Poplar Sawdust Stack Self-Heating Properties and Variations of Internal Microbial Communities
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Self-Heating Substances Test Experiments
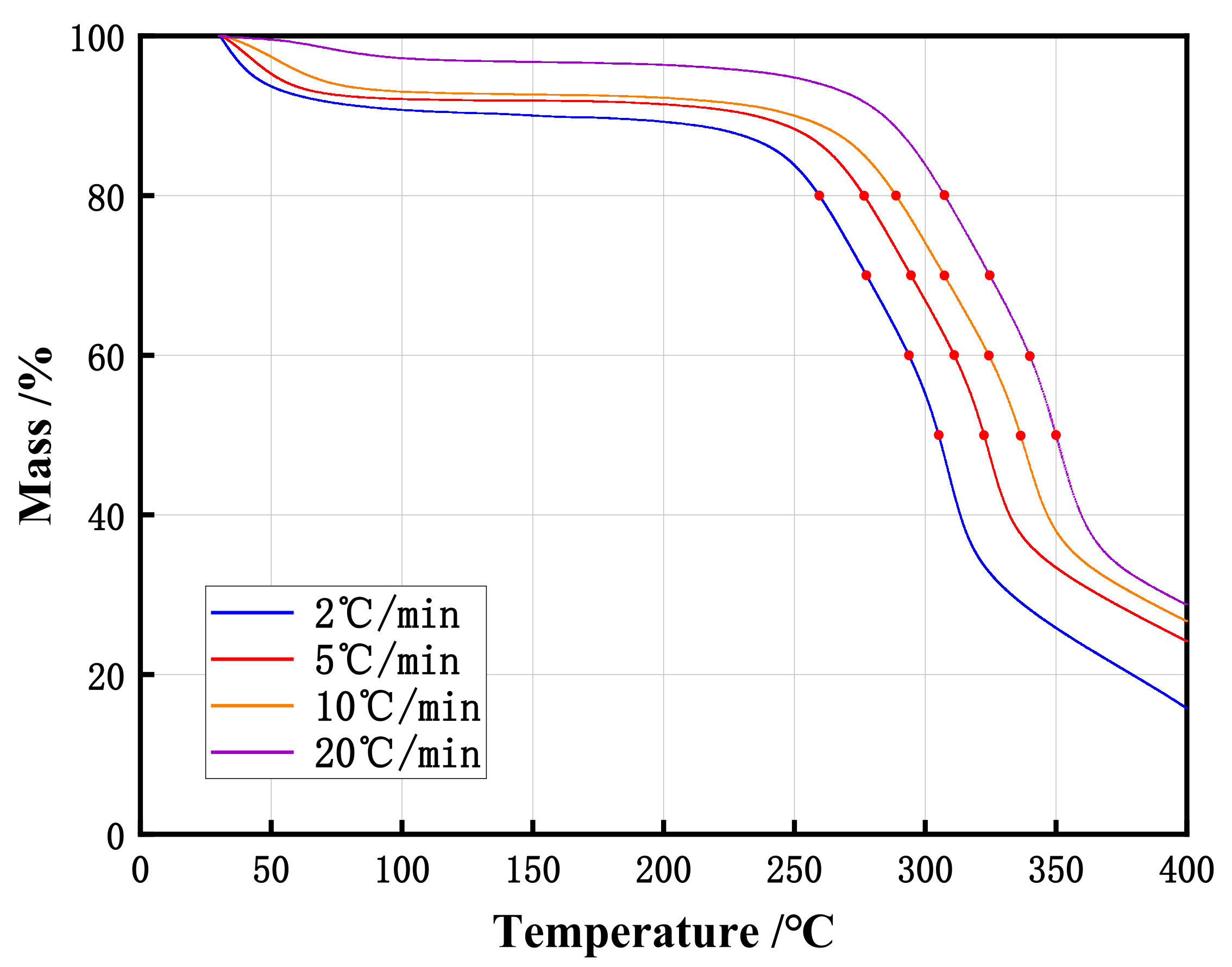
2.3. Thermogravimetry (TG) Analysis
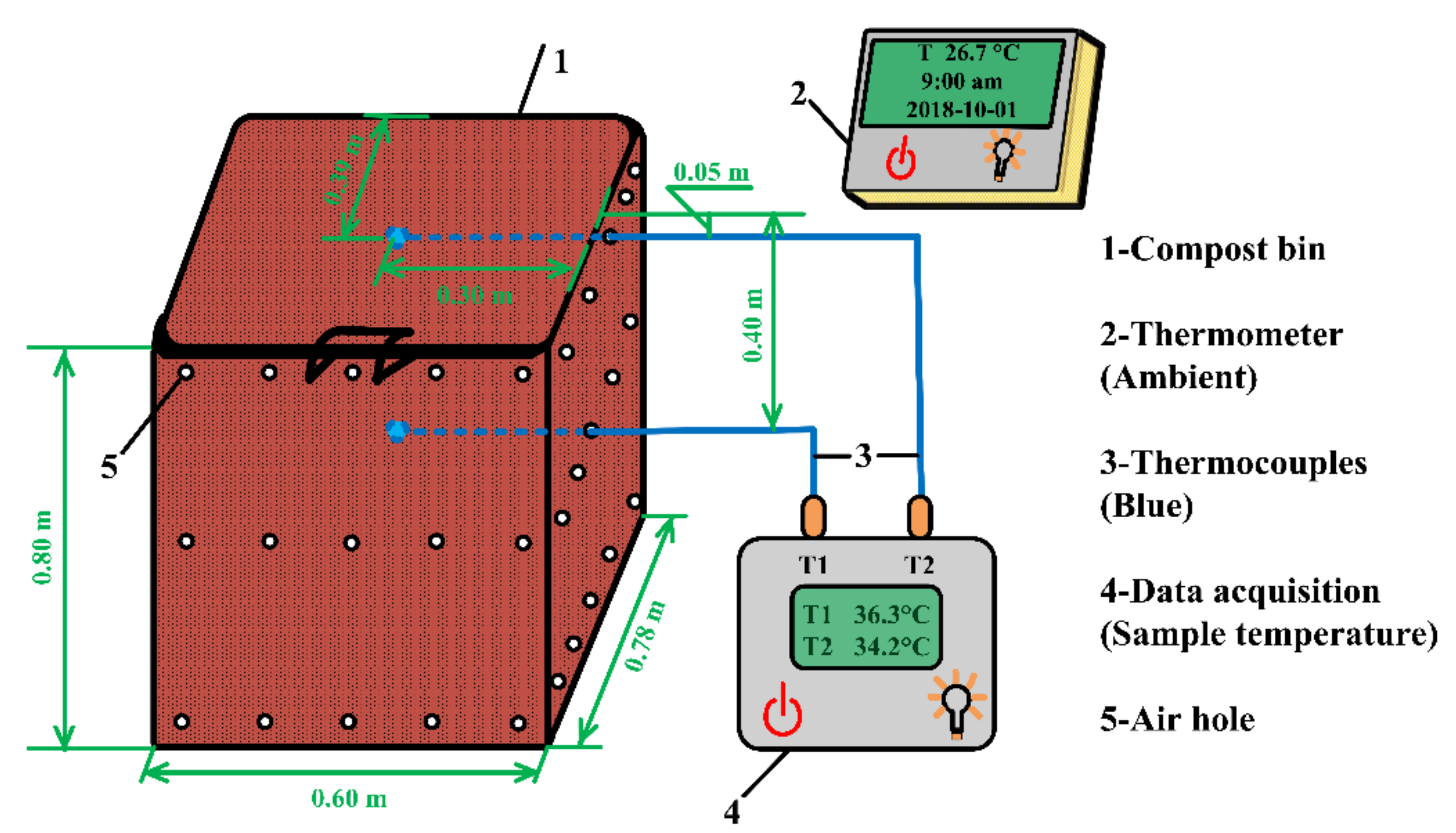
2.4. Sawdust Stack Self-Heating Experiments
2.5. Physicochemical Analysis
2.6. Microbiological Community Analysis
2.6.1. DNA Extraction, Amplification, and Sequencing
2.6.2. Processing of Sequencing Data
3. Results and Discussion
3.1. Critical Ambient Temperature (CAT)
3.1.1. CAT of 100 mm Cube Sawdust Sample
3.1.2. CAT of Larger Sawdust Stack Derived by Frank-Kamenetskii (FK) Theory
3.2. Physicochemical Properties
3.2.1. Temperature Evolution of Sawdust Stack
3.2.2. Changes of Moisture Content and pH
3.2.3. Decomposition of Cellulose, Hemicellulose and Lignin
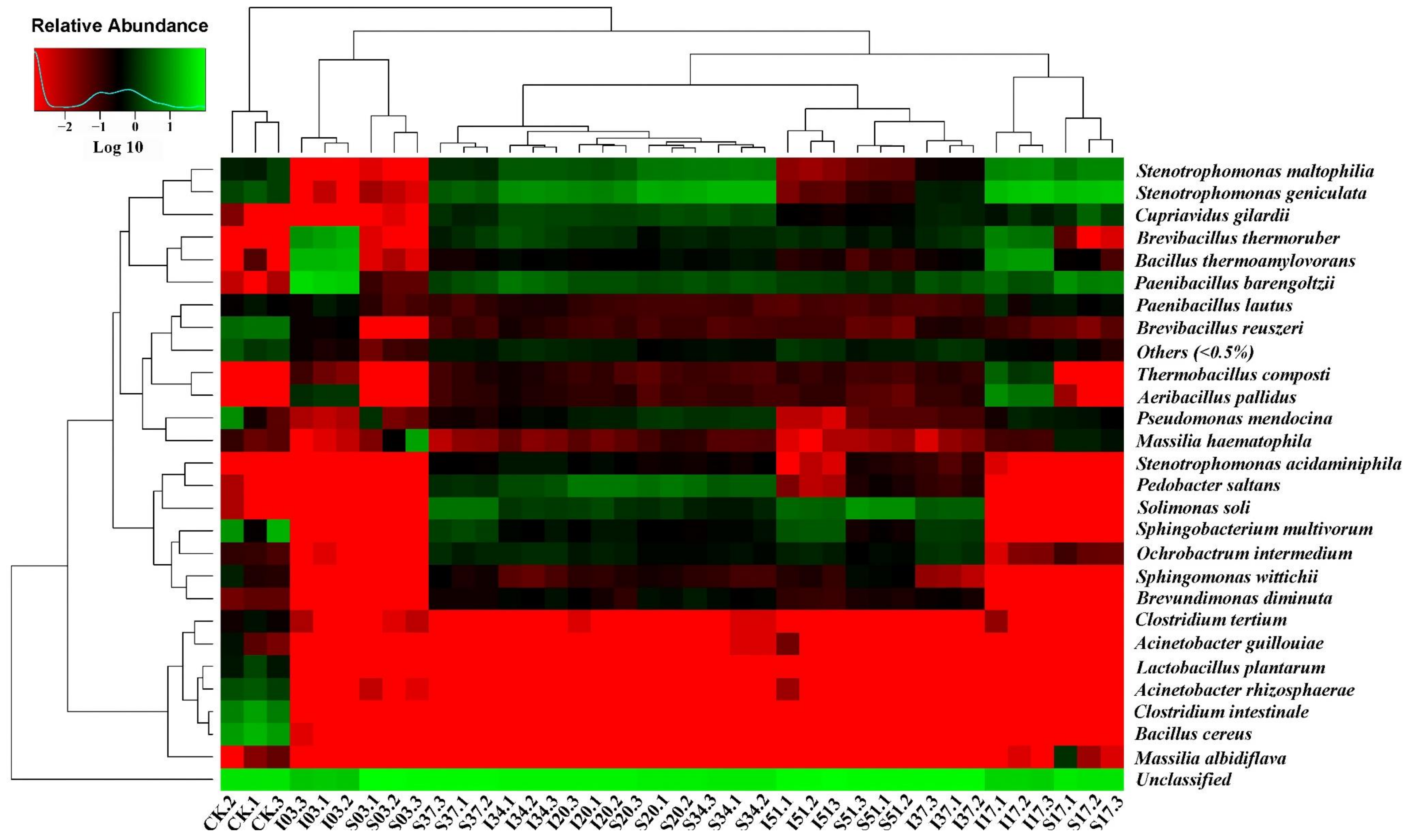
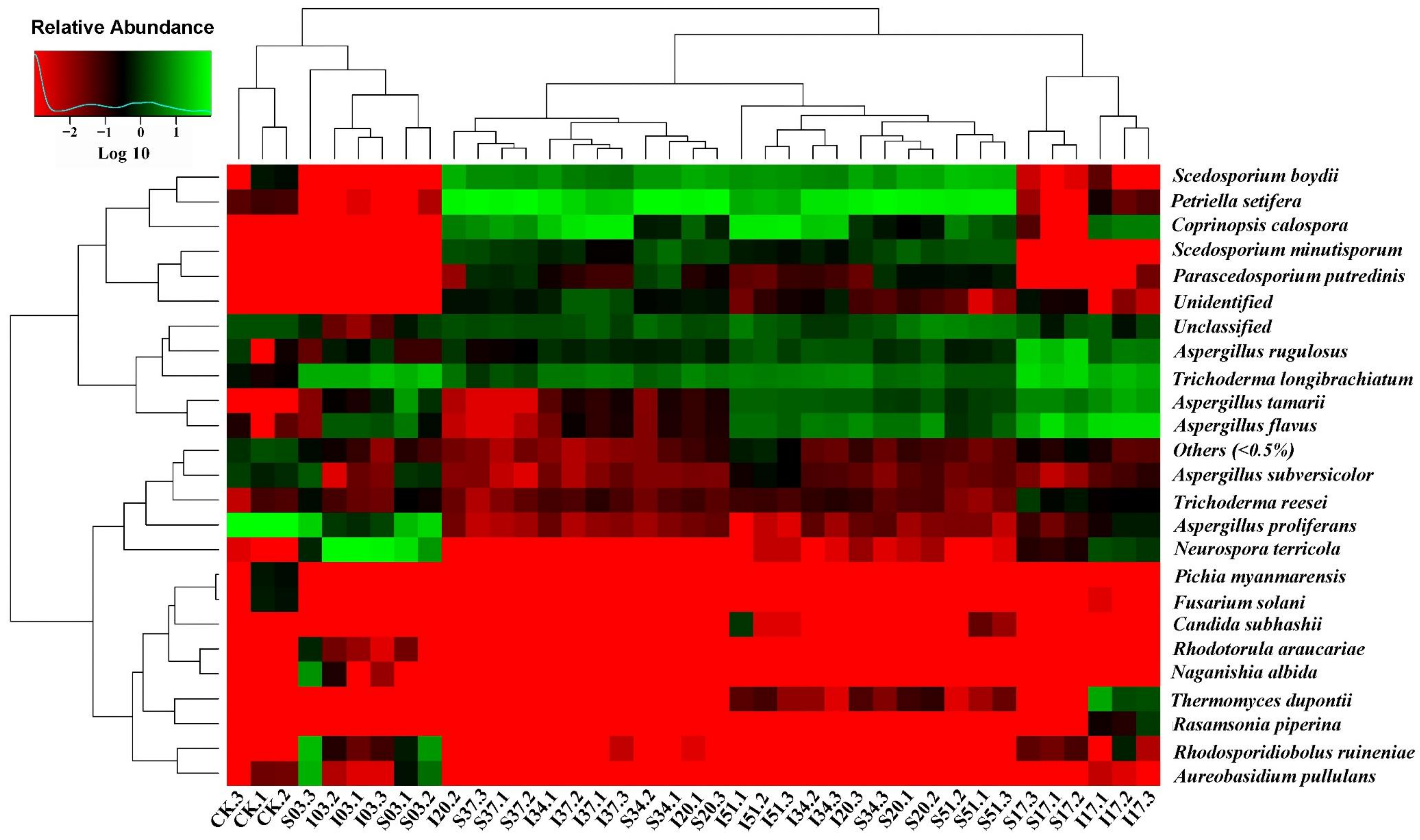
3.3. Microbial Communities
3.3.1. Diversity of the Microbial Communities
3.3.2. Changes in Microbial Community Composition
4. Conclusions
- (1)
- From the self-heating substances test experiments and Frank-Kamenetskii (FK) theory, for stacks of poplar sawdust, with the stack dimensions increased from 0.1 m to 3.2 m, the CAT of the sawdust stack would decrease from 158.27 °C to 102.46 °C. Apparently, the dimensions of sawdust stacks have a significant effect on the spontaneous combustion risk of sawdust stacks.
- (2)
- From the sawdust stack self-heating experiments, increasing water and oxygen would enhance microbial metabolic activities, which leads to a striking increase of the sawdust stack temperature and the quicker decomposition of the biochemical components (especially cellulose and hemicellulose) within three days after treatment.
- (3)
- From the microbial community analysis, different microbial populations dominated in the sawdust stacks at different stages. During the thermophilic stage (around 60 °C, large amounts of heat release in the compost bin), the dominant thermostable microbes (especially Brevibacillus thermoruber, Bacillus thermoamylovorans and Paenibacillus barengoltzii belonging to Firmicutes) played an important role in efficiently degrading organic substances.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Huang, D.; Zeng, G.; Feng, C.; Hu, S.; Lai, C.; Zhao, M.; Su, F.; Tang, L.; Liu, H. Changes of microbial population structure related to lignin degradation during lignocellulosic waste composting. Bioresour. Technol. 2010, 101, 4062–4067. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Sydor, M.; Bonenberg, A.; Doczekalska, B.; Cofta, G. Mycelium-Based Composites in Art, Architecture, and Interior Design: A Review. Polymers 2022, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.; Paredes, C.; Marhuenda-Egea, F.; Pérez-Espinosa, A.; Bernal, M.; Moral, R. Co-composting of distillery wastes with animal manures: Carbon and nitrogen transformations in the evaluation of compost stability. Chemosphere 2008, 72, 551–557. [Google Scholar] [CrossRef]
- McMahon, V.; Garg, A.; Aldred, D.; Hobbs, G.; Smith, R.; Tothill, I.E. Composting and bioremediation process evaluation of wood waste materials generated from the construction and demolition industry. Chemosphere 2008, 71, 1617–1628. [Google Scholar] [CrossRef]
- Hogland, W.; Marques, M. Physical, biological and chemical processes during storage and spontaneous combustion of waste fuel. Resour. Conserv. Recycl. 2003, 40, 53–69. [Google Scholar] [CrossRef]
- Jurado, M.; López, M.J.; Suárez-Estrella, F.; Vargas-García, M.C.; López-González, J.A.; Moreno, J. Exploiting composting biodiversity: Study of the persistent and biotechnologically relevant microorganisms from lignocellulose-based composting. Bioresour. Technol. 2014, 162, 283–293. [Google Scholar] [CrossRef]
- Xu, W.; He, Z.; Luo, Q.; Shen, H.; Liang, D.; Ren, T. The influence of soluble components on spontaneous combustion risk of sawdust samples. Thermochim. Acta 2018, 670, 219–225. [Google Scholar] [CrossRef]
- Belyaeva, O.; Haynes, R. Chemical, microbial and physical properties of manufactured soils produced by co-composting municipal green waste with coal fly ash. Bioresour. Technol. 2009, 100, 5203–5209. [Google Scholar] [CrossRef]
- Raut, M.P.; Prince William, S.P.M.; Bhattacharyya, J.K.; Chakrabarti, T.; Devotta, S. Microbial dynamics and enzyme activities during rapid composting of municipal solid waste—A compost maturity analysis perspective. Bioresour. Technol. 2008, 99, 6512–6519. [Google Scholar] [CrossRef]
- Galitskaya, P.; Biktasheva, L.; Saveliev, A.; Grigoryeva, T.; Boulygina, E.; Selivanovskaya, S. Fungal and bacterial successions in the process of co-composting of organic wastes as revealed by 454 pyrosequencing. PLoS ONE 2017, 12, e0186051. [Google Scholar] [CrossRef] [PubMed]
- Partanen, P.; Hultman, J.; Paulin, L.; Auvinen, P.; Romantschuk, M. Bacterial diversity at different stages of the composting process. BMC Microbiol. 2010, 10, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Luo, J.; Yan, S.; Chen, W.; Liu, X.; Zhang, Z. Changes in bacterial communities during two agricultural solid wastes’ co-composting processes. Ann. Microbiol. 2018, 68, 743–754. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Wei, Y.; Zhao, Y.; Lu, Q.; Liu, L.; Jiang, N.; Wei, Z. Biostimulation of nutrient additions on indigenous microbial community at the stage of nitrogen limitations during composting. Waste Manag. 2018, 74, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, X.; Xi, B.; Gao, R.; Tan, W.; Zhang, H.; Li, D. The evolution of water extractable organic matter and its association with microbial community dynamics during municipal solid waste composting. Waste Manag. 2016, 56, 79–87. [Google Scholar] [CrossRef]
- López-González, J.A.; López, M.J.; Vargas-García, M.C.; Suárez-Estrella, F.; Jurado, M.; Moreno, J. Tracking organic matter and microbiota dynamics during the stages of lignocellulosic waste composting. Bioresour. Technol. 2013, 146, 574–584. [Google Scholar] [CrossRef]
- Van Soest, P.v.; Robertson, J.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Viel, M.; Collet, F.; Lanos, C. Chemical and multi-physical characterization of agro-resources’ by-product as a possible raw building material. Ind. Crops Prod. 2018, 120, 214–237. [Google Scholar] [CrossRef]
- GB/T 21756-2008; Chemical Products for Industrial Use—Determination of Relative Temperature of Spontaneous Flammable of Solids. Standardization Administration of China: Beijing, China, 2008.
- NF T20-036; Chemical Products for Industrial Use-Determination of Relative Temperature of Spontaneous Flammable of Solids. European Committee for Standardization: Brussels, Belgium, 1985.
- United Nations. Recommendations on the Transport of Dangerous Goods: Manual of Tests and Criteria—Sixth Revised Edition, Amendment 1; United Nations Publication: New York, NY, USA; Geneva, Switzerland, 2018. [Google Scholar]
- Vargas-García, M.C.; Suárez-Estrella, F.; López, M.J.; Moreno, J. Microbial population dynamics and enzyme activities in composting processes with different starting materials. Waste Manag. 2010, 30, 771–778. [Google Scholar] [CrossRef]
- Biswas, R.; Teller, P.J.; Khan, M.U.; Ahring, B.K. Sugar Production from Hybrid Poplar Sawdust: Optimization of Enzymatic Hydrolysis and Wet Explosion Pretreatment. Molecules 2020, 25, 3396. [Google Scholar] [CrossRef]
- Bates, S.T.; Berg-Lyons, D.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. Examining the global distribution of dominant archaeal populations in soil. ISME J. 2011, 5, 908–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, H.; Jiang, H.; Krumholz, L.R.; Yang, Z. Bacterial community composition of size-fractioned aggregates within the phycosphere of cyanobacterial blooms in a eutrophic freshwater lake. PLoS ONE 2014, 9, e102879. [Google Scholar] [CrossRef] [Green Version]
- Gray, B.F. Spontaneous combustion and self-heating. In SFPE Handbook of Fire Protection Engineering; Hurley, M.J., Gottuk, D., Hall, J.R., Harada, K., Kuligowski, E., Puchovsky, M., Torero, J., Watts, J.M., Wieczorek, C., Eds.; Springer: New York, NY, USA, 2016; pp. 604–632. [Google Scholar]
- Stoessel, F. Thermal Safety of Chemical Processes: Risk Assessment and Process Design; Wiley: Weinheim, Germany, 2020; pp. 255–282. [Google Scholar]
- Vyazovkin, S. Kinetic concepts of thermally stimulated reactions in solids: A view from a historical perspective. Int. Rev. Phys. Chem. 2000, 19, 45–60. [Google Scholar] [CrossRef]
- Brown, M.; Maciejewski, M.; Vyazovkin, S.; Nomen, R.; Sempere, J.; Burnham, A.A.; Opfermann, J.; Strey, R.; Anderson, H.; Kemmler, A. Computational aspects of kinetic analysis: Part A: The ICTAC kinetics project-data, methods and results. Thermochim. Acta 2000, 355, 125–143. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry: Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics and Gaseous Products of Thermal Decomposition of Polymers. J. Macromol. Sci. Part A Chem. 1967, 1, 57–79. [Google Scholar] [CrossRef]
- Liu, X.W.; Feng, Y.L.; Li, H.R.; Zhang, P.; Wang, P. Thermal decomposition kinetics of magnesite from thermogravimetric data. J. Therm. Anal. Calorim. 2012, 107, 407–412. [Google Scholar] [CrossRef]
- Xu, M.; Guo, H.; Li, F.; Qin, Z. Thermal analysis kinetics of thermal decomposition of nickel–viscose composite fiber. J. Therm. Anal. Calorim. 2014, 118, 153–163. [Google Scholar] [CrossRef]
- Luo, Q.; Liang, D.; Mo, S. Numerical Calculation of the Critical Parameters of Frank-Kamenetskii Equation in Spontaneous Combustion Theory. Numer. Heat Transf. Part B Fundam. 2015, 68, 403–417. [Google Scholar] [CrossRef]
- Amir, S.; Hafidi, M.; Merlina, G.; Revel, J.-C. Sequential extraction of heavy metals during composting of sewage sludge. Chemosphere 2005, 59, 801–810. [Google Scholar] [CrossRef]
- Nayak, A.K.; Varma, V.S.; Kalamdhad, A.S. Effects of various C/N ratios during vermicomposting of sewage sludge using Eisenia fetida. J. Environ. Sci. Technol. 2013, 6, 63–78. [Google Scholar] [CrossRef] [Green Version]
- Ndegwa, P.M.; Thompson, S.A.; Das, K.C. Effects of stocking density and feeding rate on vermicomposting of biosolids. Bioresour. Technol. 2000, 71, 5–12. [Google Scholar] [CrossRef]
- Varma, V.S.; Das, S.; Sastri, C.V.; Kalamdhad, A.S. Microbial degradation of lignocellulosic fractions during drum composting of mixed organic waste. Sustain. Environ. Res. 2017, 27, 265–272. [Google Scholar] [CrossRef]
- Wei, H.; Wang, L.; Hassan, M.; Xie, B. Succession of the functional microbial communities and the metabolic functions in maize straw composting process. Bioresour. Technol. 2018, 256, 333–341. [Google Scholar] [CrossRef]
- Fimlaid, K.A.; Shen, A. Diverse mechanisms regulate sporulation sigma factor activity in the Firmicutes. Curr. Opin. Microbiol. 2015, 24, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, H.; Wang, Z.; Chen, G.; Wang, L. Dynamic changes of the dominant functioning microbial community in the compost of a 90-m3 aerobic solid state fermentor revealed by integrated meta-omics. Bioresour. Technol. 2016, 203, 1–10. [Google Scholar] [CrossRef]
- Dees, P.M.; Ghiorse, W.C. Microbial diversity in hot synthetic compost as revealed by PCR-amplified rRNA sequences from cultivated isolates and extracted DNA. Fems Microbiol. Ecol. 2001, 35, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-M.; Shyu, C.-L.; Ho, S.-P.; Chiou, S.-H. Species Diversity and Substrate Utilization Patterns of Thermophilic Bacterial Communities in Hot Aerobic Poultry and Cattle Manure Composts. Microb. Ecol. 2007, 54, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Song, Y.; Li, D.; Liu, R.; Niu, Q. The auto fluorescence characteristics, specific activity, and microbial community structure in batch tests of mono-chicken manure digestion. Waste Manag. 2019, 83, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.K.; Hemeda, N.F.; Abdelaliem, Y.F. Symbiotic cellulolytic bacteria from the gut of the subterranean termite Psammotermes hypostoma Desneux and their role in cellulose digestion. AMB Express 2019, 9, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Mao, H.; Li, X. Functional characteristics and influence factors of microbial community in sewage sludge composting with inorganic bulking agent. Bioresour. Technol. 2018, 249, 527–535. [Google Scholar] [CrossRef]
- Pertile, G.; Panek, J.; Oszust, K.; Siczek, A.; Oleszek, M.; Gryta, A.; Frąc, M. Effect of different organic waste on cellulose-degrading enzymes secreted by Petriella setifera in the presence of cellobiose and glucose. Cellulose 2019, 26, 7905–7922. [Google Scholar] [CrossRef] [Green Version]

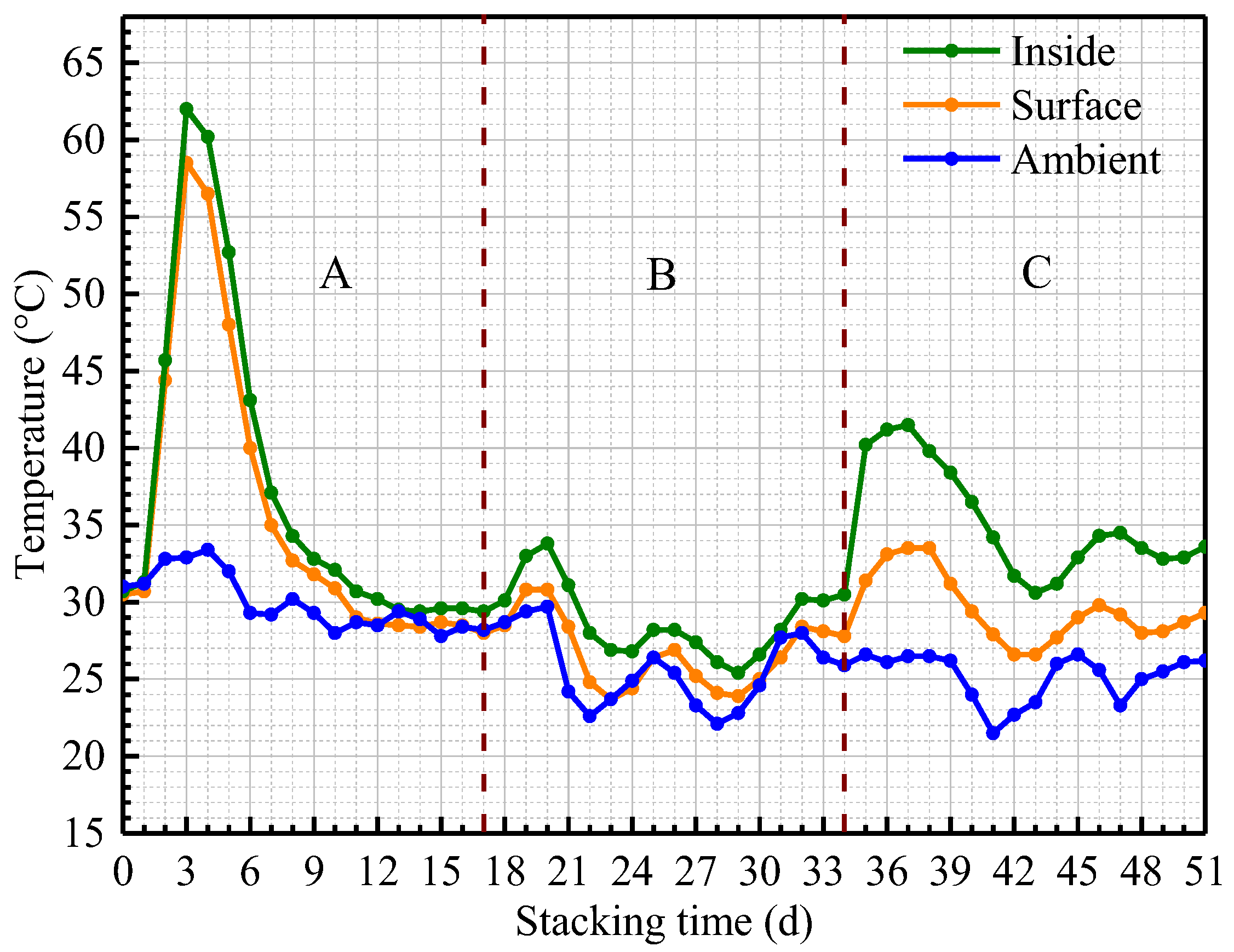
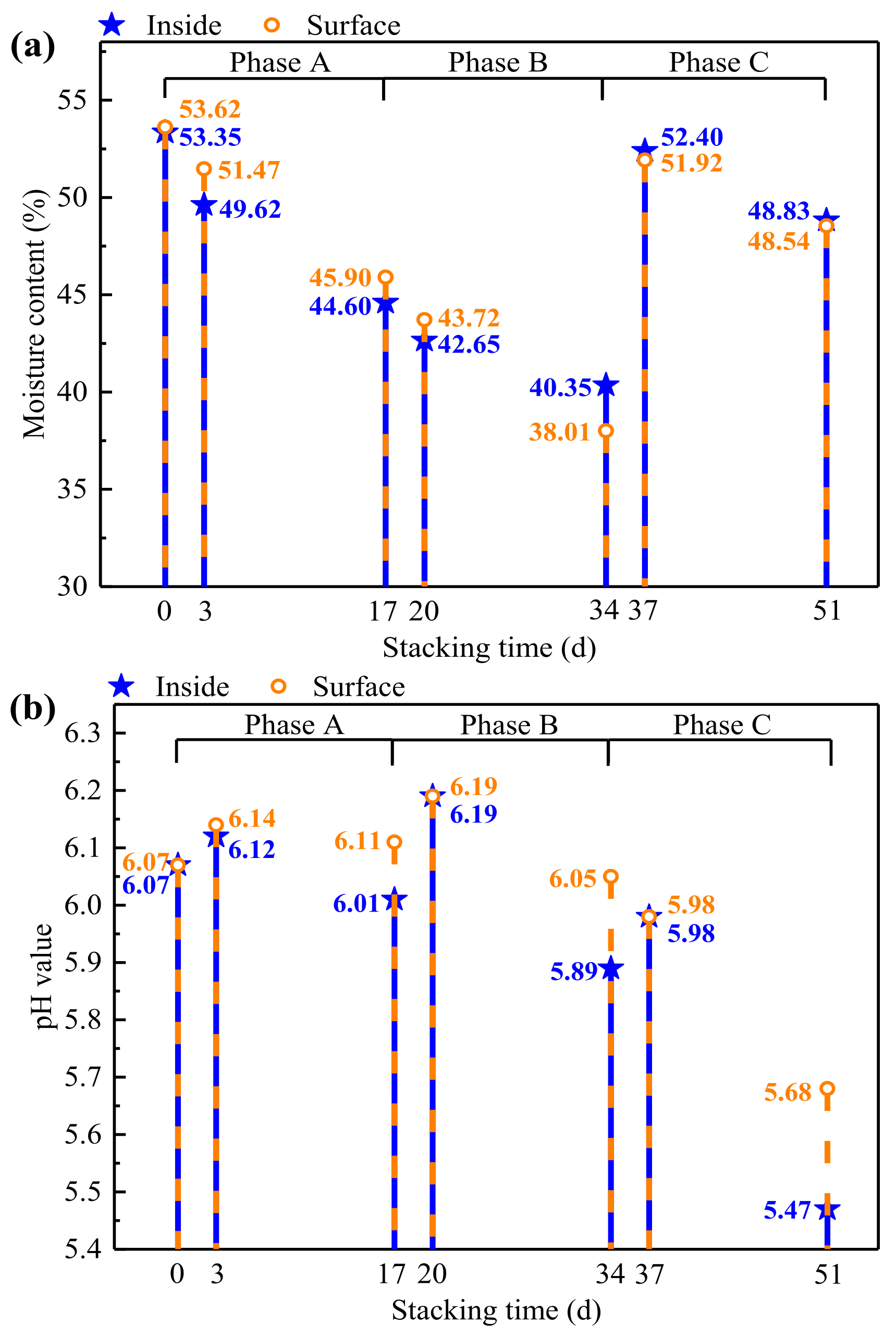
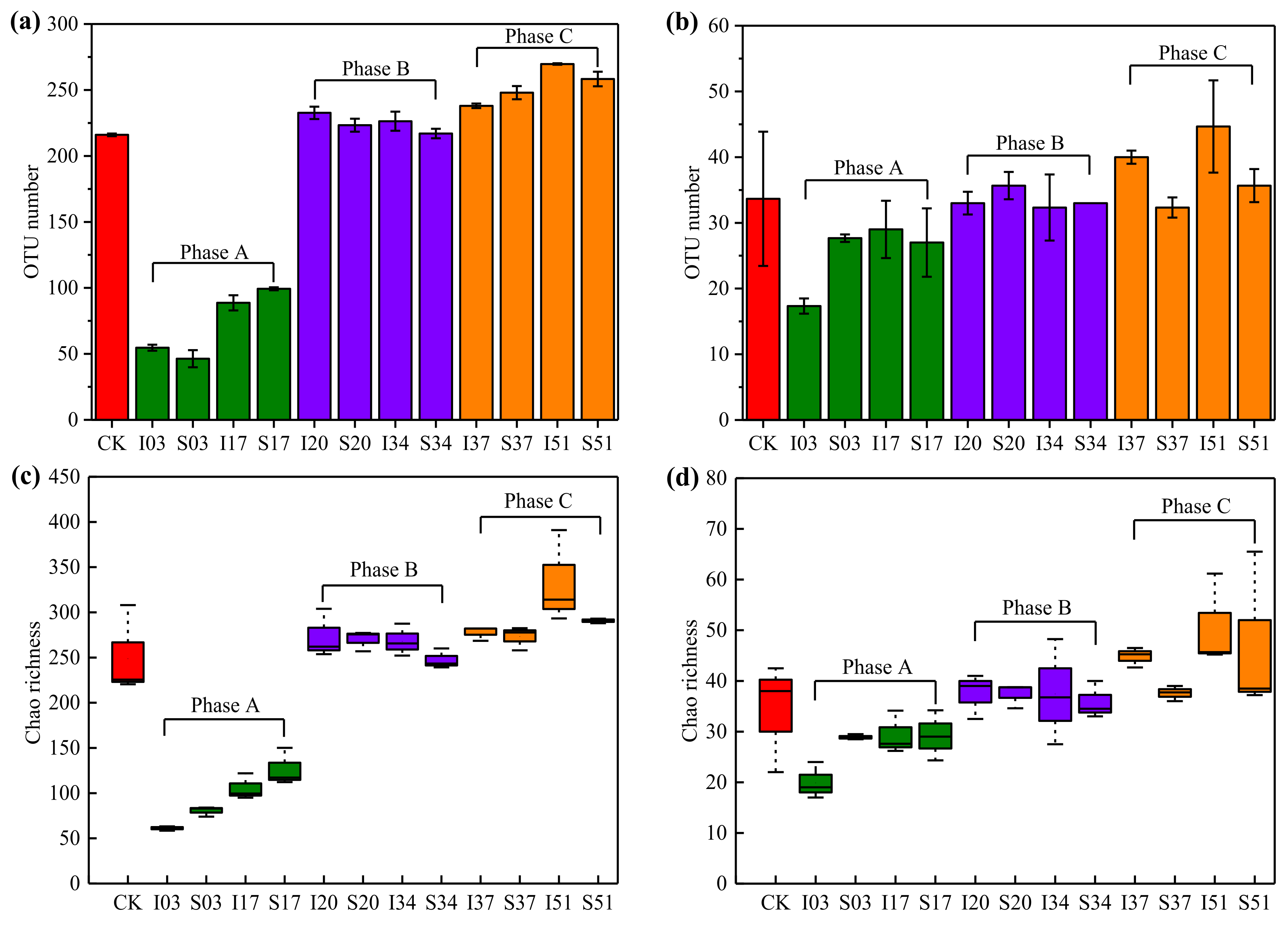
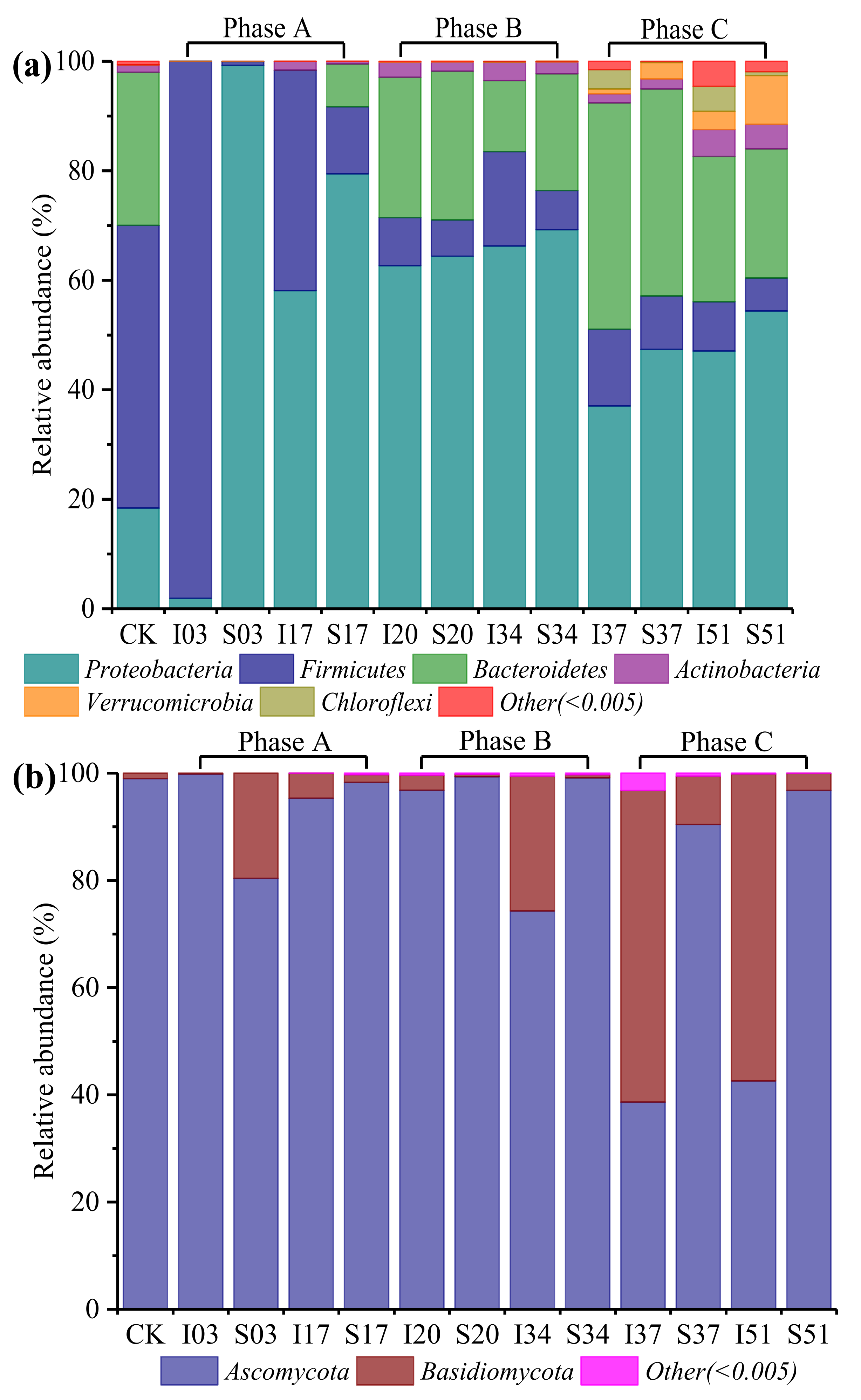
| Phase | Treatment | Days | Sample ID | Temperature (°C) |
|---|---|---|---|---|
| Raw sawdust (Day 0) | - | 0 | CK a | 30.6 |
| A (Days 1–17) | 1. Watered and Turned over (At 0st day after sampling) | 03 | I03 b | 62.0 |
| S03 c | 58.5 | |||
| 17 | I17 | 29.4 | ||
| S17 | 28.0 | |||
| B (Days 18–34) | 2. Turned over (At 17th day after sampling) | 20 | I20 | 33.8 |
| S20 | 30.8 | |||
| 34 | I34 | 30.5 | ||
| S34 | 27.8 | |||
| C (Days 35–51) | 3. Watered and Turned over (At 34th day after sampling) | 37 | I37 | 40.7 |
| S37 | 33.0 | |||
| 51 | I51 | 33.6 | ||
| S51 | 29.3 |
| Sample | α | E (kJ/mol) | Mean E (kJ/mol) |
|---|---|---|---|
| Poplar sawdust stack | 20% | 130.2 (±4.2) | 140.5 (±3.7) |
| 30% | 136.8 (±2.2) | ||
| 40% | 154.3 (±6.9) | ||
| 50% | 140.8 (±1.4) |
| Property | Sample | Stack Dimensions l (m) | |||||
| 0.1 * | 0.2 | 0.4 | 0.8 | 1.6 | 3.2 | ||
| CAT/°C (±0.01) | Poplar sawdust | 175.00 | 158.27 | 142.78 | 128.39 | 115.12 | 102.46 |
| Day | CK | Phase A | Phase B | Phase C | |||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 17 | 20 | 34 | 37 | 51 | |
| Inside | |||||||
| Cellulose (%) | 45.92 | 43.01 | 41.05 | 39.87 | 37.56 | 36.12 | 34.32 |
| Hemicellulose (%) | 20.89 | 19.58 | 18.01 | 17.64 | 16.95 | 16.42 | 15.45 |
| Lignin (%) | 25.44 | 24.95 | 24.34 | 24.08 | 23.36 | 23.01 | 22.26 |
| Others (%) * | 7.75 | 12.46 | 16.60 | 18.41 | 22.13 | 24.45 | 27.97 |
| Surface | |||||||
| Cellulose (%) | 45.92 | 43.65 | 40.23 | 38.95 | 37.31 | 35.58 | 33.20 |
| Hemicellulose (%) | 20.89 | 19.72 | 17.82 | 17.32 | 16.45 | 15.94 | 14.86 |
| Lignin (%) | 25.44 | 25.01 | 24.23 | 23.97 | 23.12 | 22.81 | 21.98 |
| Others (%) | 7.75 | 11.62 | 17.72 | 19.76 | 23.12 | 25.67 | 29.96 |
| Days | Phase A | Phase B | Phase C | |||
|---|---|---|---|---|---|---|
| 1–3 | 4–17 | 18–20 | 21–34 | 35–37 | 38–51 | |
| Inside | ||||||
| Cellulose (%) | 0.97 | 0.14 | 0.39 | 0.17 | 0.48 | 0.13 |
| Hemicellulose (%) | 0.44 | 0.11 | 0.12 | 0.05 | 0.18 | 0.07 |
| Lignin (%) | 0.16 | 0.04 | 0.09 | 0.05 | 0.12 | 0.05 |
| Total (%) | 1.57 | 0.29 | 0.60 | 0.27 | 0.78 | 0.25 |
| Surface | ||||||
| Cellulose (%) | 0.76 | 0.24 | 0.43 | 0.12 | 0.58 | 0.17 |
| Hemicellulose (%) | 0.39 | 0.14 | 0.17 | 0.06 | 0.17 | 0.08 |
| Lignin (%) | 0.14 | 0.06 | 0.09 | 0.06 | 0.10 | 0.06 |
| Total (%) | 1.29 | 0.44 | 0.69 | 0.24 | 0.85 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Xu, W.; He, Z.; Shen, H. Poplar Sawdust Stack Self-Heating Properties and Variations of Internal Microbial Communities. Materials 2022, 15, 1114. https://doi.org/10.3390/ma15031114
Yuan Z, Xu W, He Z, Shen H. Poplar Sawdust Stack Self-Heating Properties and Variations of Internal Microbial Communities. Materials. 2022; 15(3):1114. https://doi.org/10.3390/ma15031114
Chicago/Turabian StyleYuan, Zitao, Wenbin Xu, Zili He, and Hao Shen. 2022. "Poplar Sawdust Stack Self-Heating Properties and Variations of Internal Microbial Communities" Materials 15, no. 3: 1114. https://doi.org/10.3390/ma15031114
APA StyleYuan, Z., Xu, W., He, Z., & Shen, H. (2022). Poplar Sawdust Stack Self-Heating Properties and Variations of Internal Microbial Communities. Materials, 15(3), 1114. https://doi.org/10.3390/ma15031114







