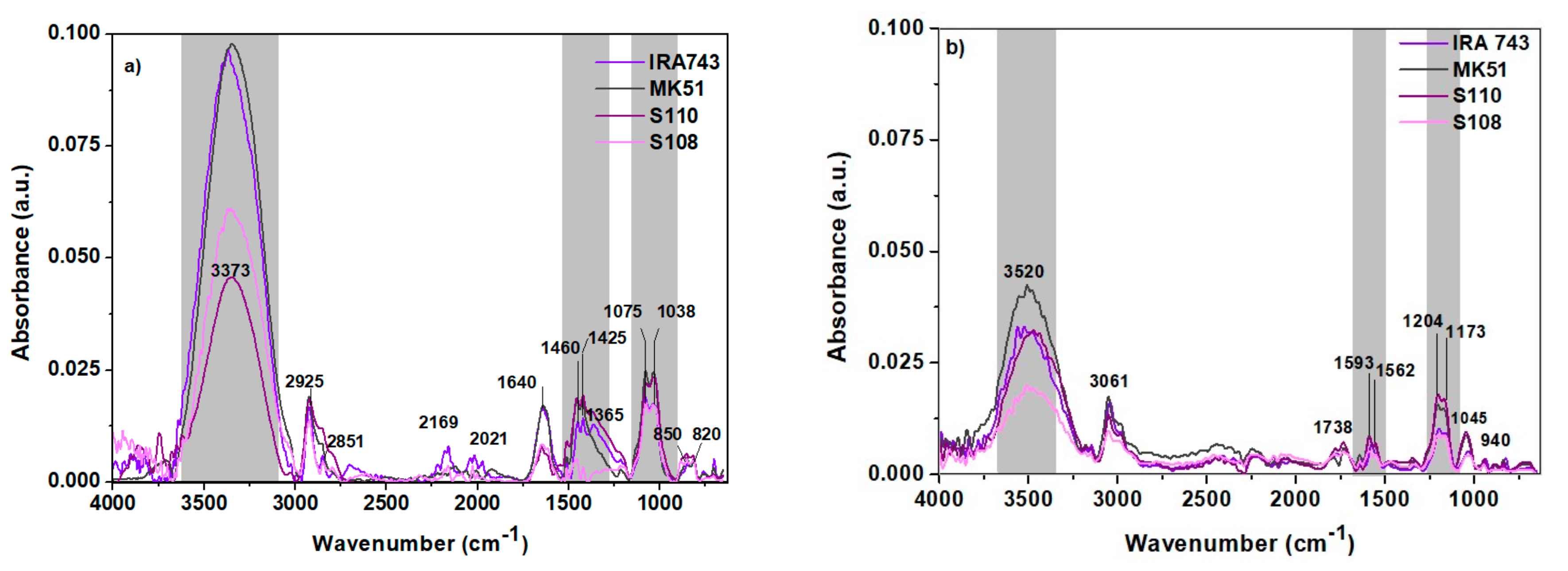
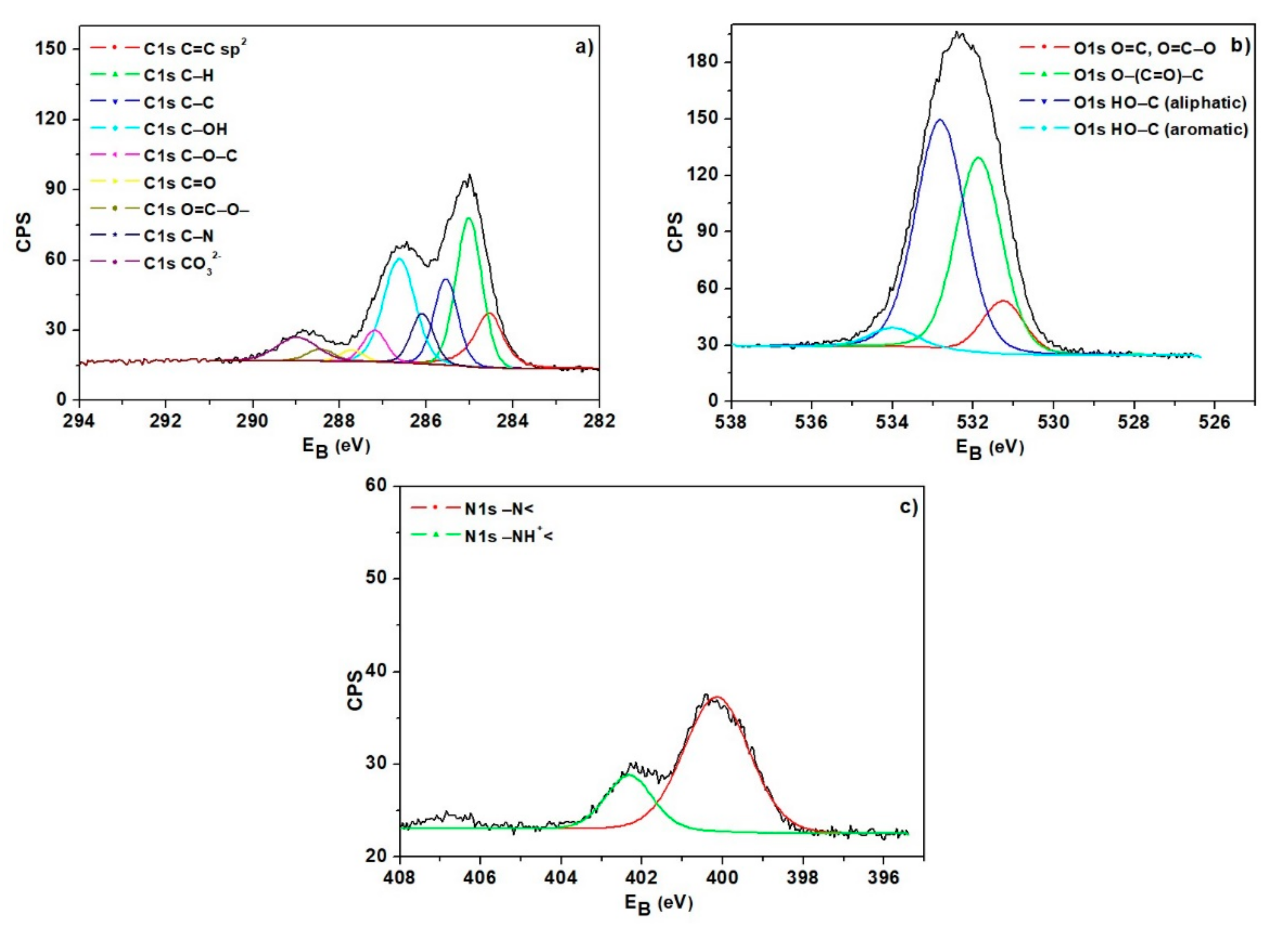
3.1. Ion Exchanger Characteristics
3.2. Adsorption Studies—Static Method
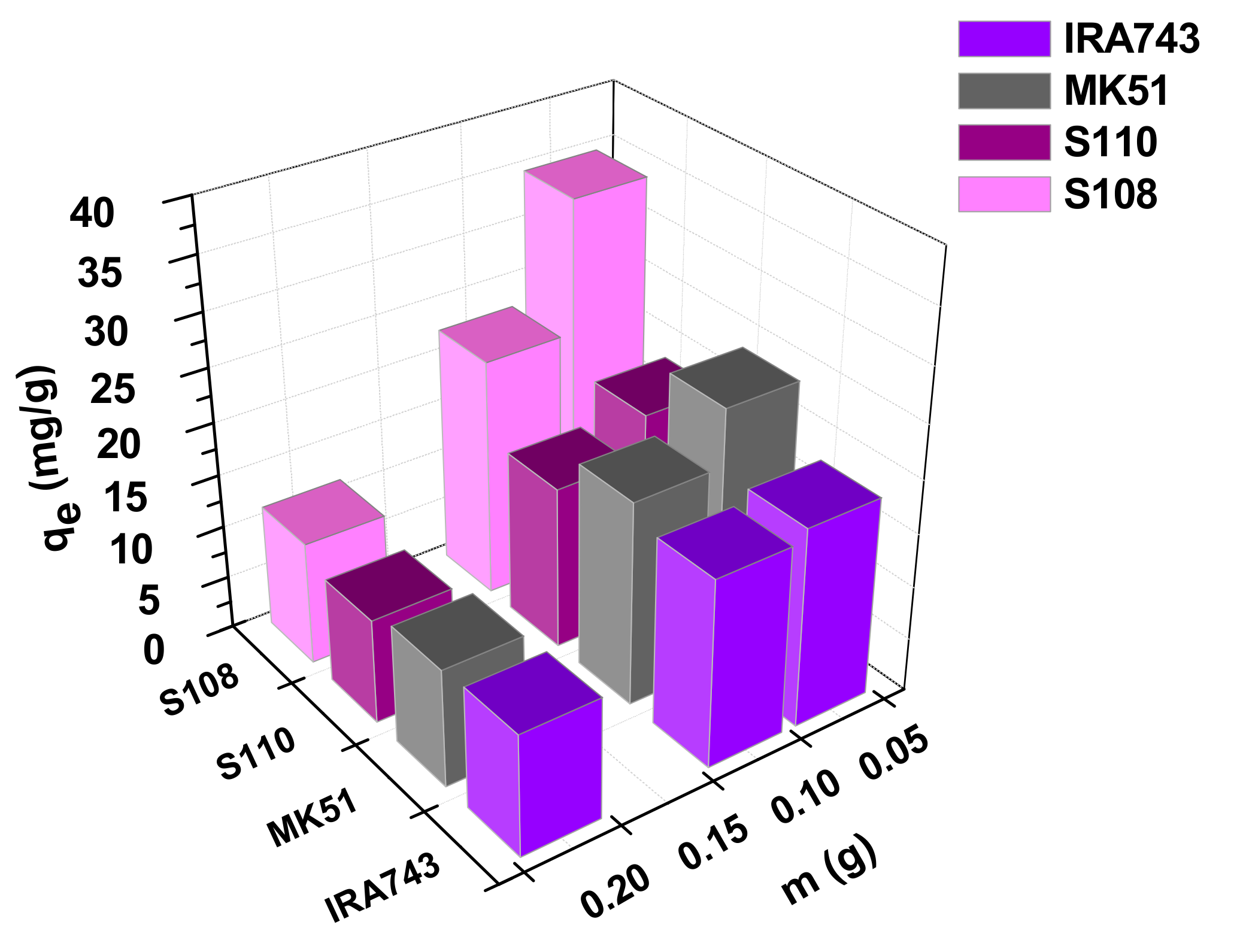
3.2.1. Effect of Adsorbent Mass
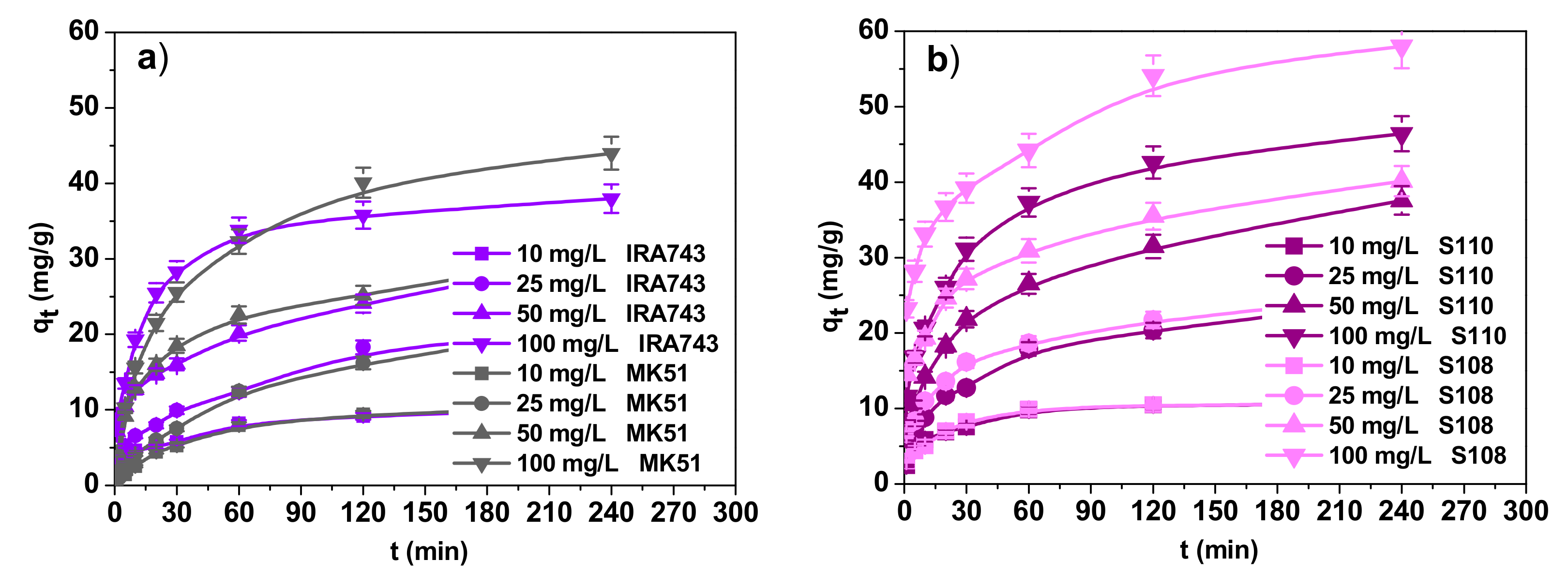
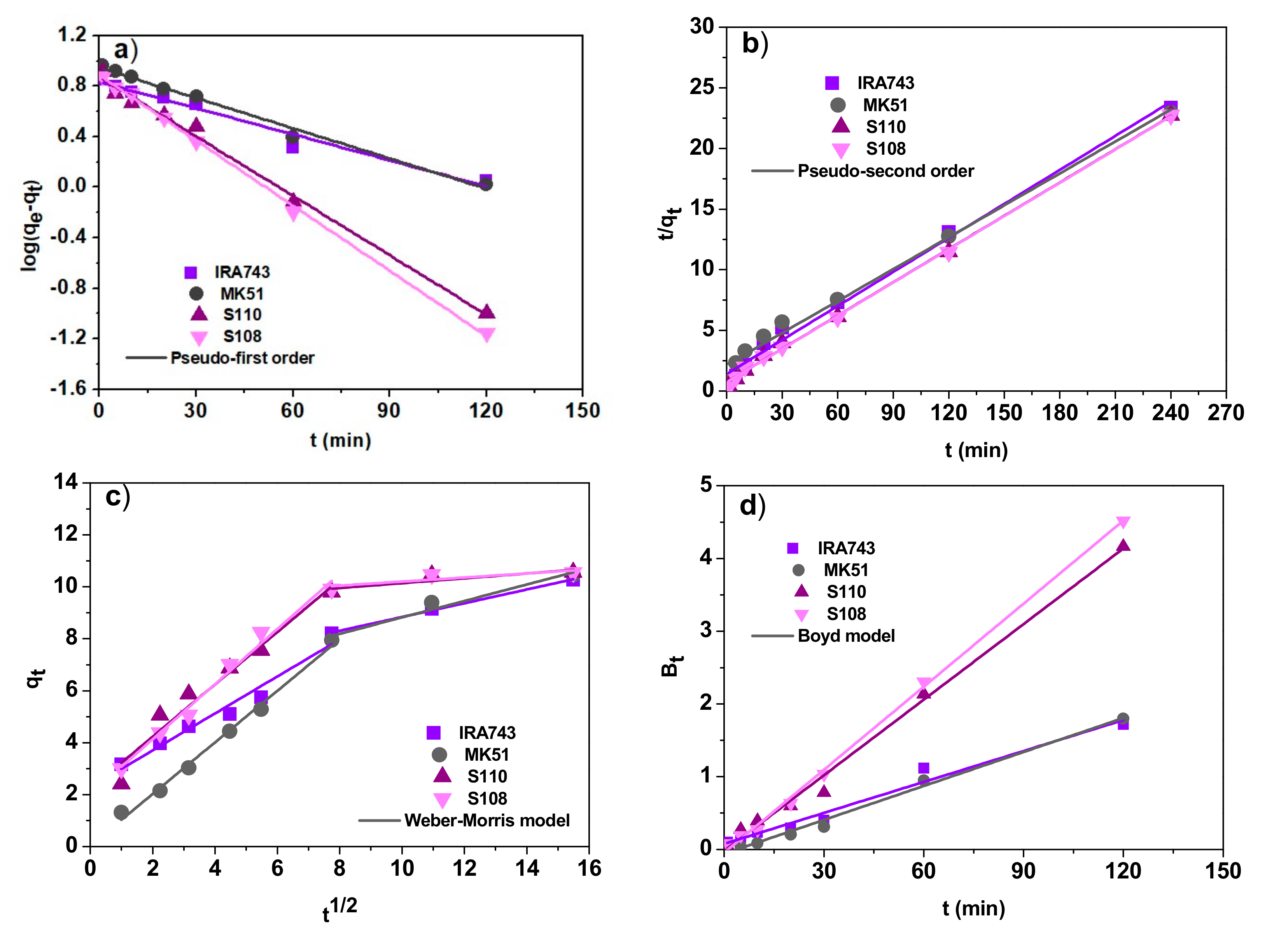
3.2.2. Effect of Contact Time and the Kinetic Studies
- The pseudo-first order model [17]:
- The pseudo-second order model [18]:
- The Weber–Morris (the intraparticle diffusion) model [19]:
- The Boyd model [20]:where: qe is the amount of V(V) ions adsorbed per amount of ion exchanger at equilibrium (mg/g), qt is the amount of V(V) adsorbed at time t (mg/g), k1 is the pseudo-first order rate constant (1/min), k2 is the pseudo-second order rate constant (g/mg min), ki is the intraparticle diffusion rate constant (mg/g min1/2), C is the intercept called the Weber–Morris diffusion constant, F is the fraction of solute adsorbed at time t (F = qt/qe).
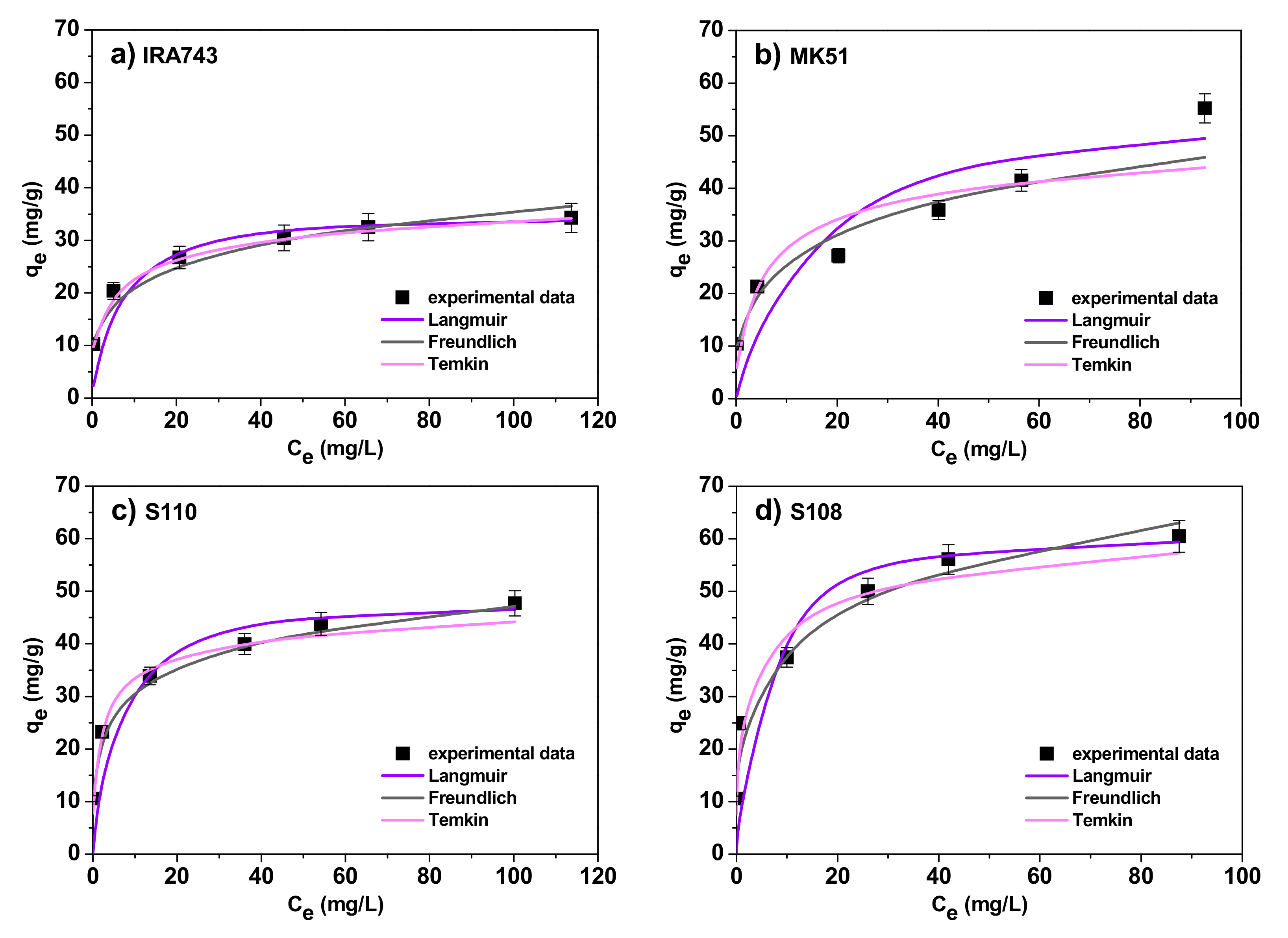
3.2.3. Adsorption Isotherms
- The Langmuir model [26]:
- The Freundlich model [27]:
- The Temkin model [28]:where qe, Ce are defined as previously, q0 is the maximum amount of V(V) ions required to form a monolayer on the surface (mg/g), KL is the Langmuir constant related to the affinity for the binding sites (L/mg), KF is the Freundlich adsorption capacity (mg/g), n is the Freundlich constant, A is the Temkin constant related to the maximum binding energy (L/g), B is the Temkin constant related to the adsorption heat (J/mol) and it can be expressed as B = RT/bT where R is the gas constant (8.314 J/mol K), T is the absolute temperature (K) and bT is a Temkin isotherm constant.
3.2.4. Thermodynamic Study
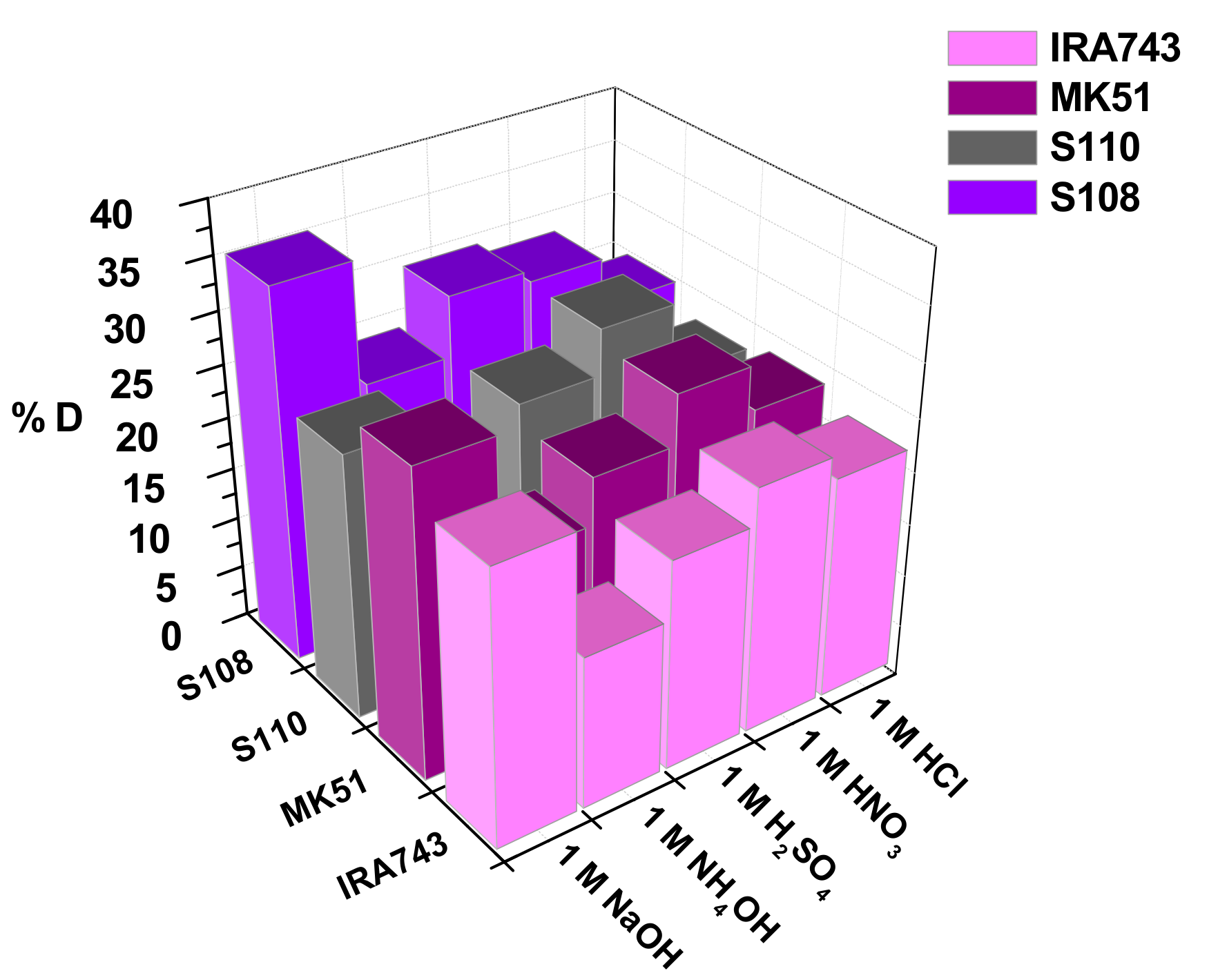
3.2.5. Effect of Interfering Ions
3.2.6. Desorption Studies
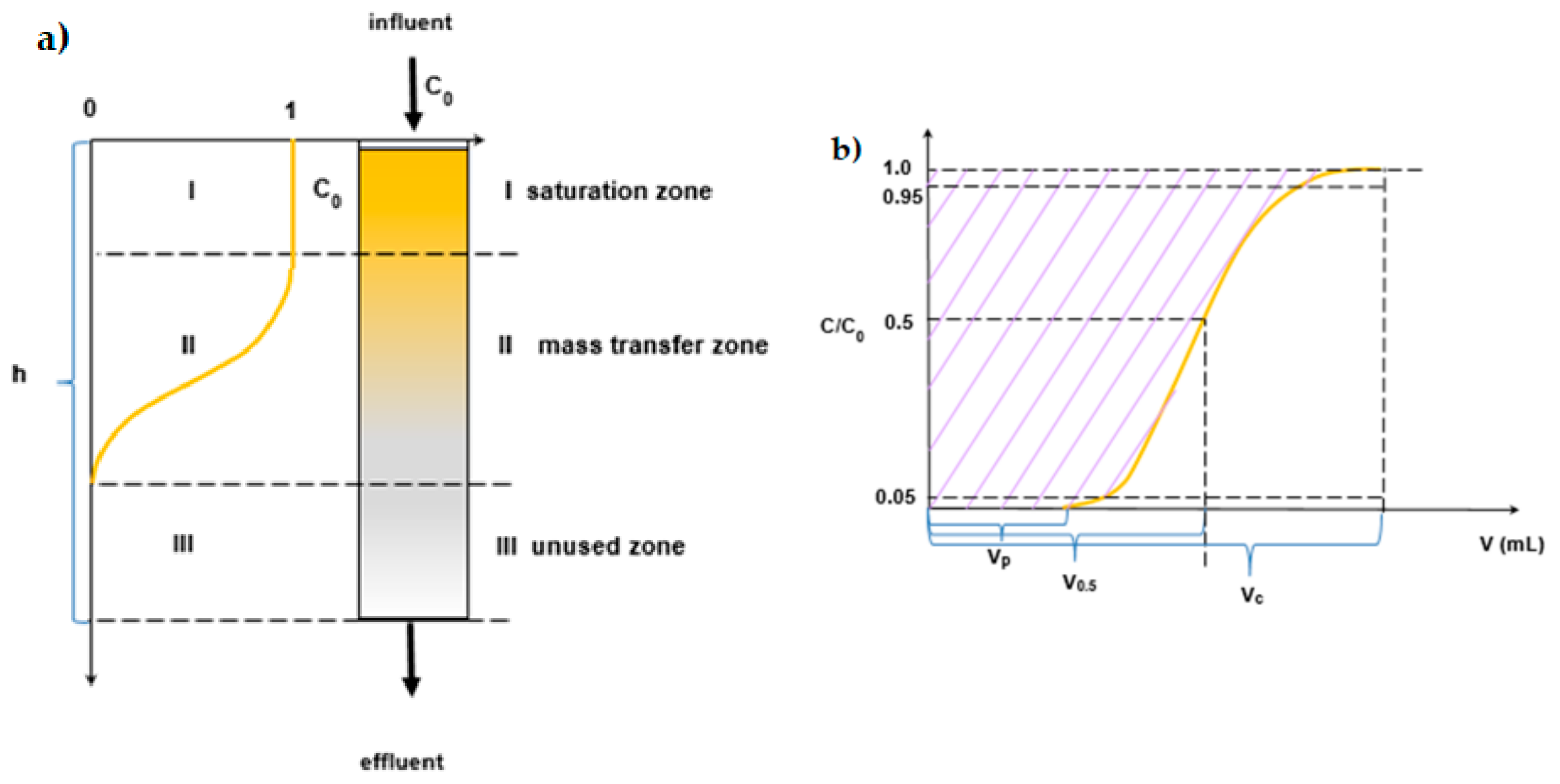
3.3. Column Studies
- Time required for the exchange zone formation (min):
- Time required for the exchange zone to become established (min):
- Rate at which the exchange zone is moving through the bed (cm/min):
- Height of exchange zone (cm):
- Fraction of the adsorbate present in the adsorption zone:where: VE is the total volume to the point of exhaustion (mL), VB is the total volume to the point of breakthrough (mL), Q is the flow rate (mL/min), h is the total bed depth (cm), tf is the time required for the exchange zone to initially form tf = (1 − F)tz (min), Sz is the amount of solute removed by the adsorption zone from the breakthrough to exhaustion and Smax is the amount of solute removed by the adsorption zone which is completely exhausted.
- The working ion exchange capacity:
- The capacity at the exhaustion point:
- The capacity from the breakthrough point to the exhaustion point:where: VB and VE are defined as previously, VEB is the volume of the eluate from the breakthrough point to the exhaustion point (mL), c0 is the initial V(V) ions concentration (mg/L) and Vj is the volume of the ion exchanger in the column (mL).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudnick, R.L.; Gao, S. Composition of the continental crust. Treatise Geochem. 2003, 3, 1–64. [Google Scholar]
- Zhang, R.; Leiviskä, T. Surface modification of pine bark with quaternary ammonium groups and its use for vanadium removal. Chem. Eng. J. 2020, 385, 123967. [Google Scholar] [CrossRef]
- Ghanim, B.; Murnane, J.G.; O’Donoghue, L.; Courtney, R.; Pembroke, J.T.; O’Dwyer, T.F. Removal of vanadium from aqueous solution using a red mud modified saw dust biochar. J. Water Process Eng. 2020, 33, 101076. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, M.; Yang, J. Characteristics of vanadium adsorption on and desorption from humic acid. Chem. Ecol. 2018, 34, 548–564. [Google Scholar] [CrossRef]
- Zhu, H.; Xiao, X.; Guo, Z.; Han, X.; Liang, Y.; Zhang, Y.; Zhou, C. Adsorption of vanadium(V) on natural kaolinite and montmorillonite: Characteristics and mechanism. Appl. Clay Sci. 2018, 161, 310–316. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Xia, W.; Zhang, W. Preparation and characterization of chitosan-zirconium(IV) composite for adsorption of vanadium(V). Int. J. Biol. Macromol. 2014, 64, 155–161. [Google Scholar] [CrossRef]
- Chen, G.; Liu, H. Understanding the reduction kinetics of aqueous vanadium(V) and transformation products using rotating ring-disk electrodes. Environ. Sci. Technol. 2017, 51, 11643–11651. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.; Hassan, H.M.A.; El-Shahat, M.F.; El Shahawy, O.; Awual, M.R. A ligand-anchored optical composite material for efficient vanadium(II) adsorption and detection in wastewater. New J. Chem. 2019, 43, 10324–10335. [Google Scholar] [CrossRef]
- Leiviskä, T.; Khalid, M.K.; Sarpola, A.; Tanskanen, J. Removal of vanadium from industrial wastewater using iron sorbents in batch and continuous flow pilot systems. J. Environ. Manag. 2017, 190, 231–242. [Google Scholar] [CrossRef]
- Guzmán, J.; Saucedo, I.; Navarro, R.; Revilla, J.; Guibal, E. Vanadium interactions with chitosan: Influence of polymer protonation and metal speciation. Langmuir 2002, 18, 1567–1573. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Aydın, S.; Güneysu, S. Adsorption isotherms and kinetics of vanadium by shale and coal waste. Adsorpt. Sci. Technol. 2018, 36, 936–952. [Google Scholar] [CrossRef]
- Padilla-Rodríguez, A.; Hernández-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Perales-Pérez, O.; Román-Velázquez, F.R. Synthesis of protonated chitosan flakes for the removal of vanadium(III, IV and V) oxyanions from aqueous solutions. Microchem. J. 2015, 118, 1–11. [Google Scholar] [CrossRef]
- Naeem, A.; Westerhoff, P.; Mustafa, S. Vanadium removal by metal (hydr)oxide adsorbents. Water Res. 2007, 41, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Keränen, A.; Leiviskä, T.; Salakka, A.; Tanskanen, J. Removal of nickel and vanadium from ammoniacal industrial wastewater by ion exchange and adsorption on activated carbon. Desalin. Water Treat. 2015, 53, 2645–2654. [Google Scholar] [CrossRef]
- Helfferich, F. Ion Exchange; McGraw-Hill: New York, USA, 1962. [Google Scholar]
- Alghamdi, A.A.; Al-Odayni, A.B.; Saeed, W.S.; Al-Kahtani, A.; Alharthi, F.A.; Aouak, T. Efficient adsorption of lead(II) from aqueous phase solutions using polypyrrole-based activated carbon. Materials 2019, 12, 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The exchange adsorption of ions from aqueous solutions by organic zeolites II. Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Staroń, P.; Chwastowski, J.; Banach, M. Sorption and desorption studies on silver ions from aqueous solution by coconut fiber. J. Clean. Prod. 2017, 149, 290–301. [Google Scholar] [CrossRef]
- Yousef, R.I.; El-Eswed, B.; Al-Muhtaseb, A.H. Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies. Chem. Eng. J. 2011, 171, 1143–1149. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Babaeivelni, K.; Khodadoust, A.P.; Bogdan, D. Adsorption and removal of arsenic(V) using crystalline manganese(II,III) oxide: Kinetics, equilibrium, effect of pH and ionic strength. J. Environ. Sci. Health Part A Toxic/Hazardous Subst. Environ. Eng. 2014, 49, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M. Synthesis and characterization of magnetic nanocomposite of chitosan/SiO2/carbon nanotubes and its application for dyes removal. J. Clean. Prod. 2017, 145, 105–113. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Temkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physicochim. URSS 1940, 12, 327–356. [Google Scholar]
- Hena, S. Removal of chromium hexavalent ion from aqueous solutions using biopolymer chitosan coated with poly 3-methyl thiophene polymer. J. Hazard. Mater. 2010, 181, 474–479. [Google Scholar] [CrossRef]
- Can, N.; Ömür, B.C.; Altındal, A. Modeling of heavy metal ion adsorption isotherms onto metallophthalocyanine film. Sens. Actuators B Chem. 2016, 237, 953–961. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Padmesh, T.V.N.; Palanivelu, K.; Velan, M. Biosorption of nickel(II) ions onto Sargassum wightii: Application of two-parameter and three-parameter isotherm models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, L.; Mao, J.; Liu, S.; Huang, J.; You, Y.; Mei, L. Kinetic and thermodynamic studies of sulforaphane adsorption on macroporous resin. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1028, 231–236. [Google Scholar]
- Mahmoud, M.E.; Nabil, G.M.; El-Mallah, N.M.; Bassiouny, H.I.; Kumar, S.; Abdel-Fattah, T.M. Kinetics, isotherm, and thermodynamic studies of the adsorption of reactive red 195 A dye from water by modified Switchgrass Biochar adsorbent. J. Ind. Eng. Chem. 2016, 37, 156–167. [Google Scholar] [CrossRef]
- Li, M.; Zhang, B.; Zou, S.; Liu, Q.; Yang, M. Highly selective adsorption of vanadium(V) by nano-hydrous zirconium oxide-modified anion exchange resin. J. Hazard. Mater. 2020, 384, 121386. [Google Scholar] [CrossRef] [PubMed]






| Ion Exchanger | V (mL) | H (cm) | Q (mL/min) | C0 (mg/L) |
|---|---|---|---|---|
| IRA73 | 10 or 100 | 12 or 25 | 0.6 or 6 | 50 or 100 |
| MK51 | 10 | 12 | 0.6 or 6 | 50 or 100 |
| S110 | 10 | 13 | 0.6 or 6 | 50 or 100 |
| S108 | 10 | 12 | 0.6 or 6 | 50 or 100 |
| Properties | IRA743 | MK51 | S110 | S108 |
|---|---|---|---|---|
| Manufacturer | Dow | Lanxess | Purolite | Purolite |
| Size (mm) | 0.5–0.7 | 0.3–1.2 | 0.5–0.7 | 0.4–0.6 |
| pH range | 0–14 | 3–10 | 0–14 | 0–14 |
| Max temp. (°C) | 75 | 60 | 60 | 60 |
| Total capacity (eq/L) | 0.7 | 0.9 | 0.8 | 0.6 |
| Applications | Removal of boric acid and borates from water based on the formation of a strong complex between the borate anion and the N-methylglucamine ligand | Softening, demineralization, decarbonization of industrial water and removal of nitrates from water | Chromatographic separation in pharmacology and biotechnology, catalysis (including catalytic oxygen removal) | Food industry, production of fruit juices, whey, glycerin, gelatin and sugar, evolution of boron from magnesium chloride solutions |
| Ion Exchanger | IRA743 | MK51 | S110 | S108 |
|---|---|---|---|---|
| %water | 27.8 | 24.5 | 29.7 | 28.1 |
| %dry mass | 72.2 | 75.5 | 70.3 | 71.2 |
| Ion Exchanger | SBET (m2/g) | Vp (mL/g) | Vmicro (mL/g) | Dp (nm) |
|---|---|---|---|---|
| IRA743 | 14.5 | 0.09 | 0.005 | 49.6 |
| MK51 | 10.3 | 0.08 | 0.003 | 28.5 |
| S110 | 12.3 | 0.07 | 0.002 | 27.8 |
| S108 | 11.4 | 0.06 | 0.004 | 32.1 |
| Ion Exchanger | pHPZC |
|---|---|
| IRA743 | 8.0 |
| MK51 | 6.9 |
| S110 | 7.8 |
| S108 | 7.7 |
| Model | Pseudo-First Order | Pseudo-Second Order | |||||||
| Ion Exchanger | C0 (mg/L) | qe.exp (mg/g) | q1.cal (mg/g) | k1 (1/min) | R2 | q2.cal (mg/g) | k2 (g/mg min) | R2 | |
| IRA743 | 10 | 10.27 | 6.80 | 0.016 | 0.971 | 10.69 | 0.0062 | 0.991 | |
| 25 | 20.41 | 17.33 | 0.017 | 0.977 | 22.08 | 0.0017 | 0.975 | ||
| 50 | 30.43 | 20.52 | 0.010 | 0.968 | 31.28 | 0.0017 | 0.978 | ||
| 100 | 38.00 | 22.58 | 0.022 | 0.927 | 39.22 | 0.0027 | 0.999 | ||
| MK51 | 10 | 10.45 | 9.38 | 0.019 | 0.986 | 11.96 | 0.0025 | 0.998 | |
| 25 | 21.28 | 19.44 | 0.012 | 0.989 | 25.02 | 0.0008 | 0.973 | ||
| 50 | 30.88 | 21.50 | 0.012 | 0.882 | 32.35 | 0.0015 | 0.992 | ||
| 100 | 44.00 | 35.57 | 0.019 | 0.990 | 47.26 | 0.0010 | 0.996 | ||
| S110 | 10 | 10.56 | 7.46 | 0.036 | 0.994 | 10.94 | 0.0113 | 0.998 | |
| 25 | 24.28 | 17.19 | 0.013 | 0.967 | 25.38 | 0.0021 | 0.987 | ||
| 50 | 37.58 | 26.56 | 0.013 | 0.968 | 39.18 | 0.0014 | 0.988 | ||
| 100 | 46.40 | 30.65 | 0.018 | 0.980 | 48.20 | 0.0016 | 0.996 | ||
| S108 | 10 | 10.55 | 7.68 | 0.040 | 0.999 | 10.95 | 0.0115 | 0.999 | |
| 25 | 24.93 | 16.11 | 0.014 | 0.963 | 25.75 | 0.0027 | 0.993 | ||
| 50 | 40.13 | 23.01 | 0.014 | 0.966 | 41.06 | 0.0021 | 0.994 | ||
| 100 | 58.00 | 32.46 | 0.017 | 0.982 | 59.48 | 0.0016 | 0.994 | ||
| Weber–Morris Intraparticle Diffusion | Boyd | ||||||||
| ki1 (mg/g min1/2) | C1 | R2 | ki2 (mg/g min1/2) | C2 | R2 | Bt | R2 | ||
| IRA743 | 10 | 0.71 | 2.28 | 0.965 | 0.26 | 6.19 | 0.998 | 1.718 | 0.975 |
| 25 | 1.32 | 2.29 | 0.995 | 1.00 | 5.60 | 0.873 | 1.764 | 0.947 | |
| 50 | 1.55 | 7.14 | 0.987 | - | - | - | 1.079 | 0.994 | |
| 100 | 3.81 | 6.26 | 0.968 | 0.54 | 29.73 | 0.995 | 2.351 | 0.951 | |
| MK51 | 10 | 0.99 | 0.04 | 0.993 | 0.32 | 5.66 | 0.964 | 1.800 | 0.993 |
| 25 | 1.42 | 0.09 | 0.990 | - | - | - | 0.950 | 0.989 | |
| 50 | 2.94 | 1.66 | 0.933 | 1.08 | 13.86 | 0.987 | 1.199 | 0.955 | |
| 100 | 4.24 | 1.19 | 0.982 | 1.47 | 22.08 | 0.919 | 1.926 | 0.997 | |
| S110 | 10 | 1.01 | 2.22 | 0.957 | 0.09 | 9.21 | 0.769 | 4.162 | 0.993 |
| 25 | 1.78 | 3.62 | 0.986 | 0.83 | 11.42 | 0.999 | 1.323 | 0.981 | |
| 50 | 2.87 | 5.04 | 0.989 | 1.42 | 15.67 | 0.999 | 1.326 | 0.994 | |
| 100 | 3.90 | 8.25 | 0.991 | 1.15 | 28.97 | 0.963 | 2.005 | 0.995 | |
| S108 | 10 | 1.06 | 2.01 | 0.986 | 0.08 | 9.42 | 0.751 | 4.518 | 0.998 |
| 25 | 1.88 | 4.83 | 0.979 | 0.80 | 12.62 | 0.992 | 1.555 | 0.987 | |
| 50 | 2.61 | 11.71 | 0.974 | 1.18 | 22.01 | 0.991 | 1.658 | 0.985 | |
| 100 | 3.10 | 21.67 | 0.968 | 1.72 | 32.49 | 0.886 | 2.202 | 0.981 | |
| Model | - | Langmuir | |||||||
| Ion Exchanger | T (K) | qe.exp (mg/g) | q0 (mg/g) | KL (L/mg) | R2 | χ2 | |||
| IRA743 | 293 | 34.30 | 34.95 | 0.245 | 0.997 | 0.01 | |||
| 313 | 50.30 | 51.07 | 0.132 | 0.975 | 0.18 | ||||
| 333 | 68.50 | 68.27 | 0.122 | 0.940 | 0.70 | ||||
| MK51 | 293 | 55.20 | 55.79 | 0.084 | 0.922 | 0.67 | |||
| 313 | 60.20 | 62.37 | 0.177 | 0.988 | 0.04 | ||||
| 333 | 70.00 | 72.51 | 0.150 | 0.975 | 0.15 | ||||
| S110 | 293 | 47.70 | 48.24 | 0.270 | 0.994 | 0.03 | |||
| 313 | 52.30 | 52.90 | 0.320 | 0.995 | 0.02 | ||||
| 333 | 65.70 | 66.42 | 0.277 | 0.989 | 0.07 | ||||
| S108 | 293 | 60.50 | 61.54 | 0.317 | 0.993 | 0.02 | |||
| 313 | 70.30 | 71.23 | 0.252 | 0.985 | 0.09 | ||||
| 333 | 76.60 | 77.03 | 0.416 | 0.992 | 0.06 | ||||
| - | - | Freundlich | Temkin | ||||||
| - | - | KF (mg/g) | n | R2 | χ2 | A (L/g) | B (J/mol) | R2 | χ2 |
| IRA743 | 293 | 13.75 | 4.85 | 0.989 | 0.13 | 35.43 | 4.12 | 0.996 | 0.01 |
| 313 | 15.52 | 4.03 | 0.997 | 0.08 | 17.32 | 6.03 | 0.936 | 0.67 | |
| 333 | 19.12 | 3.80 | 0.985 | 1.05 | 20.51 | 7.59 | 0.867 | 2.71 | |
| MK51 | 293 | 16.01 | 4.30 | 0.958 | 1.90 | 23.57 | 5.71 | 0.802 | 2.89 |
| 313 | 19.06 | 3.84 | 0.996 | 0.01 | 24.14 | 7.24 | 0.924 | 0.41 | |
| 333 | 22.45 | 4.15 | 0.975 | 0.52 | 54.69 | 7.10 | 0.846 | 1.91 | |
| S110 | 293 | 22.00 | 6.05 | 0.996 | 0.01 | 713.62 | 3.95 | 0.935 | 0.28 |
| 313 | 23.88 | 5.70 | 0.998 | 0.01 | 556.97 | 4.52 | 0.955 | 0.19 | |
| 333 | 26.76 | 4.96 | 0.995 | 0.01 | 273.55 | 5.93 | 0.928 | 0.67 | |
| S108 | 293 | 25.06 | 4.85 | 0.988 | 0.10 | 181.54 | 5.92 | 0.956 | 0.18 |
| 313 | 26.50 | 4.50 | 0.990 | 0.01 | 127.14 | 6.87 | 0.936 | 0.81 | |
| 333 | 29.81 | 4.20 | 0.986 | 0.39 | 116.10 | 7.98 | 0.979 | 0.29 | |
| Ion Exchanger | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol K) | ||
|---|---|---|---|---|---|
| 293 K | 313 K | 333 K | |||
| IRA743 | −13.91 | −16.25 | −18.71 | 21.26 | 119.98 |
| MK51 | −15.56 | −16.99 | −18.82 | 8.27 | 81.15 |
| S110 | −15.02 | −16.40 | −18.50 | 10.39 | 86.35 |
| S108 | −15.93 | −17.72 | −19.32 | 8.94 | 84.96 |
| Ion Exchanger | IRA743 | MK51 | S110 | S108 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Vj (mL) | 10 | 100 | 10 | ||||||
| C0 (mg/L) | 50 | 100 | 100 | 50 | 100 | 50 | 100 | 50 | 100 |
| qE (g/L) | 23.0 | 24.0 | 32.8 | 22.9 | 37.5 | 23.0 | 50.0 | 24.5 | 44.0 |
| qB (g/L) | 17.5 | 20.0 | 29.2 | 14.3 | 28.5 | 16.5 | 36.0 | 20.0 | 29.0 |
| qEB (g/L) | 5.5 | 4.0 | 3.7 | 8.7 | 9.0 | 6.5 | 14.0 | 4.5 | 14.0 |
| Dg | 1367.6 | 706.4 | 1018.6 | 1406.9 | 1105.7 | 1459.2 | 1449.6 | 1474.7 | 1286.6 |
| Dv | 109.6 | 39.6 | 36.6 | 173.6 | 89.6 | 129.6 | 139.6 | 89.6 | 149.6 |
| tf (min) | 1805.8 | 627.0 | 5961.7 | 2198.2 | 1116.7 | 871.0 | 1920.3 | 1404.7 | 1703.3 |
| tz (min) | 1833.3 | 666.7 | 6108.3 | 2900.0 | 1500.0 | 2166.7 | 2333.3 | 1500.0 | 2333.3 |
| te (min) | 7666.7 | 4000.0 | 54,708.3 | 7650.0 | 6250.0 | 7500.0 | 8333.3 | 8166.7 | 7166.7 |
| F | 0.02 | 0.06 | 0.02 | 0.24 | 0.26 | 0.60 | 0.18 | 0.06 | 0.27 |
| hZ (cm) | 4.0 | 2.5 | 3.1 | 6.8 | 3.7 | 4.2 | 4.6 | 2.8 | 5.4 |
| UZ (cm/min) | 0.0022 | 0.0038 | 0.0005 | 0.0023 | 0.0025 | 0.0019 | 0.0020 | 0.0019 | 0.0023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burdzy, K.; Chen, Y.-G.; Lv, G.-Y.; Chen, S.-H.; Kołodyńska, D. Application of Ion Exchangers with the N-Methyl-D-Glucamine Groups in the V(V) Ions Adsorption Process. Materials 2022, 15, 1026. https://doi.org/10.3390/ma15031026
Burdzy K, Chen Y-G, Lv G-Y, Chen S-H, Kołodyńska D. Application of Ion Exchangers with the N-Methyl-D-Glucamine Groups in the V(V) Ions Adsorption Process. Materials. 2022; 15(3):1026. https://doi.org/10.3390/ma15031026
Chicago/Turabian StyleBurdzy, Katarzyna, Yi-Gong Chen, Gui-Yuan Lv, Su-Hong Chen, and Dorota Kołodyńska. 2022. "Application of Ion Exchangers with the N-Methyl-D-Glucamine Groups in the V(V) Ions Adsorption Process" Materials 15, no. 3: 1026. https://doi.org/10.3390/ma15031026







