Influence of Gas-Flow Conditions on the Evolution of Thermally Insulating Si3N4 Nano-Felts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
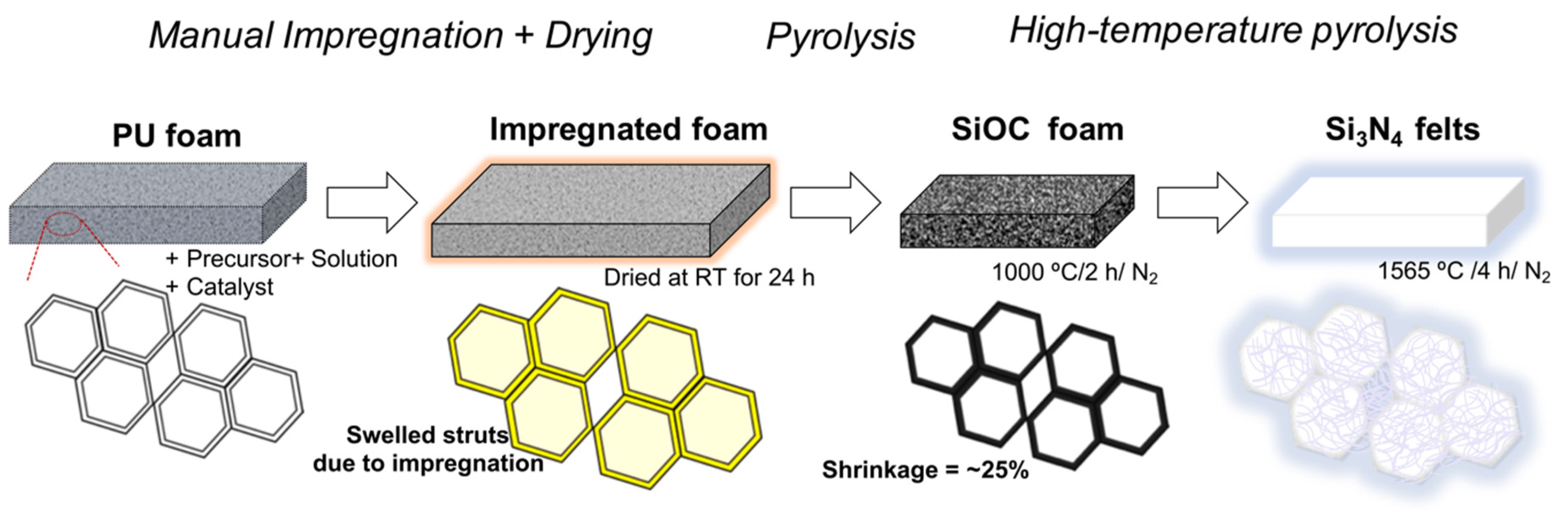
2.2. Processing the ‘Nano-Felts’
- (1)
- low (100 cm3.min−1), and
- (2)
- high (600 cm3.min−1).
2.3. Material Characterizations
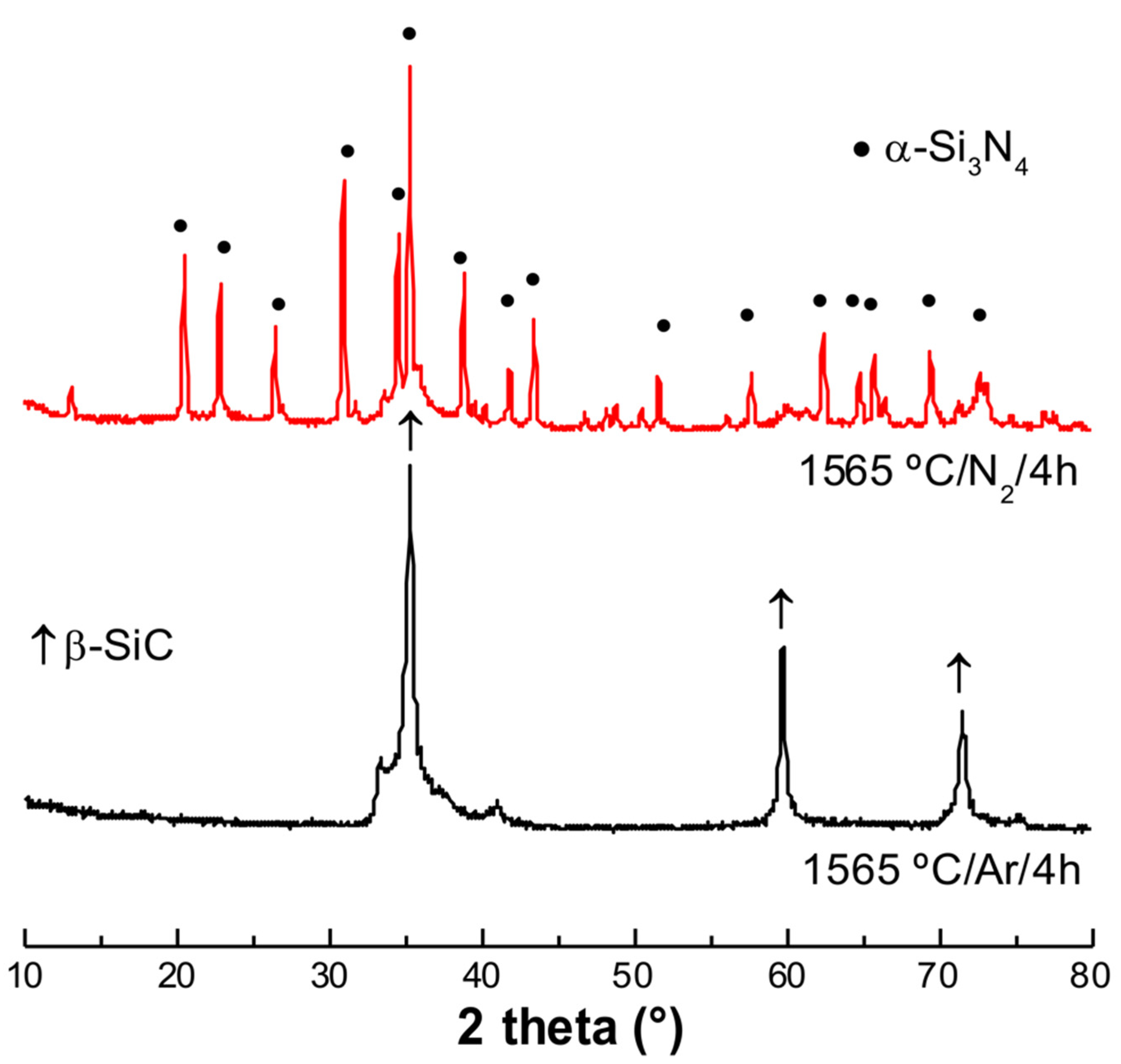
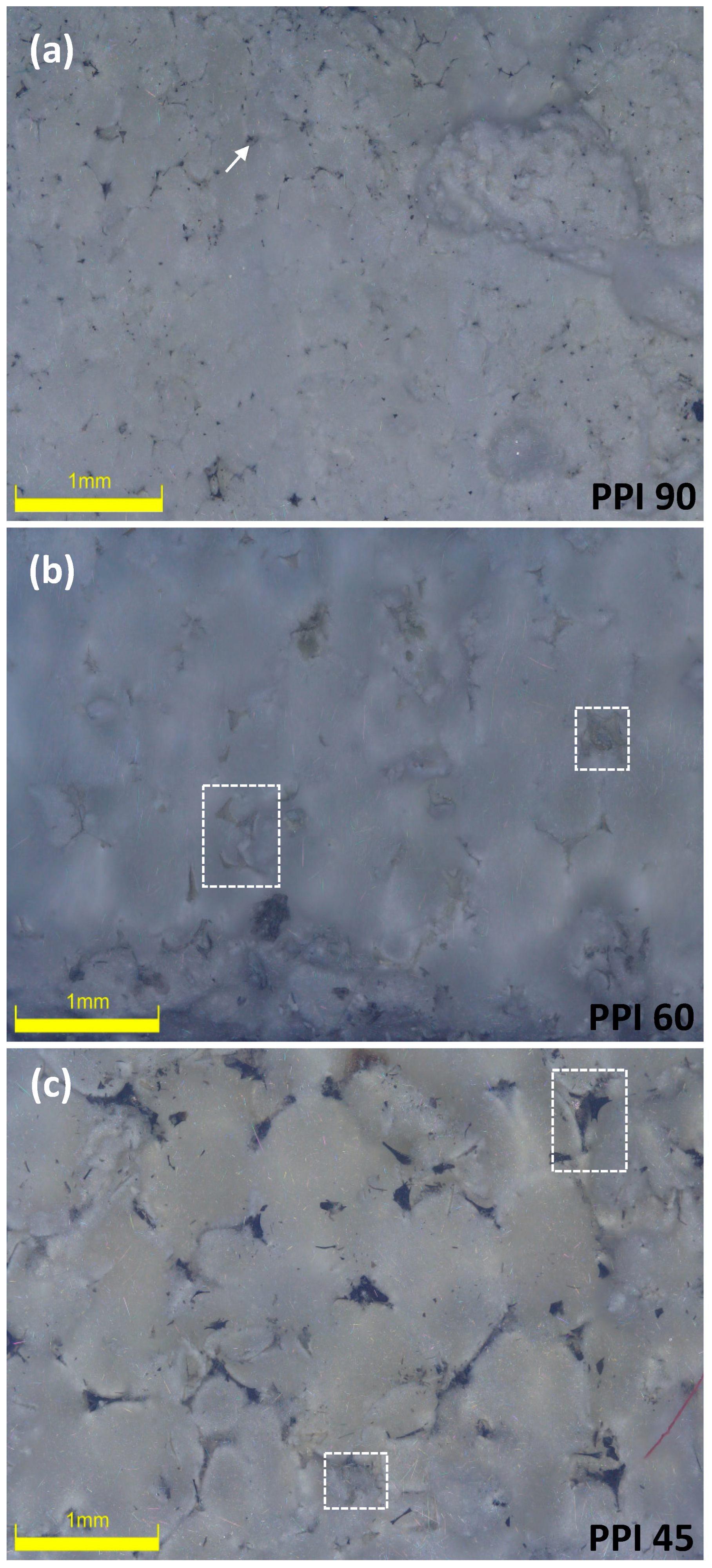
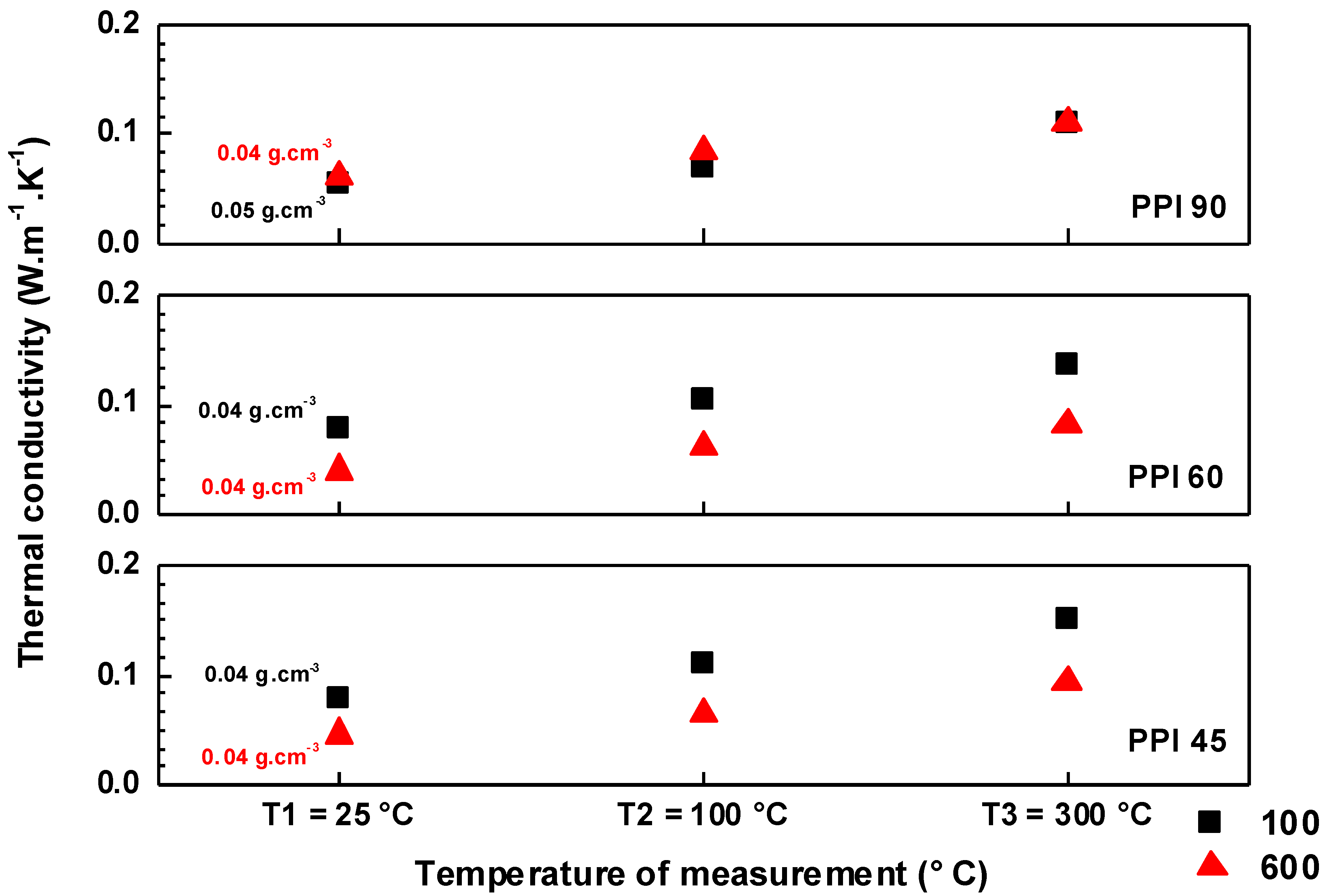
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics; John Wiley & Sons: Hoboken, NJ, USA, 1976; Volume 17, ISBN 0-471-47860-1. [Google Scholar]
- Lu, D.; Su, L.; Wang, H.; Niu, M.; Xu, L.; Ma, M.; Gao, H.; Cai, Z.; Fan, X. Scalable Fabrication of Resilient SiC Nanowires Aerogels with Exceptional High-Temperature Stability. ACS Appl. Mater. Interfaces 2019, 11, 45338–45344. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Wang, X.; Dou, L.; Yu, J.; Ding, B. Ultralight and Fire-Resistant Ceramic Nanofibrous Aerogels with Temperature-Invariant Superelasticity. Sci. Adv. 2018, 4, eaas8925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhang, X.; Wang, N.; Li, Y.; Feng, X.; Huang, Y.; Zhao, C. Ultralight, Scalable, and High-Temperature–Resilient Ceramic Nanofiber Sponges. Sci. Adv. 2017, 3, e1603170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Zhang, Q.; Hao, M.; Hu, Y.; Lin, Z.; Peng, L.; Wang, T.; Ren, X.; Wang, C.; Zhao, Z.; et al. Double-Negative-Index Ceramic Aerogels for Thermal Superinsulation. Science 2019, 363, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Hrubesh, L.W. Aerogel Applications. J. Non-Cryst. Solids 1998, 225, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Santhosh, B.; Vakifahmetoglu, C.; Ionescu, E.; Reitz, A.; Albert, B.; Sorarù, G.D. Processing and Thermal Characterization of Polymer Derived SiCN(O) and SiOC Reticulated Foams. Ceram. Int. 2020, 46, 5594–5601. [Google Scholar] [CrossRef]
- Jana, P.; Zera, E.; Sorarù, G.D. Processing of Preceramic Polymer to Low Density Silicon Carbide Foam. Mater. Des. 2017, 116, 278–286. [Google Scholar] [CrossRef]
- de Guinoa, A.S.; Zambrana-Vasquez, D.; Alcalde, A.; Corradini, M.; Zabalza-Bribián, I. Environmental Assessment of a Nano-Technological Aerogel-Based Panel for Building Insulation. J. Clean. Prod. 2017, 161, 1404–1415. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Hu, P.; Zhang, X.; Cheng, Y.; Fang, C.; Xu, J.; Chen, G. Facile Synthesis of Silicon Nitride Nanowires with Flexible Mechanical Properties and with Diameters Controlled by Flow Rate. Sci. Rep. 2017, 7, 45538. [Google Scholar] [CrossRef]
- Lu, J.; Guo, K.; Song, Q.; Li, Y.; Zhang, L.; Li, H. In-Situ Synthesis Silicon Nitride Nanowires in Carbon Fiber Felts and Their Effect on the Mechanical Properties of Carbon/Carbon Composites. Mater. Des. 2016, 99, 389–395. [Google Scholar] [CrossRef]
- Li, D.; Guzi de Moraes, E.; Guo, P.; Zou, J.; Zhang, J.; Colombo, P.; Shen, Z. Rapid Sintering of Silicon Nitride Foams Decorated with One-Dimensional Nanostructures by Intense Thermal Radiation. Sci. Technol. Adv. Mater. 2014, 15, 045003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaloyeros, A.E.; Jové, F.A.; Goff, J.; Arkles, B. Silicon Nitride and Silicon Nitride-Rich Thin Film Technologies: Trends in Deposition Techniques and Related Applications. ECS J. Solid State Sci. Technol. 2017, 6, P691. [Google Scholar] [CrossRef]
- Su, L.; Li, M.; Wang, H.; Niu, M.; Lu, D.; Cai, Z. Resilient Si3N4 Nanobelt Aerogel as Fire-Resistant and Electromagnetic Wave-Transparent Thermal Insulator. ACS Appl. Mater. Interfaces 2019, 11, 15795–15803. [Google Scholar] [CrossRef] [PubMed]
- Biesuz, M.; Zera, E.; Tomasi, M.; Jana, P.; Ersen, O.; Baaziz, W.; Lindemann, A.; Sorarù, G.D. Polymer-Derived Si3N4 Nanofelts for Flexible, High Temperature, Lightweight and Easy-Manufacturable Super-Thermal Insulators. Appl. Mater. Today 2020, 20, 100648. [Google Scholar] [CrossRef]
- Tong, Z.; Zhang, B.; Yu, H.; Yan, X.; Xu, H.; Li, X.; Ji, H. Si3N4 Nanofibrous Aerogel with In Situ Growth of SiOx Coating and Nanowires for Oil/Water Separation and Thermal Insulation. ACS Appl. Mater. Interfaces 2021, 13, 22765–22773. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, W.; Fan, Y.; An, L. Mass Production of Very Thin Single-Crystal Silicon Nitride Nanobelts. J. Solid State Chem. 2008, 181, 211–215. [Google Scholar] [CrossRef]
- Santhosh, B.; Ionescu, E.; Andreolli, F.; Biesuz, M.; Reitz, A.; Albert, B.; Sorarú, G.D. Effect of Pyrolysis Temperature on the Microstructure and Thermal Conductivity of Polymer-Derived Monolithic and Porous SiC Ceramics. J. Eur. Ceram. Soc. 2021, 41, 1151–1162. [Google Scholar] [CrossRef]
- Semerci, T.; de Mello Innocentini, M.D.; Marsola, G.A.; Lasso, P.R.O.; Soraru, G.D.; Vakifahmetoglu, C. Hot Air Permeable Preceramic Polymer Derived Reticulated Ceramic Foams. ACS Appl. Polym. Mater. 2020, 2, 4118–4126. [Google Scholar] [CrossRef]
- Valentini, F.; Dorigato, A.; Pegoretti, A.; Tomasi, M.; Sorarù, G.D.; Biesuz, M. Si3N4 Nanofelts/Paraffin Composites as Novel Thermal Energy Storage Architecture. J. Mater. Sci. 2021, 56, 1537–1550. [Google Scholar] [CrossRef]
- Biesuz, M.; Tomasi, M.; Santhosh, B.; Sglavo, V.M.; Sorarù, G.D. Polymer-Derived Si3N4 Nanofelts as a Novel Oil Spills Clean-up Architecture. J. Environ. Chem. Eng. 2020, 8, 104134. [Google Scholar] [CrossRef]
- Sorarù, G.D.; Girardini, K.; Narisawa, M.; Biesuz, M. Effect of Anionic Substitution on the High Temperature Stability of Polymer-derived SiOC Glasses. J. Am. Ceram. Soc. 2021, 104, 3097–3104. [Google Scholar] [CrossRef]
- Wei, Q.; Pippel, E.; Woltersdorf, J.; Scheffler, M.; Greil, P. Interfacial SiC Formation in Polysiloxane-Derived Si–O–C Ceramics. Mater. Chem. Phys. 2002, 73, 281–289. [Google Scholar] [CrossRef]
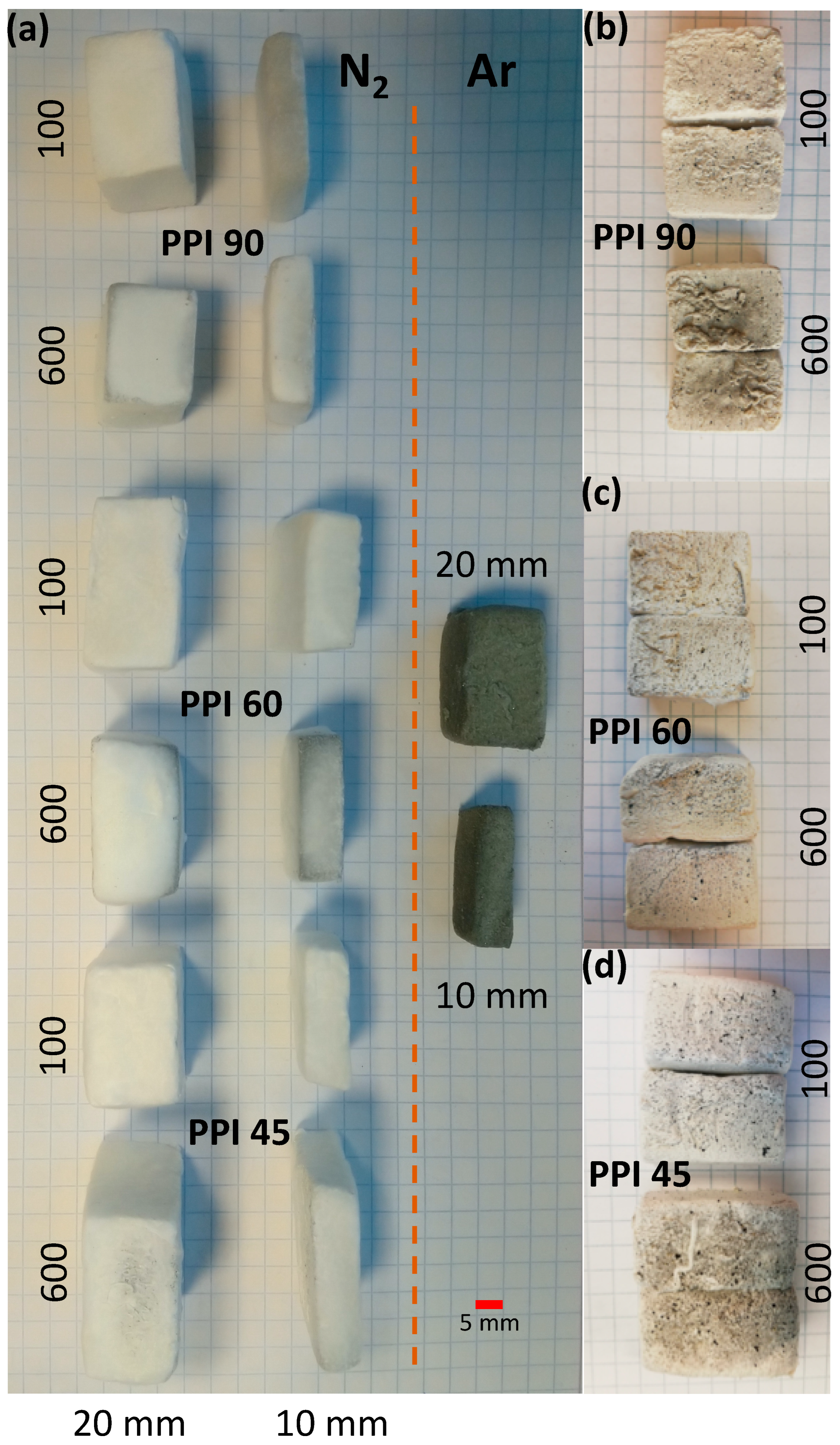

| PU Foam | PU Foam Thickness (mm) | N2-Flow Conditions (cm3.min−1) | Bulk Density (g.cm−3) | Volume Shrinkage (%) | Mass Loss (%) |
|---|---|---|---|---|---|
| PPI 90 | 10 | 100 | 0.05 | 55.3 | 67.2 |
| 20 | 100 | 0.06 | 52.5 | 64.4 | |
| 10 | 600 | 0.04 | 51.6 | 68.0 | |
| 20 | 600 | 0.04 | 53.5 | 69.3 | |
| PPI 60 | 10 | 100 | 0.04 | 42.0 | 72.4 |
| 20 | 100 | 0.04 | 48.1 | 70.7 | |
| 10 | 600 | 0.04 | 49.1 | 67.6 | |
| 20 | 600 | 0.05 | 49.6 | 65.4 | |
| PPI 45 | 10 | 100 | 0.04 | 47.6 | 69.2 |
| 20 | 100 | 0.04 | 51.4 | 70.8 | |
| 10 | 600 | 0.04 | 41.7 | 70.9 | |
| 20 | 600 | 0.05 | 47.9 | 66.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santhosh, B.; Biesuz, M.; Zambotti, A.; Sorarù, G.D. Influence of Gas-Flow Conditions on the Evolution of Thermally Insulating Si3N4 Nano-Felts. Materials 2022, 15, 1068. https://doi.org/10.3390/ma15031068
Santhosh B, Biesuz M, Zambotti A, Sorarù GD. Influence of Gas-Flow Conditions on the Evolution of Thermally Insulating Si3N4 Nano-Felts. Materials. 2022; 15(3):1068. https://doi.org/10.3390/ma15031068
Chicago/Turabian StyleSanthosh, Balanand, Mattia Biesuz, Andrea Zambotti, and Gian Domenico Sorarù. 2022. "Influence of Gas-Flow Conditions on the Evolution of Thermally Insulating Si3N4 Nano-Felts" Materials 15, no. 3: 1068. https://doi.org/10.3390/ma15031068
APA StyleSanthosh, B., Biesuz, M., Zambotti, A., & Sorarù, G. D. (2022). Influence of Gas-Flow Conditions on the Evolution of Thermally Insulating Si3N4 Nano-Felts. Materials, 15(3), 1068. https://doi.org/10.3390/ma15031068







