Alginate Hydrogels with Aloe vera: The Effects of Reaction Temperature on Morphology and Thermal Properties
Abstract
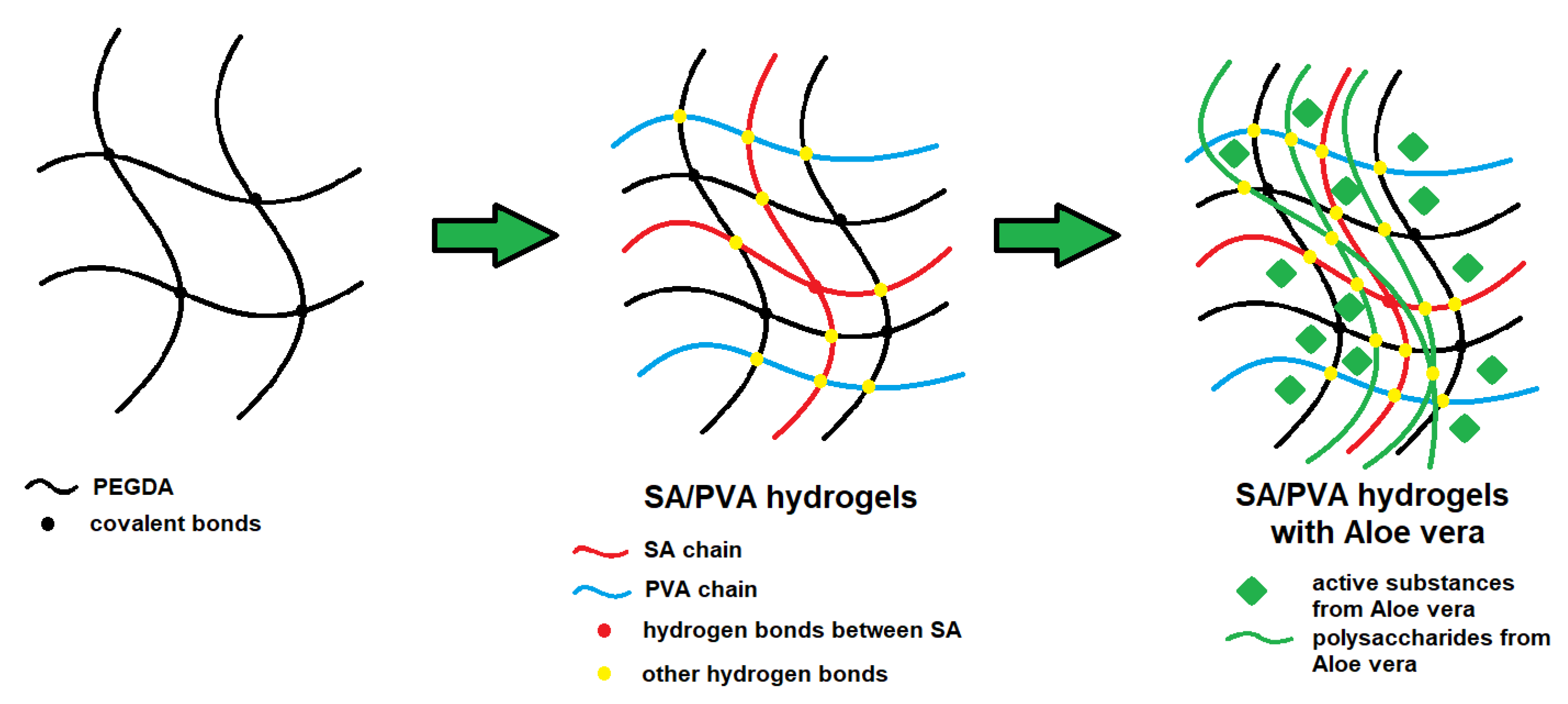
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Hydrogel Materials
2.2.2. Determination of Gel Fraction
2.2.3. Determination of Swelling Behavior
2.2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.5. Scanning Electron Microscopy (SEM)
2.2.6. Differential Scanning Calorimetry (DSC)
3. Results
3.1. Gel Fraction
3.2. Swelling Ability
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Differential Scanning Calorimetry (DSC)
3.5. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Natural polymers and the hydrogels prepared from them. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2019; pp. 17–47. [Google Scholar] [CrossRef]
- Olatunji, O. Classification of Natural Polymers. In Natural Polymers Industry Techniques and Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 1–17. [Google Scholar]
- Rajeswari, S.; Prasanthi, T.; Sudha, N.; Swain, R.P.; Panda, S.; Goka, V. Natural polymers: A recent review. World J. Pharm. Sci. 2017, 6, 472–494. [Google Scholar] [CrossRef][Green Version]
- Gyles, D.A.; Castro, L.D.; Carréra Silva, J.O., Jr.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical ad-vances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Saini, K. Preparation method, properties and crosslinking of hydrogel: A review. PharmaTutor 2017, 5, 27–36. [Google Scholar]
- Kirchmajer, D.M.; Gorkin Iii, R.; Panhuis, M.I.H. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J. Mater. Chem. B 2015, 3, 4105–4117. [Google Scholar] [CrossRef]
- Yoshida, R.; Okano, T. Stimuli-Responsive Hydrogels and Their Application to Functional Materials. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Park, K., Okano, T., Eds.; Springer: New York, NY, USA, 2010; pp. 19–43. [Google Scholar]
- Garg, S.; Garg, A. Hydrogel: Classification, properties, preparation and technical features. Asian J. Biomater. Res. 2016, 2, 163–170. [Google Scholar]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Khansari, M.M.; Sorokina, L.V.; Mukherjee, P.; Mukhtar, F.; Shirdar, M.R.; Shahidi, M.; Shokuhfar, T. Classification of Hydrogels Based on Their Source: A Review and Application in Stem Cell Regulation. JOM 2017, 69, 1340–1347. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels as Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Muhammad, Z.; Waqar, S.; Sadaf, W.; Rai, M.S.; Asif, M.; Junaid, Q.; Javed, I.; Fazal, R.S.; Muhammad, S.R.; Usman, K. Hy-drogels, their applications and polymers used for hydrogels: A review. Int. J. Biol. Pharm. Allied Sci. 2015, 4, 6581–6603. [Google Scholar]
- Nilimanka, D. Preparation methods and properties of hydrogel: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 112–117. [Google Scholar]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Park, T.G. Self-assembled and nanostructured hydrogels for drug delivery and tissue engineering. Nano Today 2009, 4, 429–437. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Figueroa-Pizano, M.; Vélaz, I.; Peñas, F.; Zavala-Rivera, P.; Rosas-Durazo, A.; Maldonado-Arce, A.; Martínez-Barbosa, M. Effect of freeze-thawing conditions for preparation of chitosan-poly (vinyl alcohol) hydrogels and drug release studies. Carbohydr. Polym. 2018, 195, 476–485. [Google Scholar] [CrossRef]
- Shi, X.; Wu, J.; Wang, Z.; Song, F.; Gao, W.; Liu, S. Synthesis and properties of a temperature-sensitive hydrogel based on physical crosslinking via stereocomplexation of PLLA-PDLA. RSC Adv. 2020, 10, 19759–19769. [Google Scholar] [CrossRef]
- Liu, S.; Oderinde, O.K.; Hussain, I.; Yao, F.; Fu, G. Dual ionic cross-linked double network hydrogel with self-healing, conductive, and force sensitive properties. Polymer 2018, 144, 111–120. [Google Scholar] [CrossRef]
- Bialik-Wąs, K.; Królicka, E.; Malina, D. Impact of the Type of crosslinking agents on the properties of modified sodium algi-nate/poly(vinyl alcohol) hydrogels. Molecules 2021, 26, 2381. [Google Scholar] [CrossRef]
- Ye, X.; Li, X.; Shen, Y.; Chang, G.; Yang, J.; Gu, Z. Self-healing pH-sensitive cytosine-and guanosine-modified hyaluronic acid hydrogels via hydrogen bonding. Polymer 2017, 108, 348–360. [Google Scholar] [CrossRef]
- Erickson, I.E.; Kestle, S.R.; Zellars, K.H.; Dodge, G.R.; Burdick, J.A.; Mauck, R.L. Improved cartilage repair via in vitro pre-maturation of MSC-seeded hyaluronic acid hydrogels. Biomed. Mater. 2012, 7, 24110. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H. Macroscopic self-assembly through molecular recognition. Nat. Chem. 2010, 3, 34–37. [Google Scholar] [CrossRef]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Ihsan, A.B.; Akasaki, T.; Sato, K.; Gong, J.P. Physical hydrogels composed of polyam-pholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef]
- Grindy, S.; Learsch, R.W.; Mozhdehi, D.; Cheng, J.; Barrett, D.G.; Guan, Z.; Messersmith, P.; Holten-Andersen, N. Control of hierarchical polymer mechanics with bioinspired metal-coordination dynamics. Nat. Mater. 2015, 14, 1210–1216. [Google Scholar] [CrossRef]
- Ke, H.; Yang, L.-P.; Xie, M.; Chen, Z.; Yao, H.; Jiang, W. Shear-induced assembly of a transient yet highly stretchable hydrogel based on pseudopolyrotaxanes. Nat. Chem. 2019, 11, 470–477. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, X.; O’Neill, S.J.K.; Wu, G.; Whitaker, D.J.; Li, J.; McCune, J.A.; Scherman, O.A. Highly compressible glass-like supramolecular polymer networks. Nat. Mater. 2021, 21, 103–109. [Google Scholar] [CrossRef]
- An, Y.M.; Liu, T.; Tian, R.; Liu, S.X.; Han, Y.N.; Wang, Q.Q.; Sheng, W.J. Synthesis of novel temperature responsive PEG-b-[PCL-gP (MEO2MA-co-OEGMA)]-b-PEG (tBG) triblock-graft copolymers and preparation of tBG/graphene oxide composite hydrogels via click chemistry. React. Funct. Polym. 2015, 94, 1–8. [Google Scholar] [CrossRef]
- Cruz, A.; García-Uriostegui, L.; Ortega, A.; Isoshima, T.; Burillo, G. Radiation grafting of N-vinylcaprolactam onto nano and macrogels of chitosan: Synthesis and characterization. Carbohydr. Polym. 2017, 155, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, J. Hydrogel brushes grafted from stainless steel via surface-initiated atom transfer radical polymerization for marine antifouling. Appl. Surf. Sci. 2016, 382, 202–216. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, J.; Liu, R.; Zhao, S. Stimulus-responsiveness and methyl violet release behaviors of poly(NIPAAm-co-AA) hydrogels chemically crosslinked with β-cyclodextrin polymer bearing methacrylates. Carbohydr. Res. 2016, 428, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.-M.S.; Voss, Y.; Schönherr, H. Rapid Detection of Escherichia coli via Enzymatically Triggered Reactions in Self-Reporting Chitosan Hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 20190–20199. [Google Scholar] [CrossRef]
- Chaykar, A.S.; Goharpey, F.; Yeganeh, J.K. Volume phase transition of electron beam cross-linked thermo-responsive PVME nanogels in the presence and absence of nanoparticles: With a view toward rheology and interactions. RSC Adv. 2016, 6, 9693–9708. [Google Scholar] [CrossRef]
- Bialik-Wąs, K.; Pluta, K.; Malina, D.; Majka, T. Alginate/PVA-based hydrogel matrices with Echinacea purpurea extract as a new approach to dermal wound healing. Int. J. Polym. Mater. 2019, 70, 195–206. [Google Scholar] [CrossRef]
- Bialik-Wąs, K.; Pluta, K.; Malina, D.; Barczewski, M.; Malarz, K.; Mrozek-Wilczkiewicz, A. Advanced SA/PVA-based hydrogel matrices with prolonged release of Aloe vera as promising wound dressings. Mater. Sci. Eng. C 2020, 120, 111667. [Google Scholar] [CrossRef]
- Monir, T.S.B.; Afroz, S.; Khan, R.A.; Miah, M.Y.; Takafuji, M.; Alam, M.A. pH-sensitive hydrogel from polyethylene oxide and acrylic acid by gamma radiation. J. Compos. Sci. 2019, 3, 58. [Google Scholar] [CrossRef]
- Elbarbary, A.M.; Ghobashy, M.M. Controlled release fertilizers using superabsorbent hydrogel prepared by gamma radiation. Radiochim. Acta 2017, 105, 865–876. [Google Scholar] [CrossRef]
- Neves, S.C.; Moroni, L.; Barrias, C.; Granja, P. Leveling Up Hydrogels: Hybrid Systems in Tissue Engineering. Trends Biotechnol. 2019, 38, 292–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Lopes, J.; Fonseca, R.; Viana, T.; Fernandes, C.; Morouço, P.; Moura, C.; Biscaia, S. Characterization of Biocompatible Poly(Ethylene Glycol)-Dimethacrylate Hydrogels for Tissue Engineering. Appl. Mech. Mater. 2019, 890, 290–300. [Google Scholar] [CrossRef]
- George, M.; Joseph, L.; Francis, L. Development and evaluation of silver sulphadiazine loaded sodium alginate gelatin film for wound dressing applications. Eur. J. Pharm. Med. Res. 2017, 4, 420–423. [Google Scholar]
- Abou-Okeil, A.; Fahmy, H.; El-Bisi, M.; Ahmed-Farid, O. Hyaluronic acid/Na-alginate films as topical bioactive wound dressings. Eur. Polym. J. 2018, 109, 101–109. [Google Scholar] [CrossRef]
- Rassu, G.; Salis, A.; Porcu, E.P.; Giunchedi, P.; Roldo, M.; Gavini, E. Composite chitosan/alginate hydrogel for controlled release of deferoxamine: A system to potentially treat iron dysregulation diseases. Carbohydr. Polym. 2016, 136, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- García-Cano, I.; Rocha-Mendoza, D.; Kosmerl, E.; Zhang, L.; Jiménez-Flores, R. Technically relevant enzymes and proteins produced by LAB suitable for industrial and biological activity. Appl. Microbiol. Biotechnol. 2020, 104, 1401–1422. [Google Scholar] [CrossRef]
- Vazquez-Morado, L.E.; Robles-Zepeda, R.E.; Ochoa-Leyva, A.; Arvizu-Flores, A.A.; Garibay-Escobar, A.; Castillo-Yañez, F.; Lopez-Zavala, A.A. Biochemical characterization and inhibition of thermolabile hemolysin from Vibrio parahaemolyticus by phenolic compounds. PeerJ 2021, 9, e10506. [Google Scholar] [CrossRef]
- Horton, J.; Klarmann-Schulz, U.; Stephens, M.; Budge, P.J.; Coulibaly, Y.; Debrah, A.; Debrah, L.B.; Krishnasastry, S.; Mwingira, U.; Ngenya, A.; et al. The design and development of a multicentric protocol to investigate the impact of adjunctive doxycycline on the management of peripheral lymphoedema caused by lymphatic filariasis and podo-coniosis. Parasites Vectors 2020, 13, 155. [Google Scholar] [CrossRef]
- Bialik-Was, K.; Malina, D.; Pluta, K. Sposób Otrzymywania Hydrożelowych Materiałów Opatrunkowych 1AD. Patent Application No. P. 432720, 28 January 2020. [Google Scholar]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y.D. Development of a polyvinyl alcohol/sodium alginate hydrogel-based scaffold incorporating bFGF-encapsulated microspheres for accelerated wound healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, C.; Shuai, Y.; Cui, X.; Nie, L. Controlled release of anticancer drug using graphene oxide as a drug-binding effector in konjac glucomannan/sodium alginate hydrogels. Colloids Surf. B Biointerfaces 2014, 113, 223–229. [Google Scholar] [CrossRef]
- Pereira, R.; Mendes, A.; Bártolo, P. Alginate/Aloe Vera Hydrogel Films for Biomedical Applications. Procedia CIRP 2013, 5, 210–215. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, A. In situ generation of sodium alginate/hydroxyapatite nanocomposite beads as drug-controlled release matrices. Acta Biomater. 2010, 6, 445–454. [Google Scholar] [CrossRef] [PubMed]
- De Moura, M.R.; Guilherme, M.R.; Campese, G.M.; Radovanovic, E.; Rubira, A.F.; Muniz, E.C. Porous alginate-Ca2+ hydrogels interpenetrated with PNIPAAm networks: Interrelationship between compressive stress and pore morphology. Eur. Polym. J. 2005, 41, 2845–2852. [Google Scholar] [CrossRef]
- Pereira, R.; Tojeira, A.; Vaz, D.B.D.M.C.; Mendes, A.; Bártolo, P. Preparation and Characterization of Films Based on Alginate and Aloe Vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Koga, A.Y.; Pereira, A.V.; Lipinski, L.; Oliveira, M.R. Evaluation of wound healing effect of alginate films containing Aloe vera (Aloe barbadensis Miller) gel. J. Biomater. Appl. 2017, 32, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Pereira, J.C.; Ramalho, A.; Pais, A.A.C.C.; Sousa, J.J.S. Films based on chitosan polyelectrolyte complexes for skin drug delivery: Development and characterization. J. Membr. Sci. 2008, 320, 268–279. [Google Scholar] [CrossRef]
- Ghadimi, A.; Amirilargani, M.; Mohammadi, T.; Kasiri, N.; Sadatnia, B. Preparation of alloyed poly(ether block am-ide)/poly(ethylene glycol diacrylate) membranes for separation of CO2/H2 (syngas application). J. Memb. Sci. 2014, 458, 14–26. [Google Scholar] [CrossRef]
- Huang, L.; Nishinari, K. Interaction between poly(ethylene glycol) and water as studied by differential scanning calorimetry. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 496–506. [Google Scholar] [CrossRef]
- Hatakeyma, T.; Kasuga, H.; Tanaka, M.; Hatakeyama, H. Cold crystallization of poly(ethylene glycol)–water systems. Thermochim. Acta 2007, 465, 59–66. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.; Tamer, T.M.; El-Meligy, M.A.; Eldin, M.M. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arab. J. Chem. 2015, 8, 38–47. [Google Scholar] [CrossRef]
- Chin, S.S.; Lyn, F.H.; Hanani, Z.N. Effect of Aloe vera (Aloe barbadensis Miller) gel on the physical and functional properties of fish gelatin films as active packaging. Food Packag. Shelf Life 2017, 12, 128–134. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bialik-Wąs, K.; Raftopoulos, K.N.; Pielichowski, K. Alginate Hydrogels with Aloe vera: The Effects of Reaction Temperature on Morphology and Thermal Properties. Materials 2022, 15, 748. https://doi.org/10.3390/ma15030748
Bialik-Wąs K, Raftopoulos KN, Pielichowski K. Alginate Hydrogels with Aloe vera: The Effects of Reaction Temperature on Morphology and Thermal Properties. Materials. 2022; 15(3):748. https://doi.org/10.3390/ma15030748
Chicago/Turabian StyleBialik-Wąs, Katarzyna, Konstantinos N. Raftopoulos, and Krzysztof Pielichowski. 2022. "Alginate Hydrogels with Aloe vera: The Effects of Reaction Temperature on Morphology and Thermal Properties" Materials 15, no. 3: 748. https://doi.org/10.3390/ma15030748
APA StyleBialik-Wąs, K., Raftopoulos, K. N., & Pielichowski, K. (2022). Alginate Hydrogels with Aloe vera: The Effects of Reaction Temperature on Morphology and Thermal Properties. Materials, 15(3), 748. https://doi.org/10.3390/ma15030748







