Mechanical, Thermal, and Fire Properties of Composite Materials Based on Gypsum and PCM
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used
2.2. Production Process
2.3. Test Methods
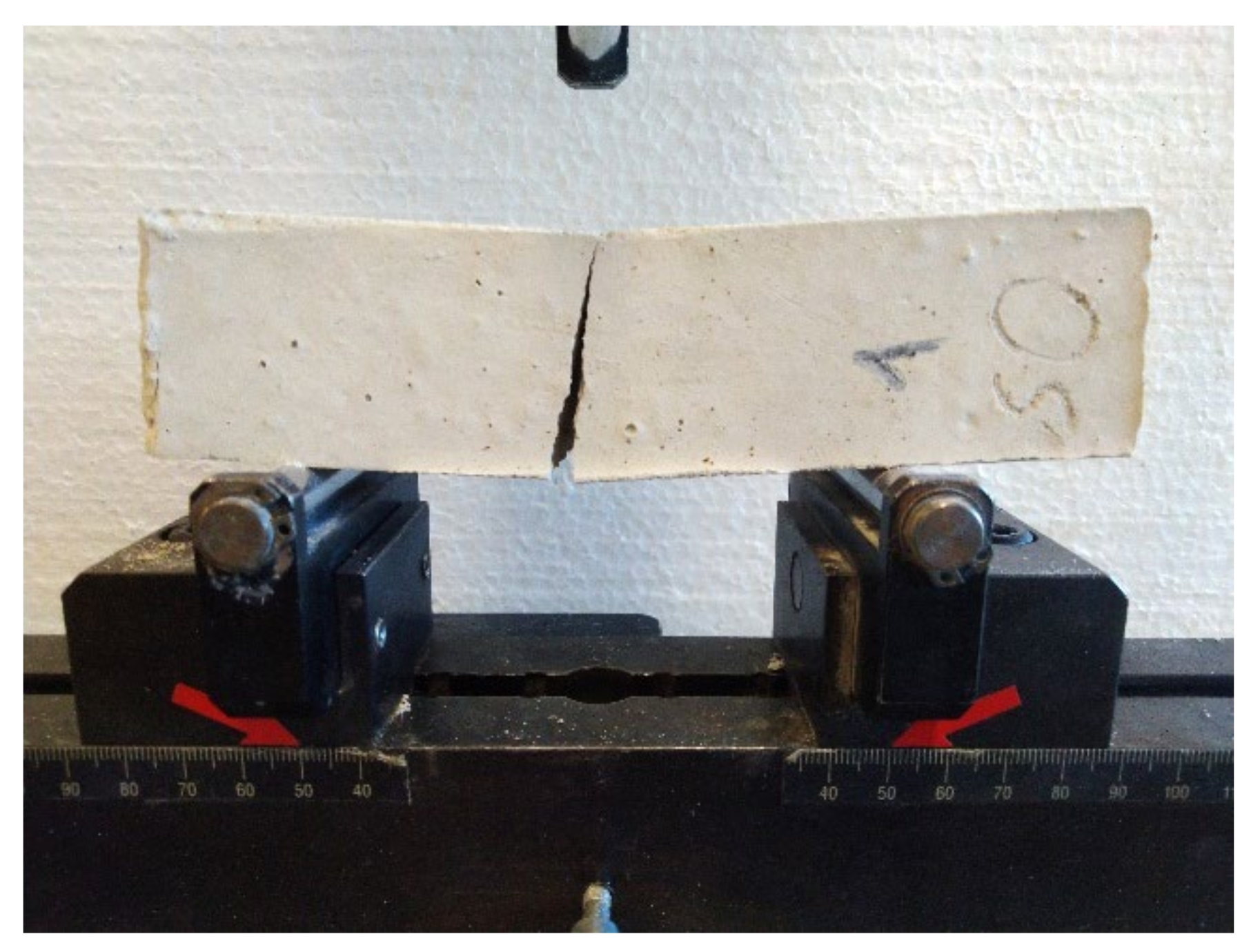
2.3.1. Flexural Strength
2.3.2. Compressive Strength
2.3.3. Tensile Strength Perpendicular to the Surface
2.3.4. Thermal Conductivity
2.3.5. Specific Heat Capacity
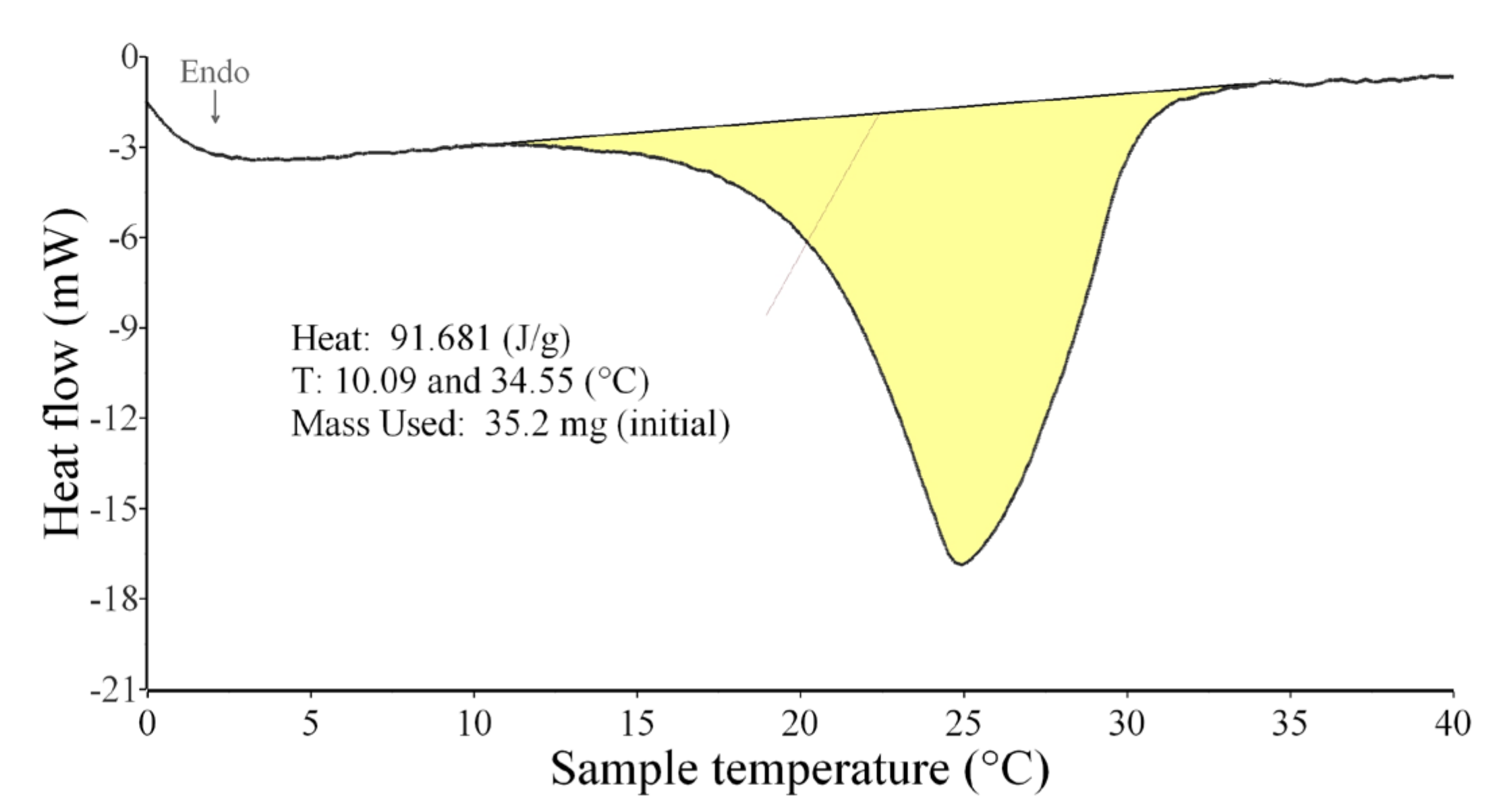
2.3.6. Differential Scanning Calorimetry
2.3.7. Single-Flame Source Fire Test
2.3.8. Gross Heat of Combustion
3. Results and Discussion
3.1. Mechanical Properties
3.2. Thermal Properties
3.3. Fire Properties
4. Conclusions
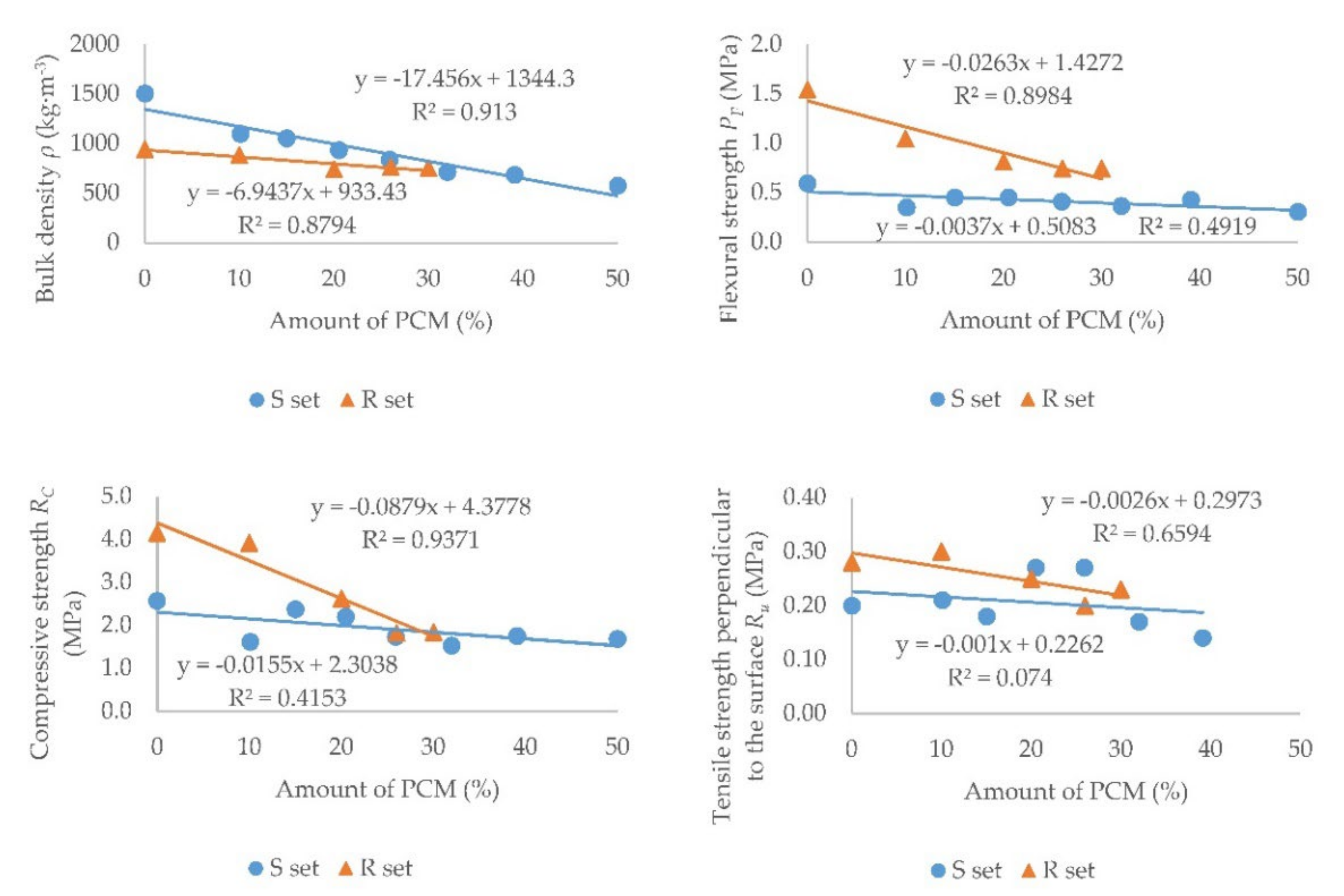
- It was observed that in samples of both sets, with an increasing proportion of PCM, the bulk density, flexural strength, compressive strength, and tensile strength perpendicular to the surface decreased.
- When comparing the trends of the S and R sets for their mechanical properties, it can be stated that in set R there was a more significant decrease in these strengths than in set S.
- With an increasing proportion of PCM in samples, the enthalpy increased. In set S, the thermal conductivity coefficient decreased and the specific heat capacity increased. In set R, the thermal conductivity coefficient slightly increased and the specific heat capacity decreased due to the low thermal conductivity of the reference sample R0 (0.171 W·m−1·K−1) caused by the expanded perlite.
- DSC analysis showed that the enthalpy was proportional to the amount of PCM contained in the plaster. Lower values of the thermal conductivity coefficient for PCM plasters are not ideal, but do not prevent their use in interiors.
- For samples with PCM, the fire properties of the plasters deteriorated, and all were classified into reaction to fire class B due to the high value of the gross heat of combustion. Class B does not prevent the use of plaster in habitable rooms, but it does not allow their use on protected escape routes.
- In terms of standard requirements, only plasters up to 10% PCM can be used in the manner of set R for common commercial plaster mixtures. However, the advantage of the R set is its easy production directly on the construction site. In the manner of set S, according to the determined trends, the applicability of plasters up to approximately 30% PCM can be hypothetically assumed after the addition of additives.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mehling, H.; Cabeza, L.F. Heat and Cold Storage with PCM: An up to Date Introduction into Basics and Applications, 1st ed.; Springer: New York, NY, USA, 2008; 308p. [Google Scholar]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Kuznik, F.; David, D.; Johannes, K.; Roux, J.-J. A review on phase change materials integrated in building walls. Renew. Sustain. Energy Rev. 2011, 15, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; De Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Cui, Y.; Xie, J.; Liu, J.; Pan, S. Review of Phase Change Materials Integrated in Building Walls for Energy Saving. Procedia Eng. 2015, 121, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Konuklu, Y.; Ostry, M.; Paksoy, H.O.; Charvat, P. Review on using microencapsulated phase change materials (PCM) in building applications. Energy Build. 2015, 106, 134–155. [Google Scholar] [CrossRef]
- Al-Absi, Z.A.A.S.; Isa, M.H.M.; Ismail, M. Application of Phase Change Materials (PCMs) in Building Walls: A Review. In The Advances in Civil Engineering Materials; Awang, M., Isa, M., Eds.; ICACE 2018. Lecture Notes in Civil Engineering; Springer: Singapore, 2019; Volume 19. [Google Scholar] [CrossRef]
- Bajare, D.; Kazjonovs, J.; Korjakins, A. Development of Latent Heat Storage Phase Change Material Containing Plaster. Mater. Sci. 2016, 22, 94–97. [Google Scholar] [CrossRef] [Green Version]
- Pavlík, Z.; Fořt, J.; Pavlíková, M.; Pokorný, J.; Trník, A.; Černý, R. Modified lime-cement plasters with enhanced thermal and hygric storage capacity for moderation of interior climate. Energy Build. 2016, 126, 113–127. [Google Scholar] [CrossRef]
- Fořt, J.; Trník, A.; Pavlík, Z. Influence of the Heating and Cooling Rate on Thermal Performance of Cement-Lime Plaster with PCM Admixture. Key Eng. Mater. 2016, 677, 150–154. [Google Scholar] [CrossRef]
- Fořt, J.; Trník, A.; Pavlík, Z. Latent Heat Storage in Plasters with Incorporated PCM Water Dispersion. Mater. Sci. Forum 2015, 824, 1–6. [Google Scholar] [CrossRef]
- Fořt, J.; Novotný, R.; Trník, A.; Černý, R. Preparation and Characterization of Novel Plaster with Improved Thermal Energy Storage Performance. Energies 2019, 12, 3318. [Google Scholar] [CrossRef] [Green Version]
- Gnanachelvam, S.; Ariyanayagam, A.; Mahendran, M. Fire resistance of light gauge steel framed wall systems lined with PCM-plasterboards. Fire Saf. J. 2019, 108, 102838. [Google Scholar] [CrossRef]
- Kolaitis, D.I.; Asimakopoulou, E.K.; Founti, M.A.; Vallerent, S.; Florence, C. Gypsum plasterboards enhanced with phase change materials: A fire safety assessment using experimental and computational techniques. MATEC Web Conf. 2013, 9, 06002. [Google Scholar] [CrossRef] [Green Version]
- Haurie, L.; Serrano, S.; Bosch, M.; Fernandez, A.I.; Cabeza, L.F. Single layer mortars with microencapsulated PCM: Study of physical and thermal properties, and fire behaviour. Energy Build. 2016, 111, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Kontogeorgos, D.A.; Semitelos, G.K.; Mandilaras, I.D.; Founti, M.A. Experimental investigation of the fire resistance of multi-layer drywall systems incorporating Vacuum Insulation Panels and Phase Change Materials. Fire Saf. J. 2016, 81, 8–16. [Google Scholar] [CrossRef]
- EN 13279-1; Gypsum Binders and Gypsum Plasters—Part 1: Definitions and Requirements. CEN: Brussels, Belgium, 2009.
- ČSN 73 0802; Fire Protection of Buildings—Non-Industrial Buildings. ÚNMZ: Prague, Czech Republic, 2009.
- Zhuk, P. Lifecycle analysis of finishing products enhanced with phase changing materials. IOP Conf. Ser. Earth Environ. Sci. 2019, 323, 012154. [Google Scholar] [CrossRef]
- RAL German Institute for Quality Assurance and Certification. Phase Change Materials Quality Assurance RAL-GZ 896; RAL Deutsches Institut für Gütesicherung und Kennzeichnung e.V.: Bonn, Germany, 2018; 47p. [Google Scholar]
- Pavlík, Z.; Zmeškal, O.; Pavlíková, M.; Černý, R. Effect of Heating and Cooling Mode on Temperature and Enthalpy of Phase Changes in PCM Modified Plaster. Appl. Mech. Mater. 2014, 595, 149–154. [Google Scholar] [CrossRef]
- Ostrý, M.; Dostálová, D.; Klubal, T.; Přikryl, R.; Charvát, P. Micro-encapsulated phase-change materials for latent-heat storage: Thermal characteristics. Mater. Tehnol. 2015, 49, 813–816. [Google Scholar] [CrossRef]
- Kusama, Y.; Ishidoya, Y. Thermal effects of a novel phase change material (PCM) plaster under different insulation and heating scenarios. Energy Build. 2017, 141, 226–237. [Google Scholar] [CrossRef]
- Fořt, J.; Trník, A.; Pavlík, Z. Cement-Lime Plaster with PCM Addition—A Perspective Material for Moderation of Interior Climate. Key Eng. Mater. 2016, 707, 43–50. [Google Scholar] [CrossRef]
- Fořt, J.; Trník, A.; Pavlík, Z. Influence of PCM Admixture on Thermal Behavior of Composite Plaster. Adv. Mater. Res. 2014, 1054, 209–214. [Google Scholar] [CrossRef]
- Fořt, J.; Trník, A.; Pavlík, Z. Utilization of the PCM latent heat for energy savings in buildings. AIP Conf. Proc. 2017, 1863, 150002. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Kaushik, S.C.; Tyagi, S.K.; Akiyama, T. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Fořt, J.; Maděra, J.; Trník, A.; Pavlíková, M.; Pavlík, Z. Computational modeling of latent-heat-storage in PCM modified interior plaster. AIP Conf. Proc. 2016, 1738, 280002. [Google Scholar] [CrossRef]
- Klimeš, L.; Charvát, P.; Ostrý, M. An optimization study into thermally activated wall system with latent heat thermal energy storage. IOP Conf. Ser. Earth Environ. Sci. 2019, 238, 012016. [Google Scholar] [CrossRef]
- Pavlíková, M.; Pavlík, Z.; Trník, A.; Pokorný, J.; Černý, R. Characterization of a lime-pozzolan plaster containing phase change material. AIP Conf. Proc. 2015, 1648, 090004. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, Y.; Wang, X.; Lin, K.; Xiao, W. An assessment of mixed type PCM-gypsum and shape-stabilized PCM plates in a building for passive solar heating. Sol. Energy 2007, 81, 1351–1360. [Google Scholar] [CrossRef]
- Theodoridou, M.; Kyriakou, L.; Ioannou, I. PCM-enhanced Lime Plasters for Vernacular and Contemporary Architecture. Energy Procedia 2016, 97, 539–545. [Google Scholar] [CrossRef] [Green Version]
- Kheradmand, M.; Azenha, M.; Castro-Gomes, J.P.; De Aguiar, J.L.B. Energy benefits of cement-based plaster containing hybrid phase-change material. Proc. Inst. Civ. Eng.—Constr. Mater. 2018, 171, 117–125. [Google Scholar] [CrossRef]
- Kusama, Y.; Ishidoya, Y. Study on a tankless solar heating system using phase-change material plaster. Build. Environ. 2018, 127, 256–267. [Google Scholar] [CrossRef]
- Carbonaro, C.; Cascone, Y.; Fantucci, S.; Serra, V.; Perino, M.; Dutto, M. Energy Assessment of a Pcm–Embedded Plaster: Embodied Energy versus Operational Energy. Energy Procedia 2015, 78, 3210–3215. [Google Scholar] [CrossRef] [Green Version]
- Mekaddem, N.; Ali, S.B.; Fois, M.; Hannachi, A. Paraffin/ Expanded Perlite/Plaster as Thermal Energy Storage Composite. Energy Procedia 2019, 157, 1118–1129. [Google Scholar] [CrossRef]
- Fořt, J.; Trník, A.; Pavlíková, M.; Pavlík, Z.; Černý, R. Fabrication of Dodecanol/Diatomite Shape-Stabilized PCM and Its Utilization in Interior Plaster. Int. J. Thermophys. 2018, 39, 137. [Google Scholar] [CrossRef]
- Sari, A.; Bicer, A.; Karaipekli, A.; Al-Sulaiman, F.A. Preparation, characterization and thermal regulation performance of cement based-composite phase change material. Sol. Energy Mater. Sol. Cells 2018, 174, 523–529. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sari, A.; Biçer, A. Thermal regulating performance of gypsum/(C18–C24) composite phase change material (CPCM) for building energy storage applications. Appl. Therm. Eng. 2016, 107, 55–62. [Google Scholar] [CrossRef]
- Gnanachelvam, S.; Ariyanayagam, A.; Mahendran, M. Fire resistance of LSF wall systems lined with different wallboards including bio-PCM mat. J. Build. Eng. 2020, 32, 101628. [Google Scholar] [CrossRef]
- Yoo, D.H.; Jeon, I.K.; Kim, H.G.; Lee, J.S.; Ryou, J.-S. Experimental evaluation of fire resistance performance of cement mortar with PCM/Mg(OH)2-based composite fine aggregate. Constr. Build. Mater. 2021, 287, 123018. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, P.; Wang, Y.; Zhou, C.; Lei, J. Form-stable phase change materials with enhanced thermal stability and fire resistance via the incorporation of phosphorus and silicon. Mater. Des. 2018, 160, 763–771. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, B.; Wang, L.; Lu, R.; Zhao, D.; Zhang, S. Novel hybrid form-stable polyether phase change materials with good fire resistance. Energy Storage Mater. 2017, 6, 46–52. [Google Scholar] [CrossRef]
- Pavlík, Z.; Trník, A.; Keppert, M.; Pavlíková, M.; Žumár, J.; Černý, R. Experimental Investigation of the Properties of Lime-Based Plaster-Containing PCM for Enhancing the Heat-Storage Capacity of Building Envelopes. Int. J. Thermophys. 2014, 35, 767–782. [Google Scholar] [CrossRef]
- EN 13914-2; Design, Preparation and Application of External Rendering and Internal Plastering—Part 2: Internal Rendering. CEN: Brussels, Belgium, 2016.
- Toppi, T.; Mazzarella, L. Gypsum based composite materials with micro-encapsulated PCM: Experimental correlations for thermal properties estimation on the basis of the composition. Energy Build. 2013, 57, 227–236. [Google Scholar] [CrossRef]
- EN 13501-1; Fire Classification of Construction Products and Building Elements—Part 1: Classification Using Test Data from Reaction to Fire Tests. CEN: Brussels, Belgium, 2019.
- ČSN 72 2301; Plaster Binders. Classification. Common Technical Requirements. Testing Methods. ÚNM: Prague, Czech Republic, 1978.
- Rigips SAINT-GOBAIN. Rimano UNI. Available online: https://www.rigips.cz/produkty/rimano-uni/ (accessed on 12 December 2021).
- EN 13279-2; Gypsum Binders and Gypsum Plasters—Part 2: Test Methods. CEN: Brussels, Belgium, 2014.
- EN ISO 11925-2; Reaction to Fire Tests—Ignitability of Products Subjected to Direct Impingement of Flame—Part 2: Single-Flame Source Test. CEN: Brussels, Belgium, 2020.
- ČSN 72 1105; Determination of the Coefficient of Thermal Conductivity by the Method of Non-Stationary Flow. FÚNM: Prague, Czech Republic, 1991.
- Applied Precision Ltd. ISOMET 2114 Thermal Properties Analyzer User’s Guide. Version 1.46, Support and Downolands. Available online: https://www.appliedp.com/download/catalog/isomet_pc_en.pdf (accessed on 3 December 2021).
- Setaram Instrumentation. SENSYS Evo DSC. Available online: https://intercovamex.com/product/sensys-evo/?lang=en (accessed on 3 December 2021).
- EN ISO 1716; Reaction to Fire Tests for Products—Determination of the Gross Heat of Combustion (Calorific Value). CEN: Brussels, Belgium, 2018.
- Lewry, A.J.; Williamson, J. The setting of gypsum plaster: Part II The development of microstructure and strength. J. Mater. Sci. 1994, 29, 5524–5528. [Google Scholar] [CrossRef]
- Lewry, A.J.; Williamson, J. The setting of gypsum plaster: Part III The effect of additives and impurities. J. Mater. Sci. 1994, 29, 6085–6090. [Google Scholar] [CrossRef]
- Raouf, Z.A.M.; Mahdy, N.; Awad, H. Improving the Properties of Gypsum by Using Additives. J. Eng. 2012, 18, 1–18. [Google Scholar]
- Aljalawi, N.; Awad, H. A Study of the Effect of Using Additives on Some Properties of Gypsum. In Proceedings of the 1st International Conference on Engineering Sciences Applications ICESA, Karbala, Iraq, 24–25 December 2014. [Google Scholar]
- Zhang, B.; Tian, Y.; Jin, X.; Lo, T.Y.; Cui, H. Thermal and Mechanical Properties of Expanded Graphite/Paraffin Gypsum-Based Composite Material Reinforced by Carbon Fiber. Materials 2018, 11, 2205. [Google Scholar] [CrossRef] [Green Version]
- Stejskalová, K.; Ostrý, M. Influence of phase change materials on thermal stability in attic rooms. Int. Multidiscip. Sci. GeoConf. Surv. Geol. Min. Ecol. Manag. SGEM 2019, 19, 183–189. [Google Scholar] [CrossRef]











| Sample | Gypsum (g) | Water (g) | Sand (g) | PCM (g) | PCM (%) |
|---|---|---|---|---|---|
| S0 | 500 | 700 | 2000 | 0 | 0 |
| S1 | 500 | 700 | 1200 | 190 | 10.1 |
| S2 | 500 | 700 | 950 | 255 | 15.0 |
| S3 | 500 | 700 | 660 | 300 | 20.5 |
| S4 | 500 | 700 | 500 | 350 | 25.9 |
| S5 | 500 | 700 | 350 | 400 | 32.0 |
| S6 | 500 | 700 | 200 | 450 | 39.1 |
| S7 | 500 | 700 | 0 | 500 | 50.0 |
| Sample | Gypsum Plaster (g) | Water (g) | PCM (g) | PCM (%) |
|---|---|---|---|---|
| R0 | 1000 | 600 | 0 | 0 |
| R1 | 900 | 540 | 100 | 10 |
| R2 | 800 | 480 | 200 | 20 |
| R3 | 700 | 420 | 250 | 26 |
| R4 | 700 | 420 | 300 | 30 |
| Sample | Bulk Density ρ (kg·m−3) | Flexural Strength PF (MPa) | Compressive Strength RC (MPa) | Tensile Strength Perpendicular to the Surface Ru (MPa) | ||||
|---|---|---|---|---|---|---|---|---|
| Average | Standard Deviation | Average | Standard Deviation | Average | Standard Deviation | Average | Standard Deviation | |
| S0 | 1502 | 22 | 0.59 | 0.02 | 2.57 | 0.12 | 0.20 | 0.03 |
| S1 | 1096 | 5 | 0.35 | 0.01 | 1.61 | 0.08 | 0.21 | 0.01 |
| S2 | 1053 | 4 | 0.45 | 0.03 | 2.37 | 0.10 | 0.18 | 0.03 |
| S3 | 933 | 9 | 0.45 | 0.07 | 2.20 | 0.08 | 0.27 | 0.04 |
| S4 | 836 | 7 | 0.41 | 0.02 | 1.74 | 0.10 | 0.27 | 0.03 |
| S5 | 714 | 8 | 0.37 | 0.04 | 1.52 | 0.10 | 0.17 | 0.02 |
| S6 | 683 | 10 | 0.43 | 0.04 | 1.75 | 0.07 | 0.14 | 0.01 |
| S7 | 575 | 7 | 0.30 | 0.05 | 1.68 | 0.09 | 0.41 | 0.04 |
| Sample | Bulk Density ρ (kg·m−3) | Flexural Strength PF (MPa) | Compressive Strength RC (MPa) | Tensile Strength Perpendicular to the Surface Ru (MPa) | ||||
|---|---|---|---|---|---|---|---|---|
| Average | Standard Deviation | Average | Standard Deviation | Average | Standard Deviation | Average | Standard Deviation | |
| R0 | 940 | 21 | 1.54 | 0.03 | 4.14 | 0.33 | 0.28 | 0.03 |
| R1 | 880 | 3 | 1.04 | 0.05 | 3.91 | 0.27 | 0.30 | 0.04 |
| R2 | 740 | 2 | 0.81 | 0.03 | 2.62 | 0.14 | 0.25 | 0.01 |
| R3 | 760 | 4 | 0.74 | 0.03 | 1.82 | 0.06 | 0.20 | 0.01 |
| R4 | 750 | 9 | 0.74 | 0.04 | 1.84 | 0.07 | 0.23 | 0.04 |
| Sample | Thermal Conductivity Coefficient λ (W·m−1·K−1) | Specific Heat Capacity c (J·kg−1·K−1) | Temperatures at Start and End (°C) | Enthalpy (J·g−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Average | Standard Deviation | Average | Standard Deviation | Melting | Solidification | Melting | Solidification | |
| S0 | 0.747 | 0.016 | 989.95 | 4.63 | – | – | 0 | 0 |
| S1 | 0.367 | 0.011 | 1340.79 | 3.79 | 12–31 | 22–7 | 8.9 | −10.1 |
| S2 | 0.394 | 0.033 | 1391.07 | 22.13 | 12–30 | 22–7 | 11.5 | −12.9 |
| S3 | 0.321 | 0.019 | 1540.62 | 13.24 | 12–31 | 21–7 | 18.2 | −19.9 |
| S4 | 0.431 | 0.018 | 1864.12 | 8.19 | 12–31 | 22–6 | 22.6 | −24.9 |
| S5 | 0.304 | 0.009 | 2029.41 | 7.33 | 10–32 | 21–5 | 28.0 | −31.2 |
| S6 | 0.210 | 0.013 | 2013.91 | 6.67 | 9–32 | 21–5 | 38.3 | −41.9 |
| S7 | 0.533 | 0.009 | 2516.52 | 26.78 | 9–33 | 21–5 | 47.2 | −50.3 |
| PCM | – | – | 9–34 | 21–5 | 92.1 | −97.3 | ||
| Sample | Thermal Conductivity Coefficient λ (W·m−1·K−1) | Specific Heat Capacity c (J·kg−1·K−1) | Temperatures at Start and End (°C) | Enthalpy (J·g−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Average | Standard Deviation | Average | Standard Deviation | Melting | Solidification | Melting | Solidification | |
| R0 | 0.171 | 0.022 | 1515.85 | 15.20 | – | – | 0 | 0 |
| R1 | 0.294 | 0.012 | 1626.36 | 6.64 | 11–30 | 22–9 | 9.2 | −10.1 |
| R2 | 0.287 | 0.015 | 2016.76 | 18.82 | 12–31 | 22–7 | 17.3 | −19.6 |
| R3 | 0.149 | 0.011 | 559.61 | 4.21 | 11–31 | 21–8 | 23.7 | −25.1 |
| R4 | 0.322 | 0.009 | 919.20 | 21.42 | 11–32 | 21–7 | 25.9 | −27.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stejskalová, K.; Bujdoš, D.; Procházka, L.; Smetana, B.; Zlá, S.; Teslík, J. Mechanical, Thermal, and Fire Properties of Composite Materials Based on Gypsum and PCM. Materials 2022, 15, 1253. https://doi.org/10.3390/ma15031253
Stejskalová K, Bujdoš D, Procházka L, Smetana B, Zlá S, Teslík J. Mechanical, Thermal, and Fire Properties of Composite Materials Based on Gypsum and PCM. Materials. 2022; 15(3):1253. https://doi.org/10.3390/ma15031253
Chicago/Turabian StyleStejskalová, Kateřina, David Bujdoš, Lukáš Procházka, Bedřich Smetana, Simona Zlá, and Jiří Teslík. 2022. "Mechanical, Thermal, and Fire Properties of Composite Materials Based on Gypsum and PCM" Materials 15, no. 3: 1253. https://doi.org/10.3390/ma15031253
APA StyleStejskalová, K., Bujdoš, D., Procházka, L., Smetana, B., Zlá, S., & Teslík, J. (2022). Mechanical, Thermal, and Fire Properties of Composite Materials Based on Gypsum and PCM. Materials, 15(3), 1253. https://doi.org/10.3390/ma15031253






