Antibacterial Electroconductive Composite Coating of Cotton Fabric
Abstract
:1. Introduction
2. Experimental Section
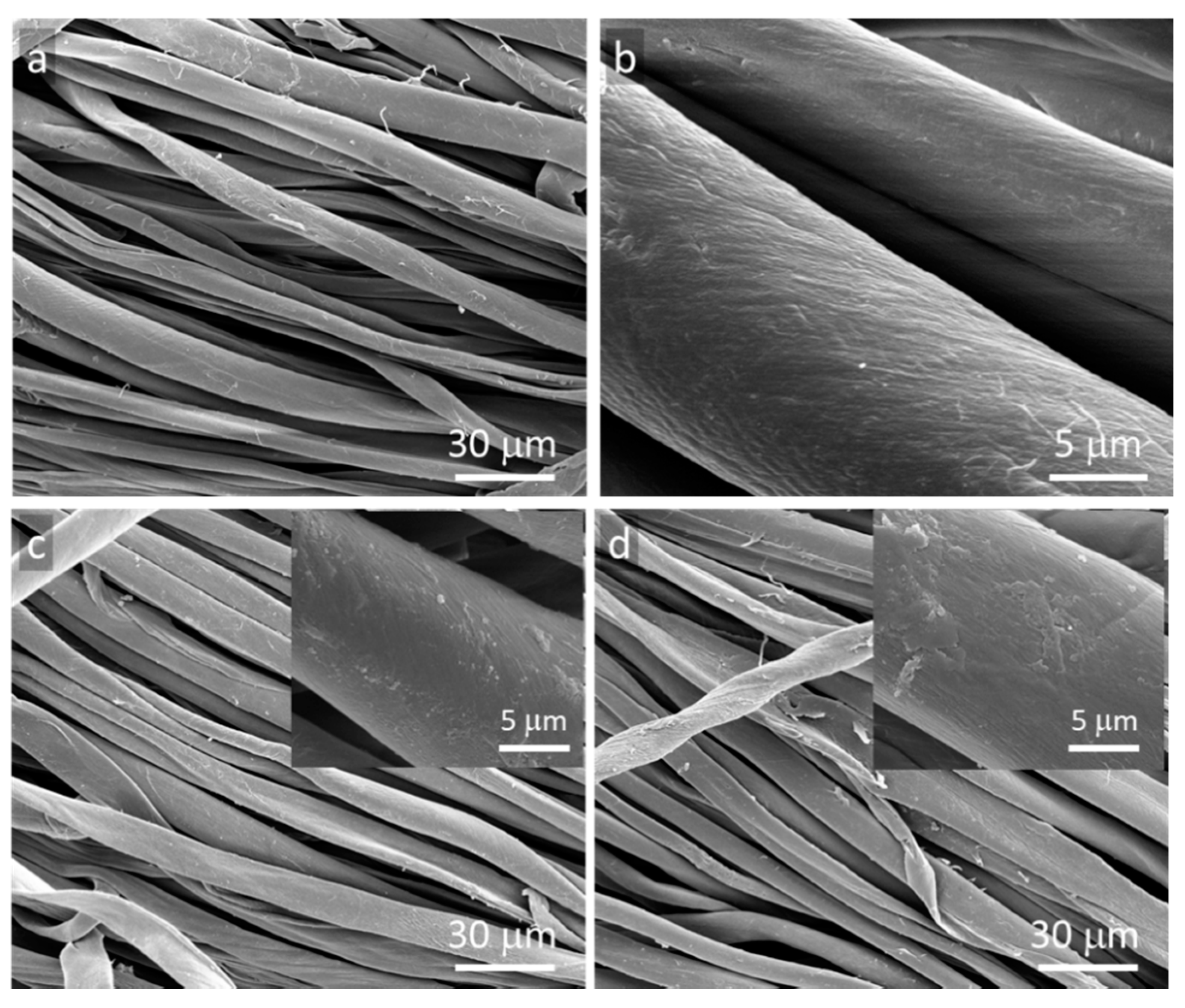
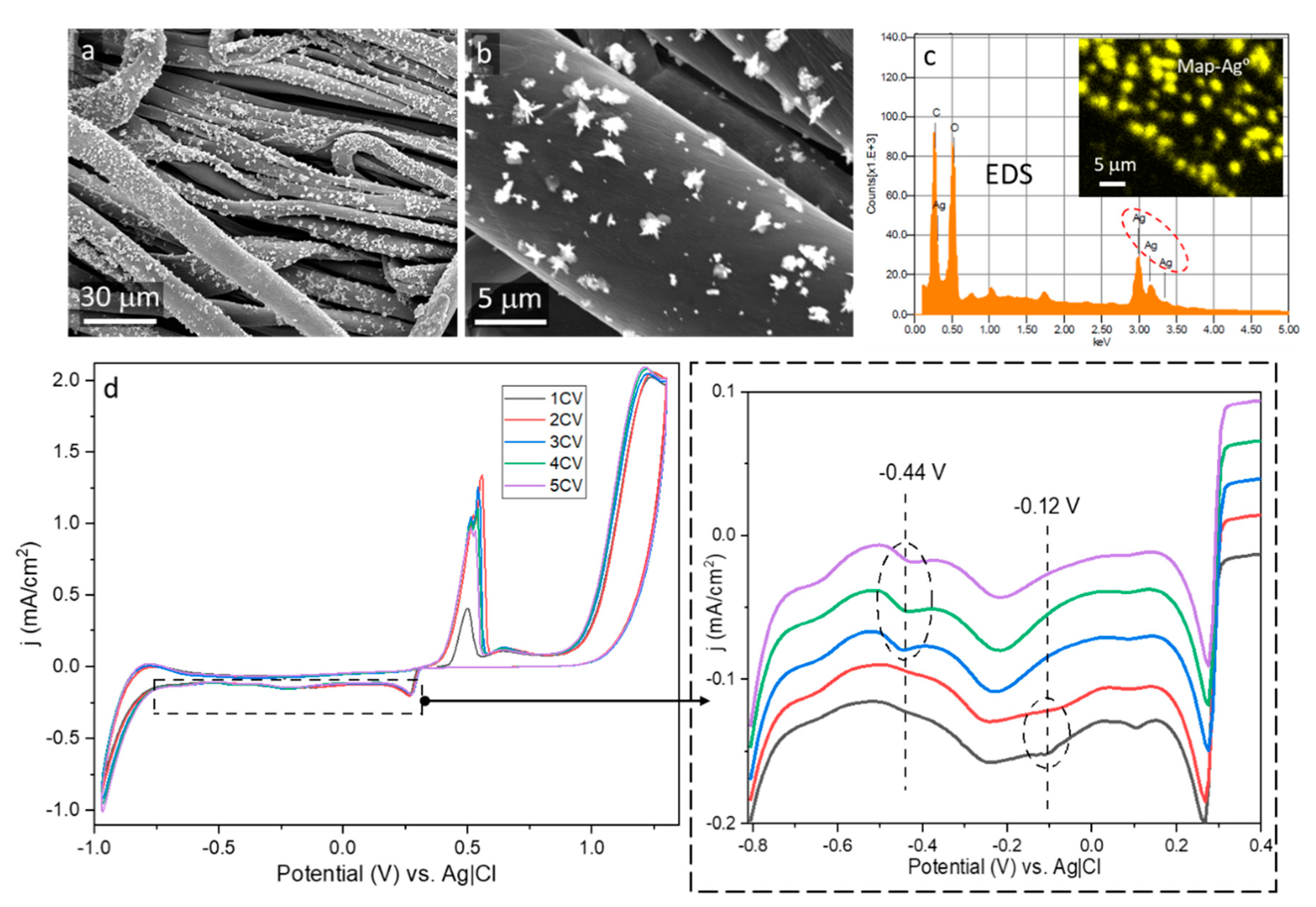
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval
References
- Islam, G.M.N.; Ali, M.A.; Collie, S. Textile sensors for wearable applications: A comprehensive review. Cellulose 2020, 27, 6103–6131. [Google Scholar] [CrossRef]
- Maity, S.; Chatterjee, A. Conductive polymer-based electro-conductive textile composites for electromagnetic interference shielding: A review. J. Ind. Text. 2016, 47, 2228–2252. [Google Scholar] [CrossRef]
- Molina, J. Graphene-based fabrics and their applications: Areview. RSC Adv. 2016, 6, 68261–68291. [Google Scholar] [CrossRef]
- Stoppa, M.; Chiolerio, A. Wearable Electronics and Smart Textiles: A Critical Review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makowski, T.; Kowalczyk, D.; Fortuniak, W.; Jeziorska, D.; Brzezinski, S.; Tracz, A. Superhydrophobic properties of cotton woven fabrics with conducting 3D networks of multiwall carbon nanotubes, MWCNTs. Cellulose 2014, 21, 4659–4670. [Google Scholar] [CrossRef] [Green Version]
- Makowski, T.; Zhang, C.; Olah, A.; Piorkowska, E.; Baer, E.; Kregiel, D. Modification of dual-component fibrous materials with carbon nanotubes and methyltrichlorosilane. Mater. Des. 2018, 162, 219–228. [Google Scholar] [CrossRef]
- Sahito, I.A.; Sun, K.C.; Arbab, A.A.; Qadir, M.B.; Choi, Y.S.; Jeong, S.H. Flexible and conductive cotton fabric counter electrode coated with graphene nanosheets for high efficiency dye sensitized solar cell. J. Power Sources 2016, 319, 90–98. [Google Scholar] [CrossRef]
- Shateri-Khalilabad, M.; Yazdanshenas, M.E. Fabricating electroconductive cotton textiles using graphene. Carbohydr. Polym. 2013, 96, 190–195. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Pei, S.F.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Huh, S.H. Thermal Reduction of Graphene Oxide. In Physics and Applications of Graphene—Experiments; Mikhailov, S., Ed.; Intech Europe: Rijeka, Croatia, 2011; pp. 73–90. [Google Scholar]
- Makowski, T.; Svyntkivska, M.; Piorkowska, E.; Mizerska, U.; Fortuniak, W.; Kowalczyk, D.; Brzezinski, S. Conductive and superhydrophobic cotton fabric through pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) assisted thermal reduction of graphene oxide and modification with methyltrichlorosilane. Cellulose 2018, 25, 5377–5388. [Google Scholar] [CrossRef]
- Jedrzejczyk, M.; Makowski, T.; Svyntkivska, M.; Piorkowska, E.; Mizerska, U.; Fortuniak, W.; Brzezinski, S.; Kowalczyk, D. Conductive cotton fabric through thermal reduction of graphene oxide enhanced by commercial antioxidants used in the plastics industry. Cellulose 2019, 26, 2191–2199. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Zattera, A.J. Structural Characteristics and Thermal Properties of Native Cellulose. In Cellulose—Fundamental Aspects; Van De Ven, T., Godbout, L., Eds.; Intech Europe: Rijeka, Croatia, 2013; pp. 45–68. [Google Scholar]
- Cao, G.H.; Yan, J.H.; Ning, X.X.; Zhang, Q.; Wu, Q.; Bi, L.; Zhang, Y.M.; Han, Y.S.; Guo, J.B. Antibacterial and antibiofilm properties of graphene and its derivatives. Colloids Surf. B Biointerfaces 2021, 200, 111588. [Google Scholar] [CrossRef] [PubMed]
- Nasirzadeh, N.; Azari, M.R.; Rasoulzadeh, Y.; Mohammadian, Y. An assessment of the cytotoxic effects of graphene nanoparticles on the epithelial cells of the human lung. Toxicol. Ind. Health 2019, 35, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Pulingam, T.; Thong, K.L.; Appaturi, J.N.; Nordin, N.I.; Dinshaw, I.J.; Lai, C.W.; Leo, B.F. Synergistic antibacterial actions of graphene oxide and antibiotics towards bacteria and the toxicological effects of graphene oxide on human epidermal keratinocytes. Eur. J. Pharm. Sci. 2019, 142, 105087. [Google Scholar] [CrossRef] [PubMed]
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F. From nanoengineering to nanomedicine: A facile route to enhance biocompatibility of graphene as a potential nano-carrier for targeted drug delivery using natural deep eutectic solvents. Chem. Eng. Sci. 2019, 195, 95–106. [Google Scholar] [CrossRef]
- Han, F.; Lv, S.; Li, Z.; Jin, L.; Fan, B.; Zhang, J.; Zhang, R.; Zhang, X.; Han, L.; Li, J. Triple-synergistic 2D material-based dual-delivery antibiotic platform. NPG Asia Mater. 2020, 12, 15. [Google Scholar] [CrossRef]
- Makowski, T.; Kowalczyk, D.; Fortuniak, W.; Brzezinski, S.; Kregiel, D. Electrochemical deposition of silver nanoparticle and polymerization of pyrrole on fabrics via conducting multiwall carbon nanotubes. Cellulose 2015, 22, 3063–3075. [Google Scholar] [CrossRef] [Green Version]
- Farouk, A.; El-Sayed Saeed, S.; Sharaf, S.; Abd El-Hady, M.M. Photocatalytic activity and antibacterial properties of linen fabric using reduced graphene oxide/silver nanocomposite. RSC Adv. 2020, 10, 41600–41611. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anuj, S.A.; Gajera, H.; Hirpara, D.G.; Golakiya, B.A. Bacterial membrane destabilization with cationic particles of nano-silver to combat efflux-mediated antibiotic resistance in Gram-negative bacteria. Life Sci. 2019, 230, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Anuj, S.A.; Gajera, H.P.; Hirpara, D.G.; Golakiya, B.A. Interruption in membrane permeability of drug-resistant Staphylococcus aureus with cationic particles of nano-silver. Eur. J. Pharm. Sci. 2019, 127, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.J.; Zheng, Y.K.; Wang, Y.H.; Du, T.Y.; Li, C.M.; Wang, X.M.; Jiang, H. Versatile roles of silver in Ag-based nanoalloys for antibacterial applications. Coord. Chem. Rev. 2021, 449, 214218. [Google Scholar] [CrossRef]
- Ul Hoque, M.I.; Chowdhury, A.-N.; Islam, M.T.; Firoz, S.H.; Luba, U.; Alowasheeir, A.; Rahman, M.M.; Rehman, A.U.; Ahmad, S.H.A.; Holze, R.; et al. Fabrication of highly and poorly oxidized silver oxide/silver/tin(IV) oxide nanocomposites and their comparative anti-pathogenic properties towards hazardous food pathogens. J. Hazard. Mater. 2021, 408, 124896. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, R.H.; Lan, J.W.; Jiang, S.X.; Zhang, Z.Y. Microwave-assisted synthesis of silver/reduced graphene oxide on cotton fabric. Cellulose 2017, 24, 4045–4055. [Google Scholar] [CrossRef]
- Karami, Z.; Youssefi, M.; Raeissi, K.; Zhiani, M. An efficient textile-based electrode utilizing silver nanoparticles/reduced graphene oxide/cotton fabric composite for high-performance wearable supercapacitors. Electrochim. Acta 2021, 368, 137647. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Macintyre, C.R.; Wen, X.Y.; Bahl, P.; Kumar, U.; Chughtai, A.A.; Joshi, R. Nanoparticles incorporated graphene-based durable cotton fabrics. Carbon 2020, 166, 148–163. [Google Scholar] [CrossRef]
- Pinho, E.; Magalhães, L.; Henriques, M.; Oliveira, R. Antimicrobial activity assessment of textiles: Standard methods comparison. Ann. Microbiol. 2011, 61, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Mizerska, U.; Fortuniak, W.; Makowski, T.; Svyntkivska, M.; Piorkowska, E.; Kowalczyk, D.; Brzezinski, S. Electrically conductive and hydrophobic rGO-containing organosilicon coating of cotton fabric. Prog. Org. Coat. 2019, 137, 105312. [Google Scholar] [CrossRef]
- Karadeniz, H.; Erdem, A.; Caliskan, A.; Pereira, C.M.; Pereira, E.M.; Ribeiro, J.A. Electrochemical sensing of silver tags labelled DNA immobilized onto disposable graphite electrodes. Electrochem. Commun. 2007, 9, 2167–2173. [Google Scholar] [CrossRef]
- Patra, S.; Pandey, A.K.; Sen, D.; Ramagiri, S.V.; Bellare, J.R.; Mazumder, S.; Goswami, A. Redox Decomposition of Silver Citrate Complex in Nanoscale Confinement: An Unusual Mechanism of Formation and Growth of Silver Nanoparticles. Langmuir 2014, 30, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.A.C.; Smith, G.C.; Banks, C.E. Graphene oxide electrochemistry: The electrochemistry of graphene oxide modified electrodes reveals coverage dependent beneficial electrocatalysis. R. Soc. Open Sci. 2017, 4, 171128. [Google Scholar] [CrossRef] [Green Version]
- Bao, Q.L.; Bao, S.J.; Li, C.M.; Qi, X.; Pan, C.X.; Zang, J.F.; Lu, Z.S.; Li, Y.B.; Tang, D.Y.; Zhang, S.; et al. Supercapacitance of Solid Carbon Nanofibers Made from Ethanol Flames. J. Phys. Chem. C 2008, 112, 3612–3618. [Google Scholar] [CrossRef]
- Stensberg, M.C.; Wei, Q.S.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepúlveda, M.S. Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 2011, 6, 879–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Khan, B.; Adeleye, A.S.; Burgess, R.M.; Russo, S.M.; Ho, K.T. Effects of graphene oxide nanomaterial exposures on the marine bivalve, Crassostrea virginica. Aquat. Toxicol. 2019, 216, 105297. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping Bacteria by Graphene Nanosheets for Isolation from Environment, Reactivation by Sonication, and Inactivation by Near-Infrared Irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makowski, T.; Svyntkivska, M.; Piorkowska, E.; Mizerska, U.; Fortuniak, W.; Kowalczyk, D.; Brzezinski, S.; Kregiel, D. Antibacterial Electroconductive Composite Coating of Cotton Fabric. Materials 2022, 15, 1072. https://doi.org/10.3390/ma15031072
Makowski T, Svyntkivska M, Piorkowska E, Mizerska U, Fortuniak W, Kowalczyk D, Brzezinski S, Kregiel D. Antibacterial Electroconductive Composite Coating of Cotton Fabric. Materials. 2022; 15(3):1072. https://doi.org/10.3390/ma15031072
Chicago/Turabian StyleMakowski, Tomasz, Mariia Svyntkivska, Ewa Piorkowska, Urszula Mizerska, Witold Fortuniak, Dorota Kowalczyk, Stefan Brzezinski, and Dorota Kregiel. 2022. "Antibacterial Electroconductive Composite Coating of Cotton Fabric" Materials 15, no. 3: 1072. https://doi.org/10.3390/ma15031072
APA StyleMakowski, T., Svyntkivska, M., Piorkowska, E., Mizerska, U., Fortuniak, W., Kowalczyk, D., Brzezinski, S., & Kregiel, D. (2022). Antibacterial Electroconductive Composite Coating of Cotton Fabric. Materials, 15(3), 1072. https://doi.org/10.3390/ma15031072






