Synthesis of Novel Nano-Sulfonamide Metal-Based Corrosion Inhibitor Surfactants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Test Samples
2.1.1. Synthesis of Sulfonamide Hydrogen Selenites IIa
2.1.2. Synthesis of Metal Complexes
- (a)
- Synthesis of cobalt and platinum hydrogen selenite dehydrate [35].PtCl2·2H2O + Na2CO3 → PtCO3·2H2O + 2NaClCoCl2·2H2O + Na2CO3 → CoCO3·2H2O + 2NaClPtCO3·2H2O + 2H2SeO3 → Pt(HSeO3)2 +2H2OCoCO3·2H2O + 2H2SeO3 → Co(HSeO3)2 + 2H2O
- (b)
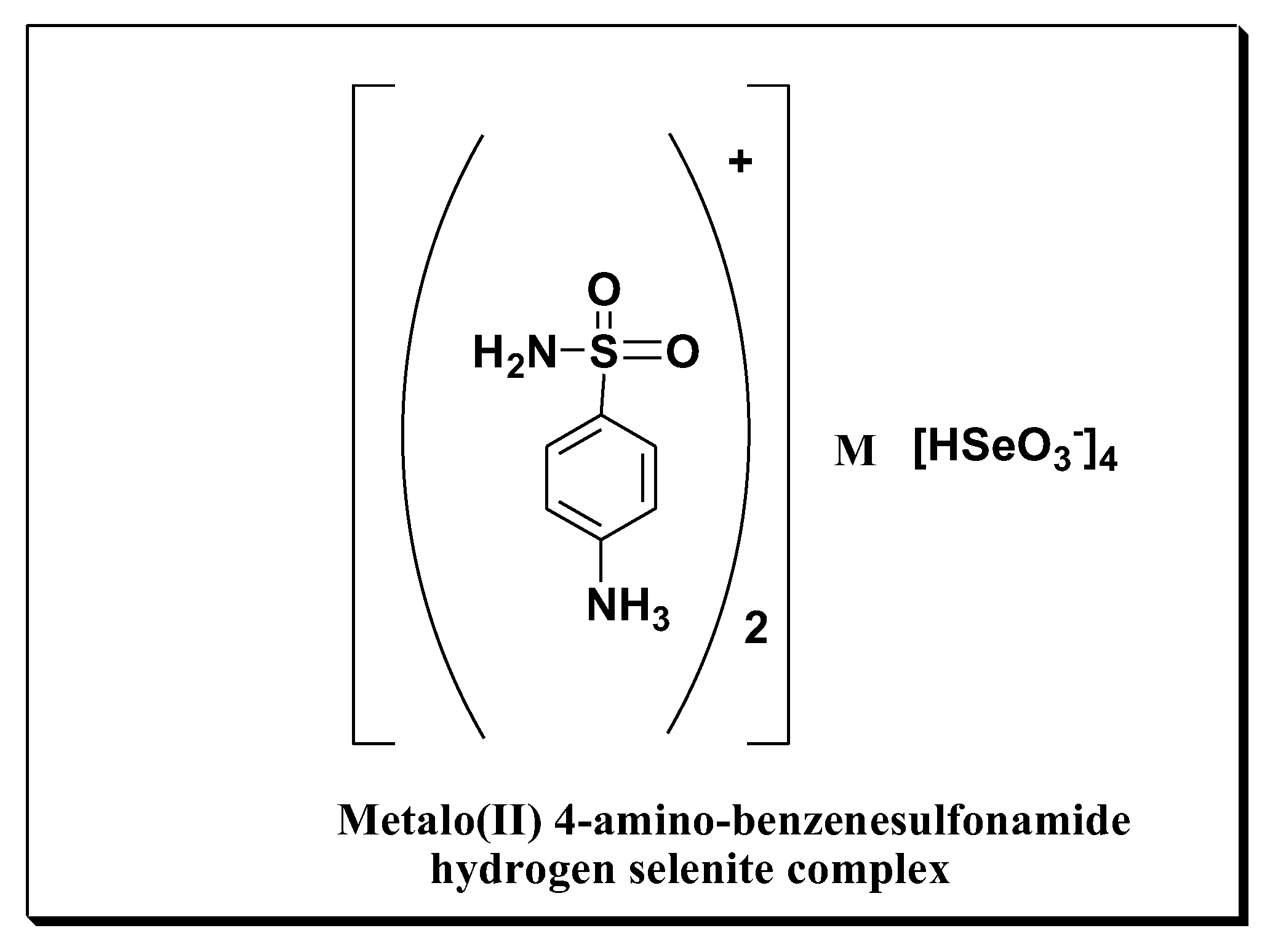
- Synthesis of platinum and cobalt ammonium hydrogen selenite complexes IIb,c.
2.1.3. General Formula for the Metal Complexes
2.1.4. Green Synthesis with Solid State Reaction in Ball Mill for Complex Nanoparticles
2.1.5. Evaluation of Anti-Sulfate-Reducing Bacteria Activity
2.2. Corrosion Inhibition Measurements
2.2.1. Weight Loss Measurements
2.2.2. Polarization Measurements
2.2.3. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Surface Properties of the Prepared Cationic Surfactants
3.2. Antibacterial Activity of the Prepared Surfactants against Sulfate-Reducing Bacteria
Corrosion Inhibitor Activity for Oil Pipes
3.3. Results of Weight Loss (Gravimeteric) Method and Effect of Inhibitor Concentration
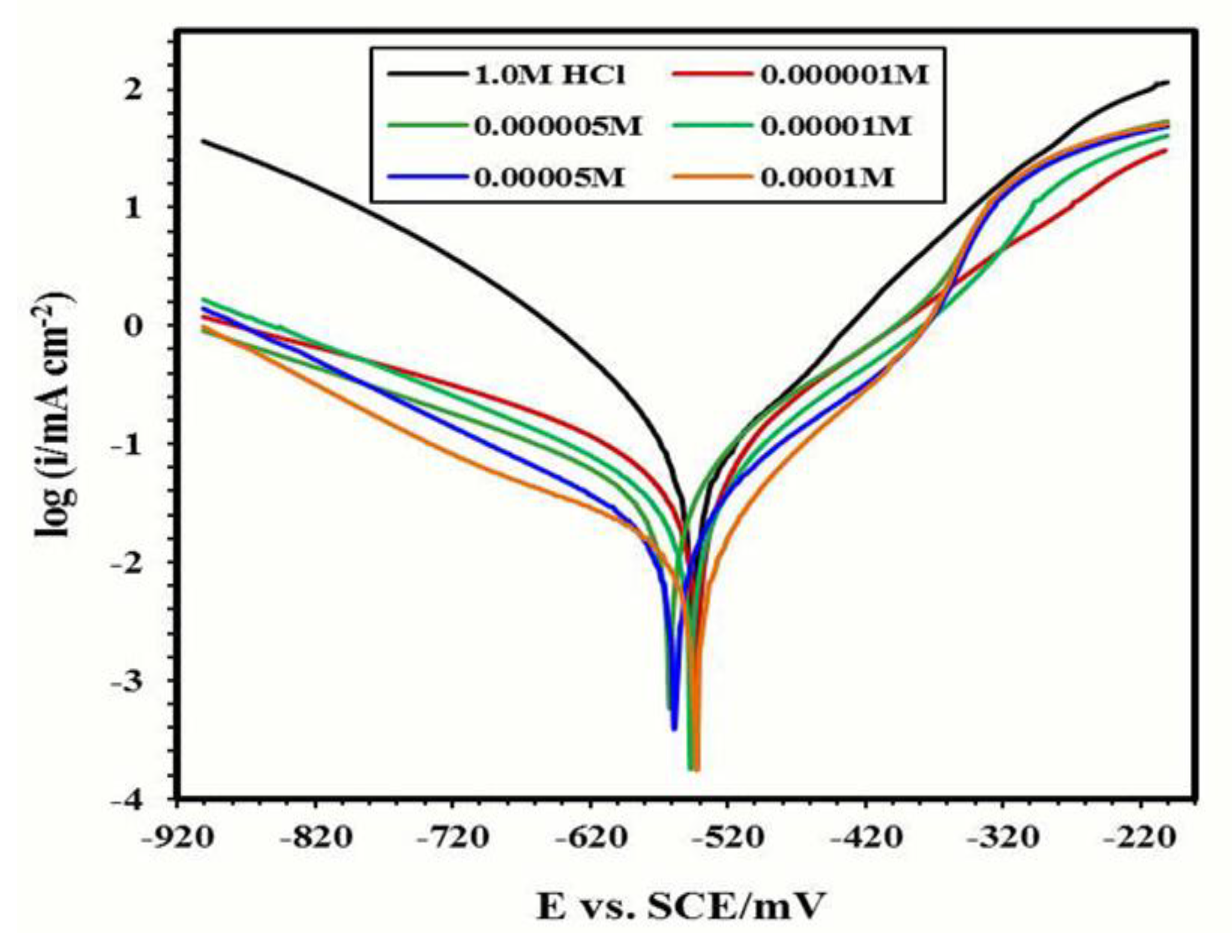
3.4. Electrochemical Evaluation
Potentiodynamic Polarization Spectroscopy
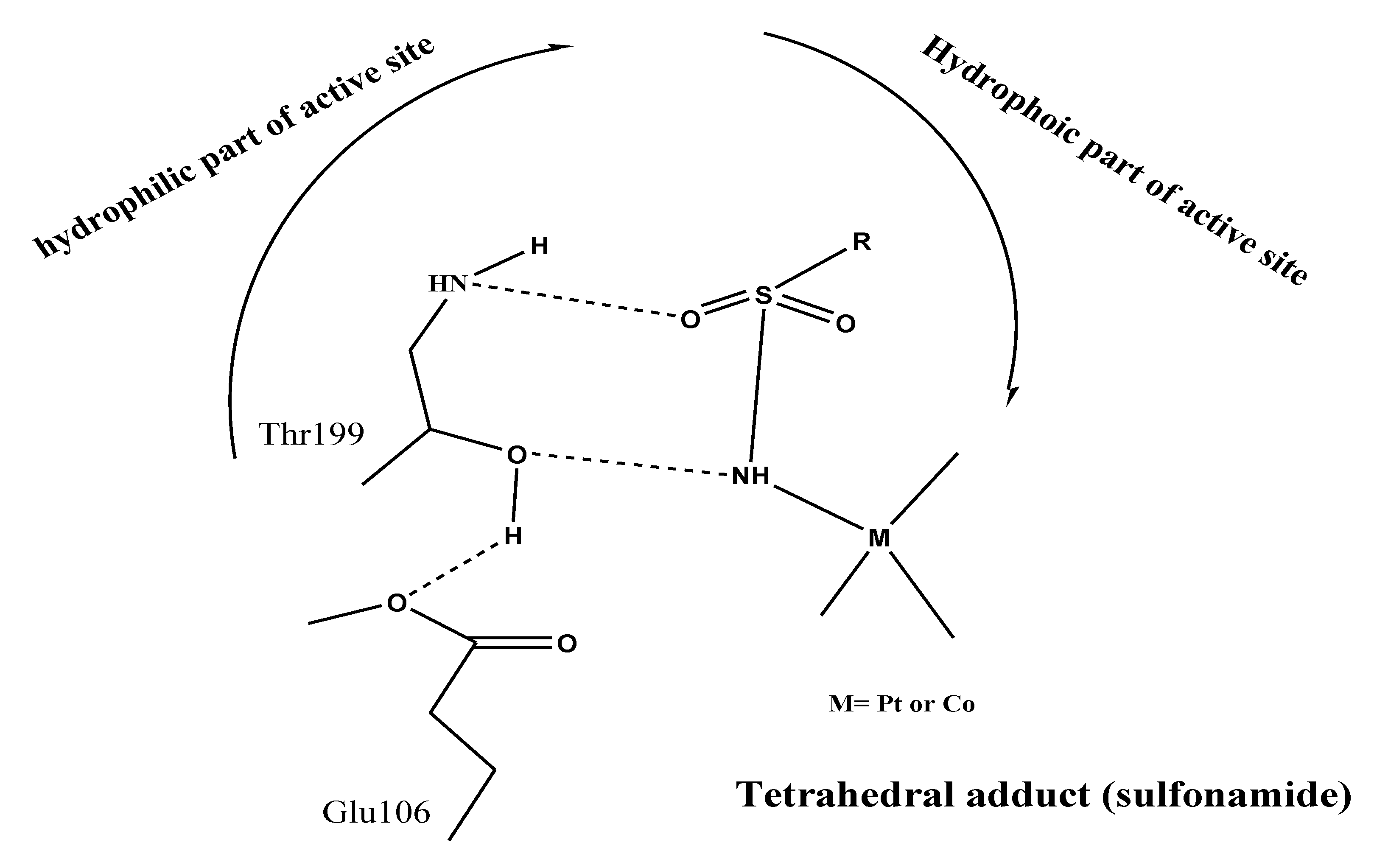
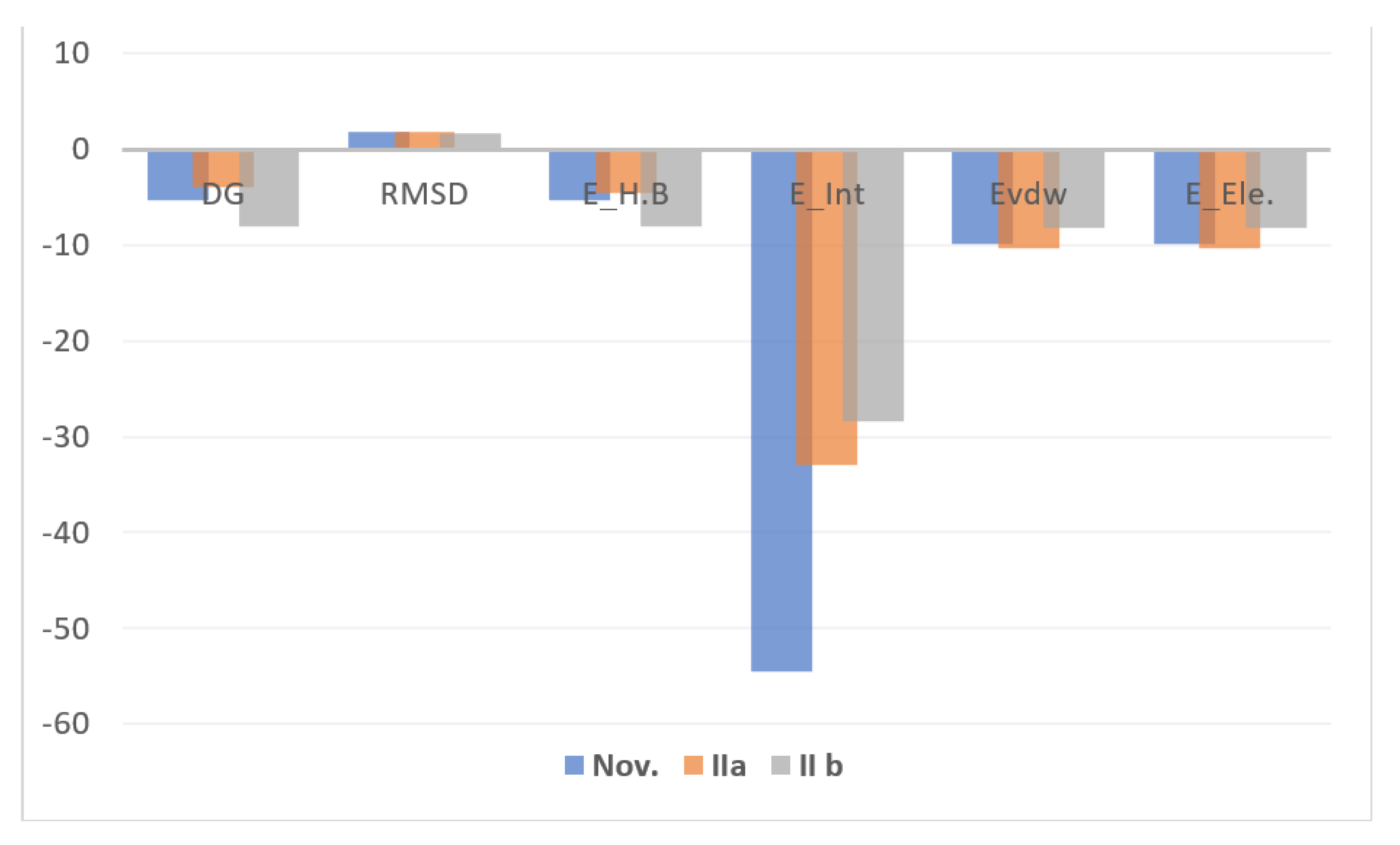
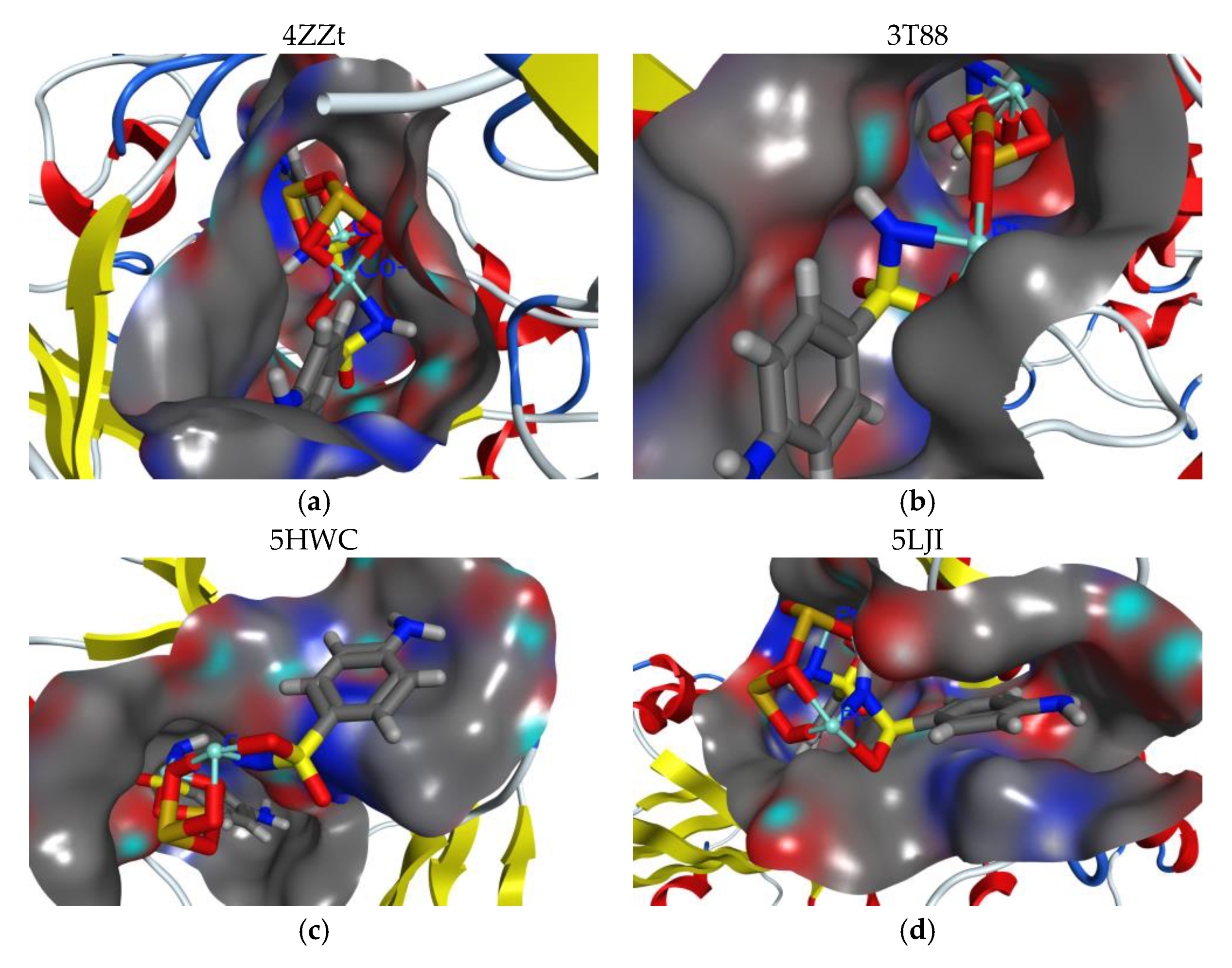
3.5. Docking Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, C.; Haque, J.; Quraishi, M.A.; Ebenso, E.E. Aqueous phase environmental friendly organic corrosion inhibitors derived from one step multicomponent reactions: A review. J. Mol. Liq. 2018, 275, 18–40. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M. Ionic liquids as green and sustainable corrosion inhibitors for metals and alloys: An overview. J. Mol. Liq. 2017, 233, 403–414. [Google Scholar] [CrossRef]
- Popova, A. Temperature effect on mild steel corrosion in acid media in presence of azoles. Corros. Sci. 2007, 49, 2144–2158. [Google Scholar] [CrossRef]
- Ahamad, I.; Quraishi, M.A. Bis-(benzimidazol-2-yl) disulphide: An efficient water soluble inhibitor for corrosion of mild steel in acid media. Corros. Sci. 2009, 51, 2006–2013. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O. Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: Experimental and theoretical investigation. Corros. Sci. 2010, 52, 198–204. [Google Scholar] [CrossRef]
- Popova, A.; Sokolova, E.; Raicheva, S.; Christov, M. AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros. Sci. 2003, 45, 33–58. [Google Scholar] [CrossRef]
- Shukla, S.K.; Quraishi, M.A. 4-Substituted anilinomethyl propionate: New and efficient corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2009, 51, 1990–1997. [Google Scholar] [CrossRef]
- Behpour, M.; Ghoreishi, S.M.; Soltani, N.; Salavati-Niasari, M.; Hamadanian, M.; Gandomi, A. Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid solution. Corros. Sci. 2008, 50, 2172–2181. [Google Scholar] [CrossRef]
- Qiu, L.; Wu, Y.; Wang, Y.; Jiang, X. Synergistic effect between cationic gemini surfactant and chloride ion for the corrosion inhibition of steel in sulphuric acid. Corros. Sci. 2008, 50, 576–582. [Google Scholar] [CrossRef]
- Hosseini, S.M.A.; Azimi, A. The inhibition of mild steel corrosion in acidic medium by 1-methyl-3-pyridin-2-yl-thiourea. Corros. Sci. 2009, 51, 728–732. [Google Scholar] [CrossRef]
- Ali, S.A.; Saeed, M.T.; Rahman, S.U. The isoxazolidines: A new class of corrosion inhibitors of mild steel in acidic medium Corros. Sci. 2003, 45, 253. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Liu, F.; Hou, B. Investigation on inhibition behavior of S-triazole–trizaole derivatives in acidic solution. Corros. Sci. 2008, 50, 3261–3266. [Google Scholar] [CrossRef]
- Palomar-Pardavé, M.; Romero-Romo, M.; Herrera-Hernández, H.; Abreu-Quijano, M.A.; Likhanova, N.V.; Uruchurtu, J.; Juárez-García, J.M. Influence of the alkyl chain length of 2-amino-5-alkyl-1,3,4- thiadiazole compounds on the corrosion inhibition of steel immersed in sulfuric acid solutions. Corros. Sci. 2012, 54, 231–243. [Google Scholar] [CrossRef]
- Chetouani, A.; Hammouti, B.; Benhadda, T.; Daoudi, M. Inhibitive action of bipyrazolic type organic compounds towards corrosion of pure iron in acidic media. Appl. Surf. Sci. 2005, 249, 375–385. [Google Scholar] [CrossRef]
- Popova, A.; Christov, M.; Raicheva, S.; Sokolova, E. Adsorption and inhibitive properties of benzimidazole derivatives in acid mild steel corrosion. Corros. Sci. 2004, 46, 1333–1350. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O.; Umoren, S.Z. The synergistic inhibitive effect and some quantum chemical parameters of 2,3-diaminonaphthalene and iodide ions on the hydrochloric acid corrosion of aluminium. Corros. Sci. 2009, 51, 276–282. [Google Scholar] [CrossRef]
- Herrag, L.; Hammouti, B.; Elkadiri, S.; Aouniti, A.; Jama, C.; Vezin, H.; Bentiss, F. Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: Experimental and theoretical investigations. Corros. Sci. 2010, 52, 3042–3051. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Wang, F. Role of nitrite addition in chloride stress corrosion cracking of a super duplex stainless steel. Corros. Sci. 2011, 53, 113–121. [Google Scholar] [CrossRef]
- Negm, N.A.; Kandile, N.G.; Badr, E.A.; Mohammed, M.A. Gravimetric and electrochemical evaluation of environmentally friendly nonionic corrosion inhibitors for carbon steel in 1M HCl. Corros. Sci. 2012, 65, 94–103. [Google Scholar] [CrossRef]
- Gust, J. Application of infrared spectroscopy for investigation of rust phase component conversion by agents containing oak tannin and phosphoric acid. NACE 1991, 47, 453–457. [Google Scholar] [CrossRef]
- Jaen, J.A.; Saldana, G.; Hernandez, C. Characterization of reaction products of iron and aqueous plant extracts. Hyperfine Interact. 1999, 122, 139–145. [Google Scholar] [CrossRef]
- Rahim, A.A.; Rocca, E.; Steinmetz, J.; Kassim, M.J. Inhibitive action of mangrove tannins and phosphoric acid on pre-rusted steel via electrochemical methods. Corros. Sci. 2008, 50, 1546–1550. [Google Scholar] [CrossRef]
- Ostovari, A.; Hoseinieh, S.M.; Peikari, M.; Shadizadeh, S.R.; Hashemi, S.J. Corrosion inhibition of mild steel in 1M HCl solution by henna extract: A comparative study of the inhibition by henna and its constituents. Corros. Sci. 2009, 51, 1935–1944. [Google Scholar] [CrossRef]
- Saroja, N.; Shamala, T.R.; Tharanathan, R.N. Biodegradation of starch-g-polyacrylonitrile, a packaging material, by Bacillus cereus. Process Biochem. 2000, 36, 119–125. [Google Scholar] [CrossRef]
- Rezende, R.; Dias, J.; Ferraz, V.; Basic, J. Metabolism of benzonitrile by Cryptococcus sp. UFMG-Y28. J. Basic Microbiol. 2000, 40, 389–392. [Google Scholar] [CrossRef]
- Holmes, P.M. Factors Affecting the Selection of Cutting Fluids. Ind. Lubr. Tribol. 1971, 2, 47–55. [Google Scholar] [CrossRef]
- Kumar, R.; Chopra, R.; Singh, G. Electrochemical, morphological and theoretical insights of a new environmentally benign organic inhibitor for mild steel corrosion in acidic media. J. Mol. Liq. 2017, 241, 9–19. [Google Scholar] [CrossRef]
- Fouda, A.; Ismail, M.; EL-Elewady, G.; Abousalem, A. Evaluation of 4-amidinophenyl2, 2′-bithiophene and its aza-analogue as novel corrosion inhibitors for CS in acidic media: Experimental and theoretical study. J. Mol. Liq. 2017, 240, 372–388. [Google Scholar] [CrossRef]
- Haque, J.; Jafar Mazumder, M.A.; Quraishi, M.A. Pyrrolidine-based quaternary ammonium salts containing propargyl and hydrophobic C-12 and C-16 alkyl chains as corrosion inhibitors in aqueous acidic media. J. Mol. Liq. 2020, 320, 11473. [Google Scholar] [CrossRef]
- Huilong, W.; Jiashen, Z.; Jing, L. Inhibition of the corrosion of carbon steel in hydrochloric acid solution by bisquaternary ammonium salt. Anti-Corros. Method. M 2002, 49, 127–132. [Google Scholar] [CrossRef]
- Walker, M.L. Method and Composition for Acidizing Subterranean Formations. U.S. Patent 4498997, 12 February 1985. [Google Scholar]
- Ali, S.A.; El-Shareef, A.; Al-Ghamdi, R.; Saeed, M. The isoxazolidines: The effects of steric factor and hydrophobic chain length on the corrosion inhibition of mild steel in acidic medium. Corros. Sci. 2005, 47, 2659–2678. [Google Scholar] [CrossRef]
- Jing, C.; Wang, Z.; Gong, Y.; Huang, H.; Ma, Y.; Xie, H.; Li, H.; Zhang, S.; Gao, F. Photo and thermally stable branched corrosion inhibitors containing two benzotriazole groups for copper in 3.5 wt% sodium chloride solution. Corros. Sci. 2018, 138, 353–371. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Milić, S.M.; Petrović, M.B. Films formed on copper surface in chloride media in the presence of azoles. Corros. Sci. 2009, 51, 1228–1237. [Google Scholar] [CrossRef]
- Badawi, A.M.; Mekawi, M.A.; Mohamed, M.Z.; Mohamed, A.S.; Khowdairy, M.M. Surface and Biological Activity of Organoammonium Hydrogen Selenite Surfactants. J. Surfactants Deterg. 2007, 10, 257–267. [Google Scholar] [CrossRef]
- Supuran, C.T.; Scozzafava, A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg. Med. Chem. 2007, 15, 4336–4350. [Google Scholar] [CrossRef]
- Khowdiary, M.M.; El-Henawy, A.A.; Shawky, A.M.; Negm, N.A. Evaluation of cationic inhibitors derived from recycled polyethylene terephthalate wastes in protection of carbon steel against acidic corrosion in oil fields. J. Mol. Liq. 2020, 320, 114504. [Google Scholar] [CrossRef]
- Khowdiary, M.M.; El-Henawy, A.A.; Shawky, A.M.; Sameeh, M.Y.; Negm, N.A. Synthesis, characterization of silver nanoparticles-cationic quaternary ammonium polymers and evaluation as biocides against sulfate reducing bacteria. J. Mol. Liq. 2016, 12, 939. [Google Scholar]
- Aljourani, J.; Raeissi, K.; Golozar, M.A. Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1M HCl solution. Corros. Sci. 2009, 51, 1836–1843. [Google Scholar] [CrossRef]
- Ashassi Sorkhabi, H.; Shaabani, B.; Seifzadeh, D. Corrosion inhibition of mild steel by some Schiff base compounds in hydrochloric acid. Appl. Surf. Sci. 2005, 239, 154. [Google Scholar] [CrossRef]
- Moretti, G.; Guidi, F.; Grion, G. Tryptamine as a green iron corrosion inhibitor in 0.5 M deaerated sulphuric acid. Corros. Sci. 2004, 46, 387. [Google Scholar] [CrossRef]
- Cachet, C.; Keddam, M.; Mariotte, V.; Wiart, R. Influence of perfluorinated and hydrogenated surfactants upon hydrogen evolution on gold electrodes. Electrochim. Acta 1994, 39, 2743–2750. [Google Scholar] [CrossRef]
- Bentiss, F.; Bouanis, M.; Mernari, B.; Traisnel, M.; Vezin, H.; Lagrenee, M. Understanding the adsorption of 4H–1,2,4–triazole derivatives on mild steel surface in molar hydrochloric acid. Appl. Surf. Sci. 2006, 253, 3696–3703. [Google Scholar] [CrossRef]
- Negm, N.A.; Al Sabagh, A.M.; Migahed, M.A.; Abdel Bary, H.M.; El Din, H.M. Effectiveness of some diquaternary ammonium surfactants as corrosion inhibitors for carbon steel in 0.5 M HCl solution. Corros. Sci. 2010, 52, 2122–2132. [Google Scholar] [CrossRef]
- Behpour, M.; Ghoreishi, S.M.; Salavati-Niasari, M.; Ebrahimi, B. Evaluating two new synthesized S–N Schiff bases on the corrosion of copper in 15% hydrochloric acid. Mater. Chem. Phys. 2008, 107, 153–157. [Google Scholar] [CrossRef]
- Negm, N.A.; Ghuiba, E.M.; Mahmoud, S.A.; Tawfik, S.M. Biocidal and anti-corrosive activities of benzoimidazol-3-ium cationic Schiff base surfactants. Eng. Life Sci. 2011, 11, 496–510. [Google Scholar] [CrossRef]
- Tonthat, N.K.; Juvvadi, P.R.; Zhang, H.; Lee, S.C.; Venters, R.; Spicer, L.; Steinbach, W.J.; Heitman, J.; Schumacher, M.A. Structures of pathogenic fungal FKBP12s reveal possible self-catalysis function. MBio 2016, 7, e00492-16. [Google Scholar] [CrossRef] [Green Version]
- Borisova, A.S.; Eneyskaya, E.V.; Bobrov, K.S.; Jana, S.; Logachev, A.; Polev, D.E.; Lapidus, A.L.; Ibatullin, F.M.; Saleem, U.; Sandgren, M.; et al. Sequencing, biochemical characterization, crystal structure and molecular dynamics of cellobiohydrolase Cel7A from Geotrichum candidum 3C. FEBS J. 2015, 282, 4515–4537. [Google Scholar] [CrossRef]
- Rodríguez-Cárdenas, Á.; Rojas, A.L.; Conde-Giménez, M.; Velázquez-Campoy, A.; Hurtado-Guerrero, R.; Sancho, J. Streptococcus pneumoniae TIGR4 flavodoxin: Structural and biophysical characterization of a novel drug target. PLoS ONE 2016, 11, e0161020. [Google Scholar] [CrossRef] [Green Version]
- Li, H.J.; Li, X.; Liu, N.; Zhang, H.; Truglio, J.J.; Mishra, S.; Kisker, C.; Garcia-Diaz, M.; Tonge, P.J. Mechanism of the intramolecular Claisen condensation reaction catalyzed by MenB, a crotonase superfamily member. Biochemistry 2011, 50, 9532–9544. [Google Scholar] [CrossRef] [Green Version]
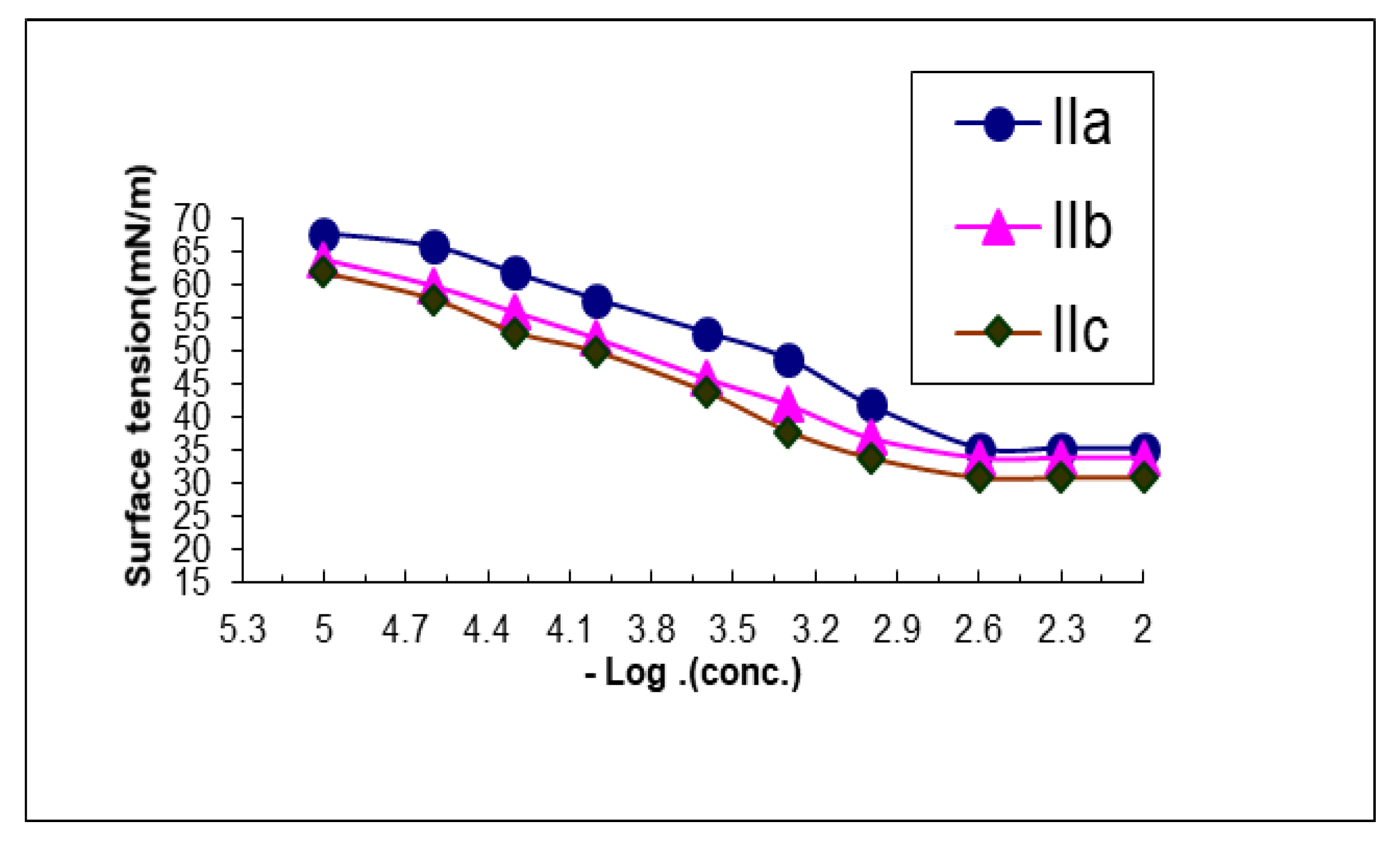

| Comp. No. | CMC X 10−3 | Γcmc (mN/m) | Πcmc (mN/m) | PC20 (Mole/L) | Γmax X 10−11 (Mole/cm2) | Amin (nm2) | Δ Gads | Δ Gmic | ΔGads/Amin |
|---|---|---|---|---|---|---|---|---|---|
| IIa | 1.2 | 32 | 40 | 3.9 | 10.4 | 1.5 | −67.7 | −34.1 | −46.8 |
| IIb | 1.1 | 30 | 42 | 4.1 | 10.2 | 1.5 | −69.9 | −34.8 | −49.1 |
| IIc | 0.80 | 29 | 43 | 4.3 | 11.1 | 1.45 | −71.1 | −35.3 | −50.2 |
| Sample | Inhibition Zone Diameter (Iz D) (mm/mg Sample) Sulfate-Reducing Bacteria |
|---|---|
| IIa | 22 |
| IIb | 20 |
| IIc | 18 |
| Temperature °C | Conc. of Inhibitor M | K mg cm−2 h−2 | ηw % |
|---|---|---|---|
| 30 | 0.00 | 1.307 | - |
| 1 × 10−4 | 0.838 | 35.86 | |
| 5 × 10−4 | 0.643 | 50.80 | |
| 1 × 10−3 | 0.402 | 69.26 | |
| 5 × 10−3 | 0.298 | 77.16 | |
| 1 × 10−2 | 0.241 | 81.54 |
| Temperature °C | Conc. of Inhibitor M | K mg cm−2 h−2 | ηw % |
|---|---|---|---|
| 30 | 0.00 | 1.4 | - |
| 1 × 10−4 | 0.5 | 61.82 | |
| 5 × 10−4 | 0.261 | 80.01 | |
| 1 × 10−3 | 0.222 | 83.01 | |
| 5 × 10−3 | 0.103 | 92.11 | |
| 1 × 10−2 | 0.070 | 94.68 |
| Inhibitor Name | Conc. of Inhibitor (M) | Ecorr (mV) | Icorr (mAcm−2) | βa (mV/Decade) | βc (mV/Decade) | θ | ηP% |
|---|---|---|---|---|---|---|---|
| Without inhibitor | 0.00 | −487.3 | 2.02 | 208.1 | 202.4 | - | - |
| IIb | 1 × 10−4 | −499.0 | 0.0646 | 118.0 | −137.4 | 0.72 | 71.55 |
| 5 × 10−4 | −485.5 | 0.0345 | 114.8 | −161.6 | 0.85 | 84.81 | |
| 1 × 10−3 | −485.4 | 0.0299 | 137.2 | −194.2 | 0.87 | 86.83 | |
| 5 × 10−3 | −477.2 | 0.0280 | 117.6 | −104.2 | 0.88 | 87.67 | |
| 1 × 10−2 | −504.7 | 0.0243 | 128.1 | −140.3 | 0.89 | 89.30 | |
| IIc | 1 × 10−4 | −563.6 | 0.0530 | 116.5 | −176.3 | 0.77 | 76.66 |
| 5 × 10−4 | −475.7 | 0.0315 | 128.2 | −112.7 | 0.86 | 86.13 | |
| 1 × 10−3 | −478.8 | 0.0277 | 127.6 | −106.3 | 0.88 | 87.80 | |
| 5 × 10−3 | −509.6 | 0.0244 | 108.8 | −170.1 | 0.89 | 89.26 | |
| 1 × 10−2 | −496.9 | 0.0233 | 125.8 | −196.5 | 0.90 | 89.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khowdiary, M.M.; Taha, N.A.; Saleh, N.M.; Elhenawy, A.A. Synthesis of Novel Nano-Sulfonamide Metal-Based Corrosion Inhibitor Surfactants. Materials 2022, 15, 1146. https://doi.org/10.3390/ma15031146
Khowdiary MM, Taha NA, Saleh NM, Elhenawy AA. Synthesis of Novel Nano-Sulfonamide Metal-Based Corrosion Inhibitor Surfactants. Materials. 2022; 15(3):1146. https://doi.org/10.3390/ma15031146
Chicago/Turabian StyleKhowdiary, Manal M., Nahla A. Taha, Nashwa M. Saleh, and Ahmed A. Elhenawy. 2022. "Synthesis of Novel Nano-Sulfonamide Metal-Based Corrosion Inhibitor Surfactants" Materials 15, no. 3: 1146. https://doi.org/10.3390/ma15031146





