Spectroscopic Properties of Inorganic Glasses Doped with Pr3+: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
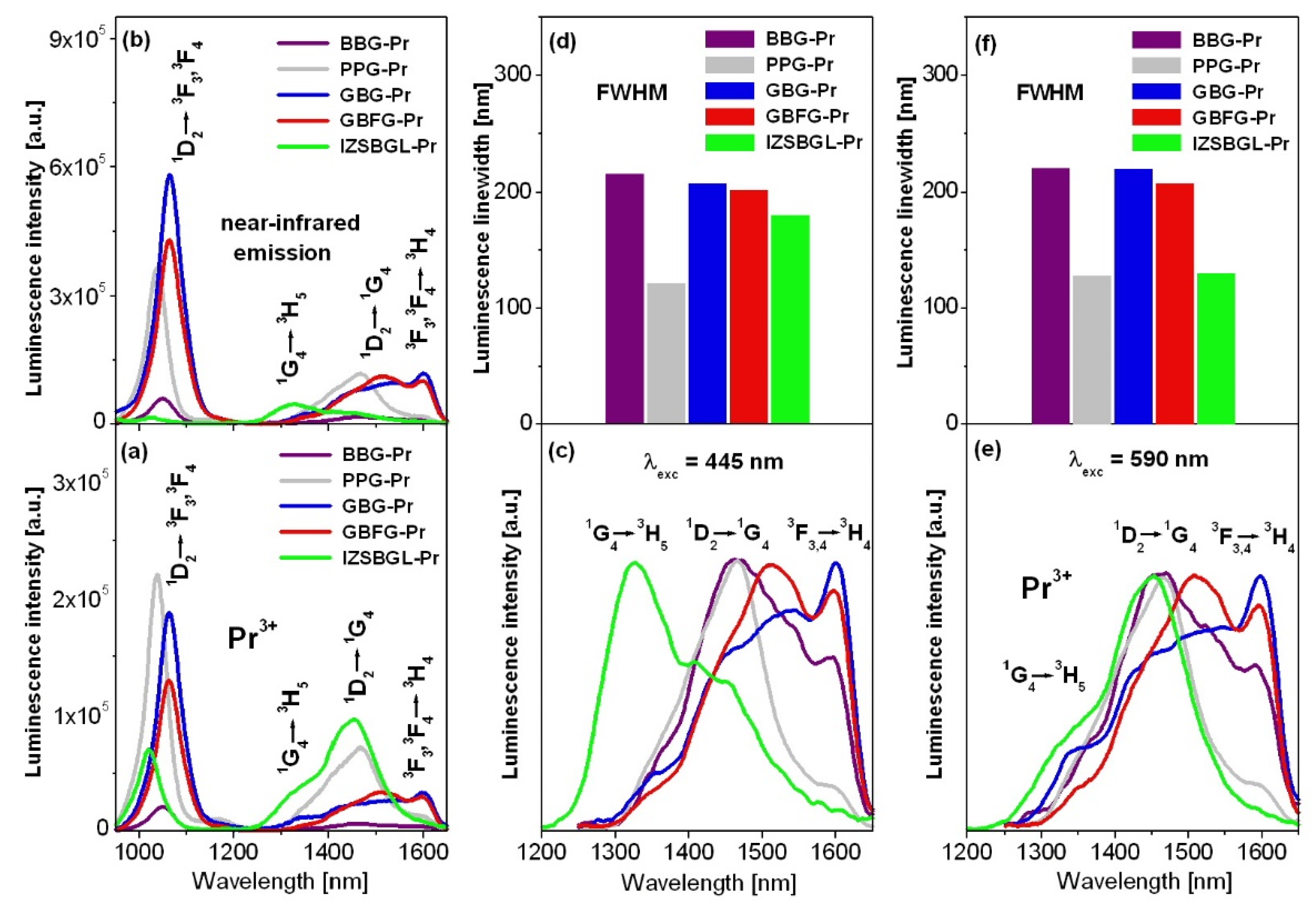
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babu, P.; Jayasankar, C.K. Spectroscopy of Pr3+ ions in lithium borate and lithium fluoroborate glasses. Phys. B 2001, 301, 326–340. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, J.; Tong, Y.; Wang, T.; Xu, W.; Chen, G. Observation of intra- and inter-configurational luminescence of Pr3+-doped strontium phosphate glasses. J. Am. Ceram. Soc. 2012, 95, 41–44. [Google Scholar] [CrossRef]
- Niu, L.; Zhou, Y.; Zhu, C.; He, Z.; Meng, X. Pr3+ doped oxyfluoride silicate glasses for LEDs. Ceram. Int. 2019, 45, 4108–4112. [Google Scholar] [CrossRef]
- Rai, V.K.; Rai, S.B.; Rai, D.K. Spectroscopic properties of Pr3+ doped in tellurite glass. Spectrochim. Acta A 2005, 62, 302–306. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Pisarska, J.; Dorosz, D.; Dorosz, J. Rare earths in lead-free oxyfluoride germanate glasses. Spectrochim. Acta A 2015, 134, 587–591. [Google Scholar] [CrossRef]
- Seeber, W.; Downing, E.A.; Hesselink, L.; Fejer, M.M.; Ehrt, D. Pr3+-doped fluoride glasses. J. Non-Cryst. Solids 1995, 189, 218–226. [Google Scholar] [CrossRef]
- Binnemans, K.; Verboven, D.; Görller-Walrand, C.; Lucas, J.; Duhamel-Henry, N.; Adam, J.L. Absorption and magnetic circular dichroism spectra of praseodymium doped fluorozirconate (ZBLAN) glass. J. Alloys Compd. 1997, 250, 321–325. [Google Scholar] [CrossRef]
- Olivier, M.; Parastesh Pirasteh, P.; Doualan, J.-L.; Camy, P.; Lhermite, H.; Adam, J.-L.; Nazabal, V. Pr3+-doped ZBLA fluoride glasses for visible laser emission. Opt. Mater. 2011, 33, 980–984. [Google Scholar] [CrossRef]
- Manzani, D.; Paboeuf, D.; Ribeiro, S.J.L.; Goldner, P.; Bretenaker, F. Orange emission in Pr3+-doped fluoroindate glasses. Opt. Mater. 2013, 35, 383–386. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Guo, H.; Xu, Y.; Wu, Z.; Li, M.; Jia, X.; Nie, Q. Effect of glass composition on the physical properties and luminescence of Pr3+ ion-doped chalcogenide glasses. J. Am. Ceram. Soc. 2019, 102, 6794–6801. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, F.; Liao, M.; Wang, X.; Li, Y.; Hu, L.; Knight, J. Emission properties of Pr3+-doped aluminosilicate glasses at visible wavelengths. J. Lumin. 2020, 220, 117013. [Google Scholar] [CrossRef]
- Rao, V.H.; Prasad, P.S.; Babu, K.S. Visible luminescence characteristics of Pr3+ ions in TeO2-Sb2O3-WO3 glasses. Opt. Mater. 2020, 101, 109740. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Y.; Shen, X.; Wei, W. Concentration dependence of visible luminescence from Pr3+-doped phosphate glasses. Spectrochim. Acta A 2019, 206, 454–459. [Google Scholar] [CrossRef]
- Ramteke, D.D.; Swart, H.C.; Gedam, R.S. Spectroscopic properties of Pr3+ ions embedded in lithium borate glasses. Phys. B 2016, 480, 111–115. [Google Scholar] [CrossRef]
- Babu, S.; Rajput, P.; Ratnakaram, Y.C. Compositional-dependent properties of Pr3+-doped multicomponent fluoro-phosphate glasses for visible applications: A photoluminescence study. J. Mater. Sci. 2016, 51, 8037–8054. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, G.; Peng, M.; Qiu, J. Comparative investigation on the spectroscopic properties of Pr3+-doped boro-phosphate, boro-germo-silicate and tellurite glasses. Spectrochim. Acta A 2012, 93, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Mahato, K.K.; Rai, D.K.; Rai, S.B. Optical studies of Pr3+ doped oxyfluoroborate glass. Phys. Stat. Sol. A 1999, 174, 277–289. [Google Scholar] [CrossRef]
- Shaw, L.B.; Harbison, B.B.; Cole, B.; Sanghera, J.S.; Aggarwal, I.D. Spectroscopy of the IR transitions in Pr3+ doped heavy metal selenide glasses. Opt. Express 1997, 1, 87–96. [Google Scholar] [CrossRef]
- Choi, Y.G.; Park, B.J.; Kim, K.H.; Heo, J. Crossrelaxations between and multiphonon relaxation of near-infrared excited states of Pr3+ ions in selenide glasses. Chem. Phys. Lett. 2003, 368, 625–629. [Google Scholar] [CrossRef]
- Oswald, J.; Kuldova, K.; Frumarova, B.; Frumar, M. Near and mid-infrared luminescence of new chalcohalide glasses doped with Pr3+ ions. Mater. Sci. Eng. B 2008, 146, 107–109. [Google Scholar] [CrossRef]
- Sourkova, P.; Frumarova, B.; Frumar, M.; Nemec, P.; Kincl, M.; Nazabal, V.; Moizan, V.; Doualan, J.-L.; Moncorge, R. Spectroscopy of infrared transitions of Pr3+ ions in Ga-Ge-Sb-Se glasses. J. Lumin. 2009, 129, 1148–1153. [Google Scholar] [CrossRef]
- Nachimuthu, P.; Vithal, M.; Jagannathan, R. Absorption and emission spectral properties of Pr3+, Nd3+, and Eu3+ ions in heavy-metal oxide glasses. J. Am. Ceram. Soc. 2000, 83, 597–604. [Google Scholar] [CrossRef]
- Balda, R.; Fernandez, J.; De Pablos, A.; Fdez-Navarro, J.M. Spectroscopic properties of Pr3+ ions in lead germanate glass. J. Phys. Condens. Matter 1999, 11, 7411–7421. [Google Scholar] [CrossRef]
- Balda, R.; Saez de Ocariz, I.; Fernandez, J.; Fdez-Navarro, J.M.; Arriandiaga, M.A. Spectroscopy and orange-blue frequency upconversion in Pr3+-doped GeO2-PbO-Nb2O5 glass. J. Phys. Condens. Matter 2000, 12, 10623–10632. [Google Scholar] [CrossRef]
- Burtan, B.; Cisowski, J.; Mazurak, Z.; Jarzabek, B.; Czaja, M.; Reben, M.; Grelowska, I. Concentration-dependent spectroscopic properties of Pr3+ ions in TeO2-WO3-PbO-La2O3 glass. J. Non-Cryst. Solids 2014, 400, 21–26. [Google Scholar] [CrossRef]
- Mitra, S.; Jana, S. Intense orange emission in Pr3+ doped lead phosphate glass. J. Phys. Chem. Solids 2015, 85, 245–253. [Google Scholar] [CrossRef]
- Sharma, R.; Rao, A.S.; Deopa, N.; Venkateswarlu, M.; Jayasimhadri, M.; Haranath, D.; Vijaya Prakash, G. Spectroscopic study of Pr3+ ions doped zinc lead tungsten tellurite glasses for visible photonic device applications. Opt. Mater. 2018, 78, 457–464. [Google Scholar] [CrossRef]
- Basavapoornima, C.; Kesavulu, C.R.; Maheswari, T.; Pecharapa, W.; Depuru, S.R.; Jayasankar, C.K. Spectral characteristics of Pr3+-doped lead based phosphate glasses for optical display device applications. J. Lumin. 2020, 228, 117585. [Google Scholar] [CrossRef]
- Bhargavi, K.; Sudarsan, V.; Brik, M.G.; Rao, M.S.; Gandhi, Y.; Rao, P.N.; Veeraiah, N. Influence of Al declustering on the photoluminescent properties of Pr3+ ions in PbO-SiO2 glasses. J. Non-Cryst. Solids 2013, 362, 201–206. [Google Scholar] [CrossRef]
- Bhargavi, K.; Sanyal, B.; Rao, M.S.; Kumar, V.R.; Gandhi, Y.; Baskaran, G.S.; Veeraiah, N. γ-Ray induced thermoluminescence characteristics of the PbO-Al2O3-SiO2:Pr3+ glass system. J. Lumin. 2015, 161, 417–421. [Google Scholar] [CrossRef]
- Suresh, B.; Purnachand, N.; Zhydachevskii, Y.; Brik, M.G.; Reddy, M.S.; Suchocki, A.; Piasecki, M.; Veeraiah, N. Influence of Bi3+ ions on the amplification of 1.3 μm emission of Pr3+ ions in lead silicate glasses for the applications in second telecom window communications. J. Lumin. 2017, 182, 312–322. [Google Scholar] [CrossRef]
- Naranjo, L.P.; De Araújo, C.B.; Malta, O.L.; Santa Cruz, P.A.; Kassab, L.R.P. Enhancement of Pr3+ luminescence in PbO-GeO2 glasses containing silver nanoparticles. Appl. Phys. Lett. 2005, 87, 241914. [Google Scholar] [CrossRef]
- Rai, V.K.; Menezes, L.S. De Araújo, C.B.; Kassab, L.R.P.; Da Silva, D.M.; Kobayashi, R.A. Surface-plasmon-enhanced frequency upconversion in Pr3+ doped tellurium-oxide glasses containing silver nanoparticles. J. Appl. Phys. 2008, 103, 093526. [Google Scholar] [CrossRef] [Green Version]
- Hua, C.; Zhao, X.; Pun, E.Y.B.; Lin, H. Pr3+ doped tellurite glasses incorporated with silver nanoparticles for laser illumination. RSC Adv. 2017, 7, 55691–55701. [Google Scholar] [CrossRef] [Green Version]
- Rajesh, D.; Amjad, R.J.; Reza Dousti, M.; De Camargo, A.S.S. Enhanced VIS and NIR emissions of Pr3+ ions in TZYN glasses containing silver ions and nanoparticles. J. Alloys Compd. 2017, 695, 607–612. [Google Scholar] [CrossRef]
- Herrera, A.; Balzaretti, N.M. Effect of high pressure in the luminescence of Pr3+-doped GeO2-PbO glass containing Au nanoparticles. J. Phys. Chem. C 2018, 122, 27829–27835. [Google Scholar] [CrossRef]
- Anjaiah, J.; Laxmikanth, C.; Veeraiah, N.; Kistaiah, P. Luminescence properties of Pr3+ doped Li2O-MO-B2O3 glasses. J. Lumin. 2015, 161, 147–153. [Google Scholar] [CrossRef]
- Jayasankar, C.K.; Babu, P. Compositional dependence of optical properties of Pr3+ ions in lithium borate glasses. J. Alloys Compd. 1998, 275–277, 369–373. [Google Scholar] [CrossRef]
- Boora, M.; Malik, S.; Kumar, V.; Bala, M.; Arora, S.; Rohilla, S.; Kumar, A.; Dalal, J. Investigation of structural and impedance spectroscopic properties of borate glasses with high Li+ concentration. Solid State Ion. 2021, 368, 115704. [Google Scholar] [CrossRef]
- Tijaria, M.; Sharma, Y.; Kumar, V.; Dahiya, S.; Dalal, J. Effect of Na2O on physical, structural and electrical properties of borate glasses. Mater. Today Proc. 2021, 45, 3722–3725. [Google Scholar] [CrossRef]
- Janek, J.; Sołtys, M.; Żur, L.; Pietrasik, E.; Pisarska, J.; Pisarski, W.A. Luminescence investigations of rare earth doped lead-free borate glasses modified by MO (M = Ca, Sr, Ba). Mater. Chem. Phys. 2016, 180, 237–243. [Google Scholar] [CrossRef]
- Pisarska, J.; Pisarski, W.A.; Dorosz, D.; Dorosz, J. Spectroscopic properties of Pr3+ and Er3+ ions in lead-free borate glasses modified by BaF2. Opt. Mater. 2015, 47, 548–554. [Google Scholar] [CrossRef]
- Turri, G.; Sudesh, V.; Richardson, M.; Bass, M.; Toncelli, A.; Tonelli, M. Temperature-dependent spectroscopic properties of Tm3+ in germanate, silica, and phosphate glasses: A comparative study. J. Appl. Phys. 2008, 103, 093104. [Google Scholar] [CrossRef] [Green Version]
- Yuliantini, L.; Djamal, M.; Hidayat, R.; Boonin, K.; Yasaka, P.; Kaewnuam, E.; Venkatramu, V.; Kaewkhao, J. Optical and X-ray induced luminescence of Sm3+-doped borotellurite and fluoroborotellurite glasses: A comparative study. J. Lumin. 2019, 213, 19–28. [Google Scholar] [CrossRef]
- Zaman, F.; Srisittipokakun, N.; Rooh, G.; Khattak, S.A.; Kaewkhao, J.; Rani, M.; Kim, H.J. Comparative study of Dy3+ doped borate glasses on the basis of luminescence and lasing properties for white-light generation. Opt. Mater. 2021, 119, 111308. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Y.; Shen, X.; Yang, R.; Wei, W. Investigations on the effects of the Stark splitting on the fluorescence behaviors in Yb3+-doped silicate, tellurite, germanate, and phosphate glasses. Opt. Mater. 2018, 75, 1–6. [Google Scholar] [CrossRef]
- Desirena, H.; De la Rosa, E.; Romero, V.H.; Castillo, J.F.; Diaz-Torres, L.A.; Oliva, J.R. Comparative study of the spectroscopic properties of Yb3+/Er3+ codoped tellurite glasses modified with R2O (R = Li, Na and K). J. Lumin. 2012, 132, 391–397. [Google Scholar] [CrossRef]
- Bueno, L.A.; Messaddeq, Y.; Dias Filho, F.A.; Ribeiro, S.J.L. Study of fluorine losses in oxyfluoride glasses. J. Non-Cryst. Solids 2005, 351, 3804–3808. [Google Scholar] [CrossRef]
- Lin, L.; Ren, G.; Chen, M.; Liu, Y.; Yang, Q. Study of fluorine losses and spectroscopic properties of Er3+ doped oxyfluoride silicate glasses and glass ceramics. Opt. Mater. 2009, 31, 1439–1442. [Google Scholar] [CrossRef]
- Brauer, D.S.; Mneimne, M.; Hill, R.G. Fluoride-containing bioactive glasses: Fluoride loss during melting and ion release in tris buffer solution. J. Non-Cryst. Solids 2011, 357, 3328–3333. [Google Scholar] [CrossRef]
- De Pablos-Martín, A.; Contreras Jaimes, A.T.; Wahl, S.; Meyer, S.; Brauer, D.S. Fluorine loss determination in bioactive glasses by laser-induced breakdown spectroscopy (LIBS). Int. J. Appl. Glass Sci. 2021, 12, 213–221. [Google Scholar] [CrossRef]
- Möncke, D.; Da Cruz Barbosa Neto, M.; Bradtmüller, H.; De Souza, G.B.; Rodrigues, A.M.; Elkholy, H.S.; Othman, H.O.; Moulton, B.J.A.; Kamitsos, E.I.; Rodrigues, A.C.M.; et al. NaPO3-AlF3 glasses: Fluorine evaporation during melting and the resulting variations in structure and properties. J. Chem. Technol. Metall. 2018, 53, 1047–1060. [Google Scholar]
- Kochanowicz, M.; Zmojda, J.; Baranowska, A.; Kuwik, M.; Starzyk, B.; Lesniak, M.; Miluski, P.; Pisarski, W.A.; Pisarska, J.; Dorosz, J.; et al. Fluoroindate glass co-doped with Yb3+/Ho3+ as a 2.85 µm luminescent source for MID-IR sensing. Sensors 2021, 21, 2155. [Google Scholar] [CrossRef]
- Zhou, B.; Wei, T.; Cai, M.; Tian, Y.; Zhou, J.; Deng, D.; Xu, S.; Zhang, J. Analysis on energy transfer process of Ho3+ doped fluoroaluminate glass sensitized by Yb3+ for mid-infrared 2.85 µm emission. J. Quant. Spectrosc. Radiat. Transf. 2014, 149, 41–50. [Google Scholar] [CrossRef]
- Pisarska, J.; Kuwik, M.; Górny, A.; Kochanowicz, M.; Zmojda, J.; Dorosz, J.; Dorosz, D.; Sitarz, M.; Pisarski, W.A. Holmium doped barium gallo-germanate glasses for near-infrared luminescence at 2000 nm. J. Lumin. 2019, 215, 116625. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Żur, L.; Goryczka, T.; Sołtys, M.; Pisarska, J. Structure and spectroscopy of rare earth—Doped lead phosphate glasses. J. Alloys Compd. 2014, 587, 90–98. [Google Scholar] [CrossRef]
- Pisarski, W.A. Spectroscopic analysis of praseodymium and erbium ions in heavy metal fluoride and oxide glasses. J. Mol. Struct. 2005, 744–747, 473–479. [Google Scholar] [CrossRef]
- Żur, L.; Janek, J.; Sołtys, M.; Pisarska, J.; Pisarski, W.A. Effect of BaF2 content on luminescence of rare-earth ions in borate and germanate glasses. J. Am. Ceram. Soc. 2016, 99, 2009–2016. [Google Scholar] [CrossRef]
- Pisarska, J.; Kowal, M.; Kochanowicz, M.; Zmojda, J.; Dorosz, J.; Dorosz, D.; Pisarski, W.A. Influence of BaF2 and activator concentration on broadband near-infrared luminescence of Pr3+ ions in gallo-germanate glasses. Opt. Express 2016, 24, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswari, D.; Jamalaiah, B.C.; Chengaiah, T.; Kim, I.-G.; Moorthy, L.R. Optical properties of sodium fluoroborate glasses containing Pr3+ ions for red luminescence. Phys. Chem. Glasses Eur. J. Glass Sci. Technol. B 2012, 53, 271–275. [Google Scholar]
- Naresh, V.; Ham, B.S. Influence of multiphonon and cross relaxations on 3P0 and 1D2 emission levels of Pr3+ doped borosilicate glasses for broad band signal amplification. J. Alloys Compd. 2016, 664, 321–330. [Google Scholar] [CrossRef]
- Tanner, P.A.; Zhou, L.; Duan, C.; Wong, K.-L. Misconceptions in electronic energy transfer: Bridging the gap between chemistry and physics. Chem. Soc. Rev. 2018, 47, 5234–5265. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Dai, Z.; Li, J.; Tian, Y. Energy transfer and frequency upconversion in Pr3+-doped ZBLAN glass. J. Non-Cryst. Solids 2006, 352, 5469–5474. [Google Scholar] [CrossRef]
- Annapurna, K.; Chakrabarti, R.; Buddhudu, S. Absorption and emission spectral analysis of Pr3+: Tellurite glasses. J. Mater. Sci. 2007, 42, 6755–6761. [Google Scholar] [CrossRef]
- Suthanthirakumar, P.; Basavapoornima, Ch.; Marimuthu, K. Effect of Pr3+ ions concentration on the spectroscopic properties of zinc telluro-fluoroborate glasses for laser and optical amplifier applications. J. Lumin. 2017, 187, 392–402. [Google Scholar] [CrossRef]
- Deopa, N.; Rao, A.S.; Mahamuda, Sk.; Gupta, M.; Jayasimhadri, M.; Haranath, D.; Prakash, G.V. Spectroscopic studies of Pr3+ doped lithium lead alumino borate glasses for visible reddish orange luminescent device applications. J. Alloys Compd. 2017, 708, 911–921. [Google Scholar] [CrossRef]
- Venkateswarlu, M.; Prasad, M.V.V.K.S.; Swapna, K.; Mahamuda, Sk.; Rao, A.S.; Babu, A.M.; Haranath, D. Pr3+ doped lead tungsten tellurite glasses for visible red lasers. Ceram. Int. 2014, 40, 6261–6269. [Google Scholar] [CrossRef]
- Mahamuda, Sk.; Swapna, K.; Rao, A.S.; Sasikala, T.; Moorthy, L.R. Reddish-orange emission from Pr3+ doped zinc alumino bismuth borate glasses. Phys. B 2013, 428, 36–42. [Google Scholar] [CrossRef]
- Devi, C.B.A.; Mahamuda, S.; Swapna, K.; Venkateswarlu, M.; Rao, A.S.; Prakash, G.V. Pr3+ ions doped single alkali and mixed alkali fluoro tungsten tellurite glasses for visible red luminescent devices. J. Non-Cryst. Solids 2018, 498, 345–351. [Google Scholar] [CrossRef]
- Pisarska, J.; Pisarski, W.A.; Dorosz, D.; Dorosz, J. Spectral analysis of Pr3+ doped germanate glasses modified by BaO and BaF2. J. Lumin. 2016, 171, 138–142. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Gouveia, E.A.; De Araujo, M.T.; Gouveia-Neto, A.S.; De Araujo, C.B.; Messaddeq, Y. Twentyfold blue upconversion emission enhancement through thermal effects in Pr3+/Yb3+-codoped fluoroindate glasses excited at 1.064 µm. J. Appl. Phys. 2000, 87, 4274–4278. [Google Scholar] [CrossRef] [Green Version]
- Shojiya, M.; Kawamoto, Y.; Kadono, K. Judd-Ofelt parameters and multiphonon relaxation of Ho3+ ions in ZnCl2-based glass. J. Appl. Phys. 2001, 89, 4944–4950. [Google Scholar] [CrossRef]
- Choi, Y.G.; Baik, J.H.; Heo, H.J. Spectroscopic properties of Pr3+: 1D2 → 1G4 transition in SiO2-based glasses. Chem. Phys. Lett. 2005, 406, 436–440. [Google Scholar] [CrossRef]
- Liu, X.; Chen, B.J.; Pun, E.Y.B.; Lin, H. Ultra-broadband near-infrared emission in praseodymium ion doped germanium tellurite glasses for optical fiber amplifier operating at E-, S-, C-, and L-band. J. Appl. Phys. 2012, 111, 116101. [Google Scholar] [CrossRef]
- Choi, Y.G.; Kim, K.H.; Park, B.J.; Heo, H.J. 1.6 μm emission from Pr3+: (3F3, 3F4) → 3H4 transition in Pr3+- and Pr3+/Er3+-doped selenide glasses. Appl. Phys. Lett. 2001, 78, 1249–1251. [Google Scholar] [CrossRef]
- Yu, D.C.; Chen, Q.J.; Lin, H.H.; Wang, Y.Z.; Zhang, Q.Y. New insight into two-step near-infrared quantum cutting in Pr3+ singly doped oxyfluoride glass-ceramics. Opt. Mater. Express 2016, 6, 197–205. [Google Scholar] [CrossRef]
- Belançon, M.P.; Marconi, J.D.; Ando, M.F.; Barbosa, L.C. Near-IR emission in Pr3+ single doped and tunable near-IR emission in Pr3+/Yb3+ codoped tellurite tungstate glasses for broadband optical amplifiers. Opt. Mater. 2014, 36, 1020–1026. [Google Scholar] [CrossRef]
- Shen, L.F.; Chen, B.J.; Lin, H.; Pun, E.Y.B. Praseodymium ion doped phosphate glasses for integrated broadband ion-exchanged waveguide amplifier. J. Alloys Compd. 2015, 622, 1093–1097. [Google Scholar] [CrossRef]
- Han, X.; Shen, L.; Pun, E.Y.B.; Ma, T.; Lin, H. Pr3+-doped phosphate glasses for fiber amplifiers operating at 1.38–1.53 µm of the fifth optical telecommunication window. Opt. Mater. 2014, 36, 1203–1208. [Google Scholar] [CrossRef]
- Zhou, B.; Tao, L.; Tsang, Y.H.; Jin, W.; Pun, E.Y.B. Superbroadband near-IR photoluminescence from Pr3+-doped fluorotellurite glasses. Opt. Express 2012, 20, 3803–3813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
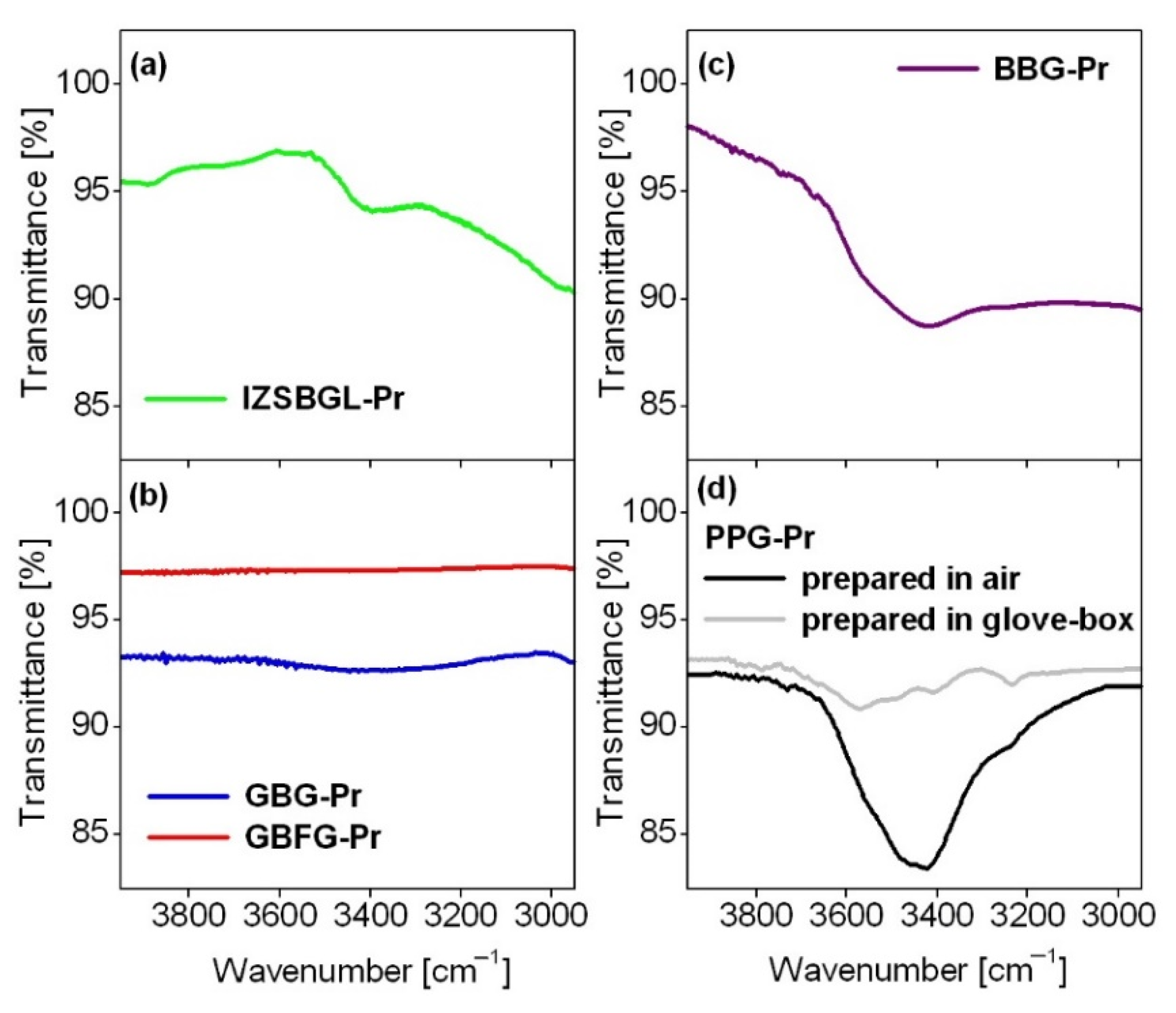
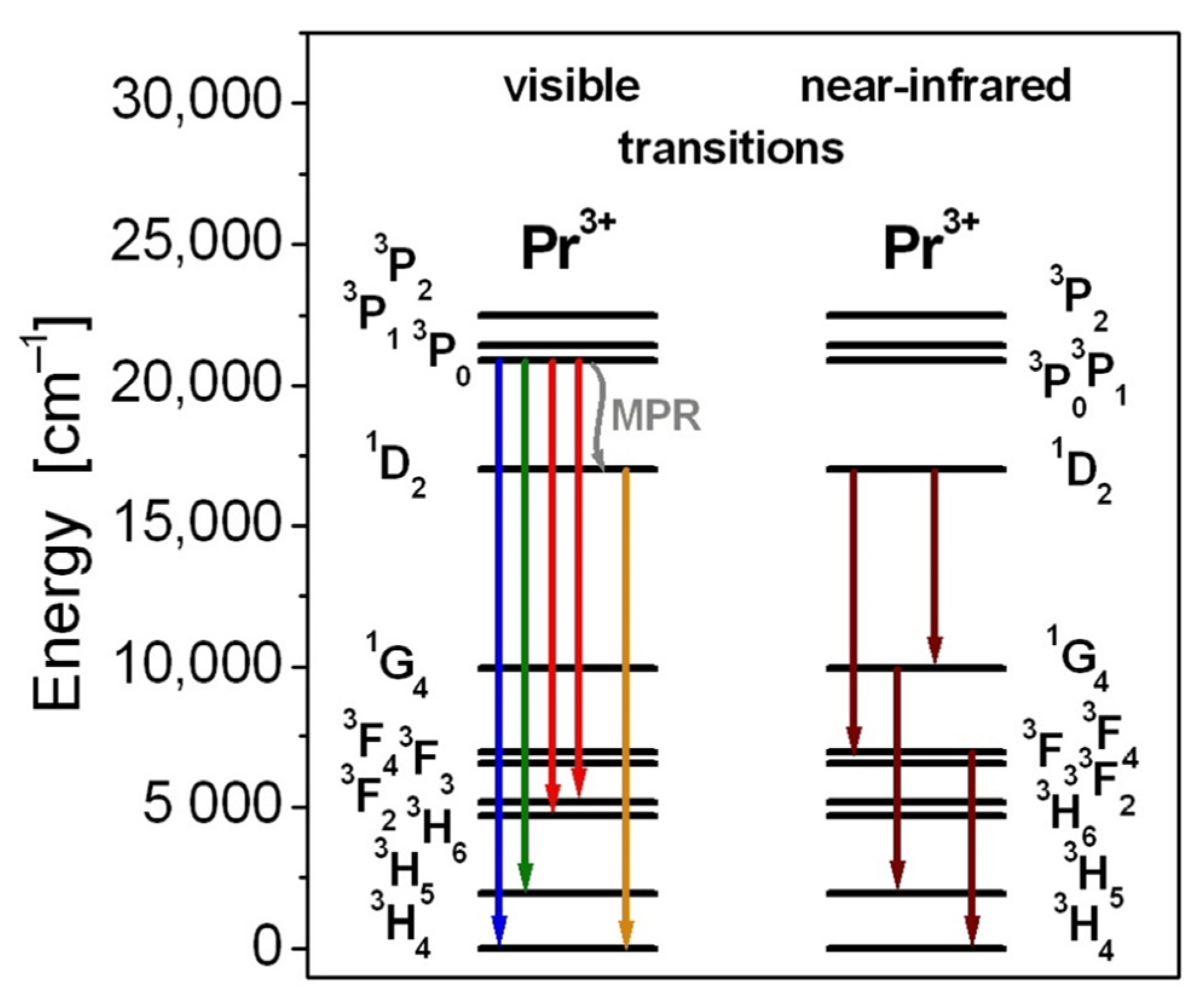
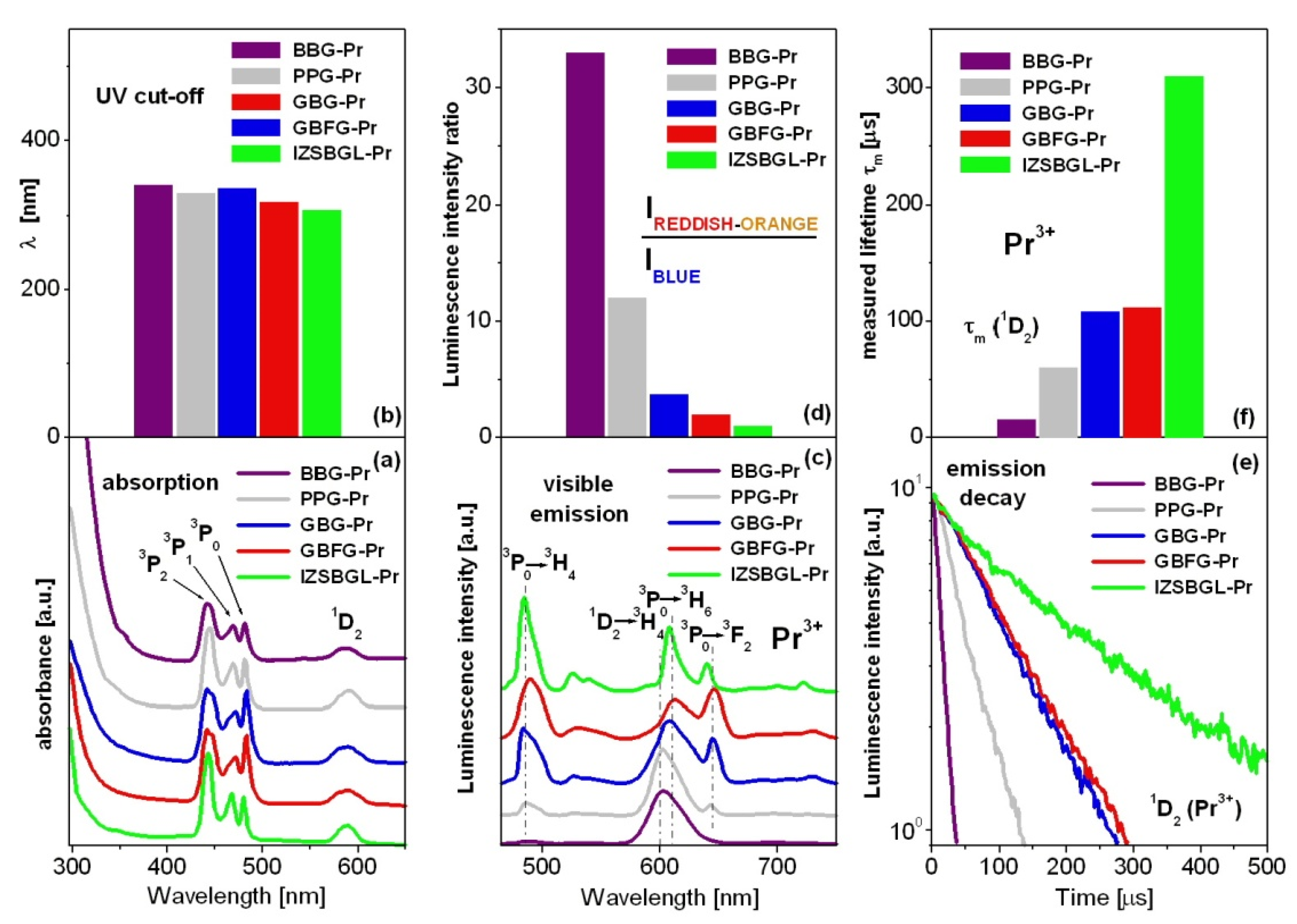

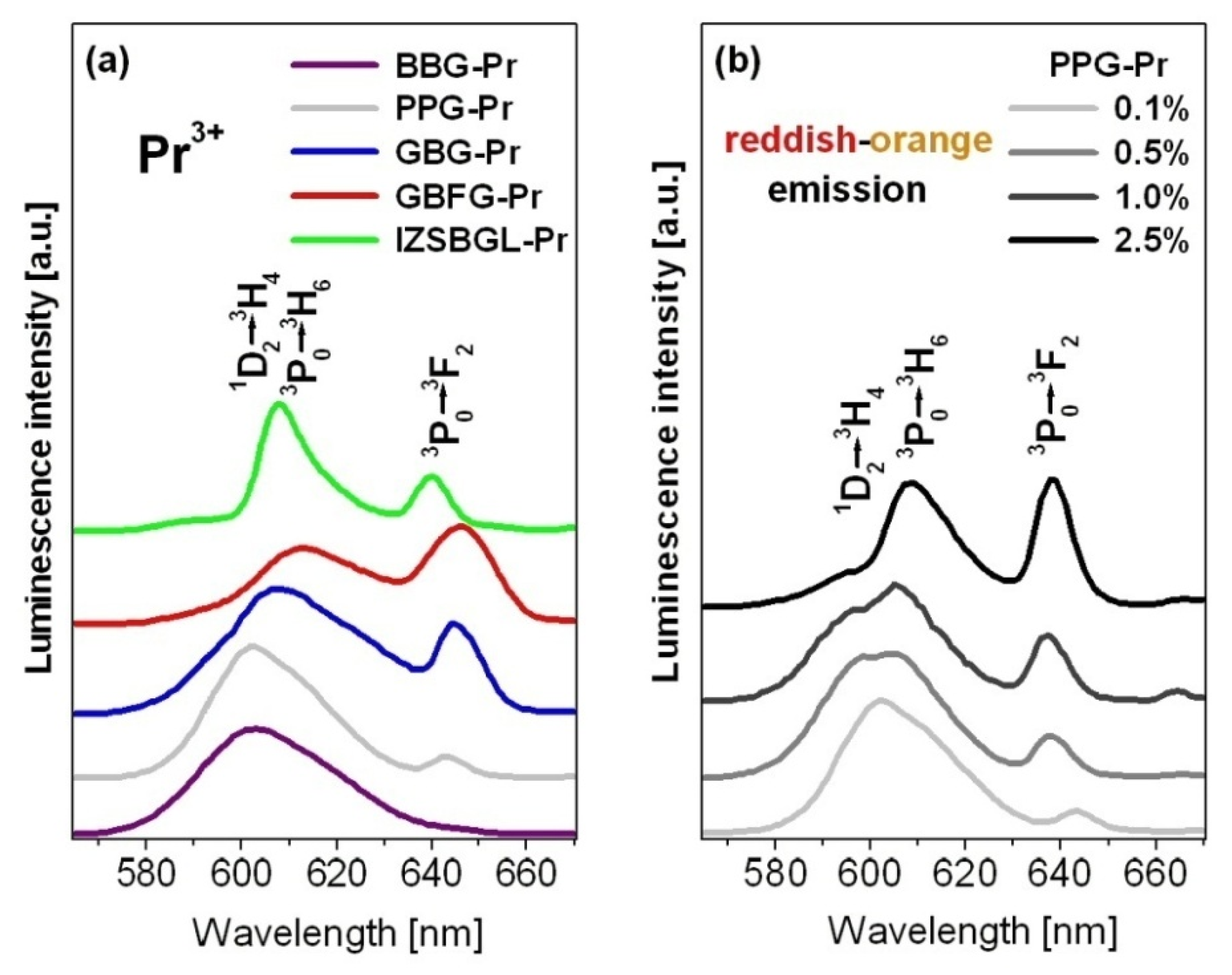
| Glass Code | Chemical Composition [mol%] | Melting Conditions |
|---|---|---|
| IZSBGL-Pr | 37.9InF3-20ZnF2-20SrF2-16BaF2-4GaF3-2LaF3-0.1PrF3 | 900 °C/60 min |
| GBFG-Pr | 60GeO2-25BaO-5BaF2-9.9Ga2O3-0.1Pr2O3 | 1200 °C/45 min |
| GBG-Pr | 60GeO2-30BaO-9.9Ga2O3-0.1Pr2O3 | 1200 °C/45 min |
| PPG-Pr | 45PbO-45P2O5-9.9Ga2O3-0.1Pr2O3 | 1100 °C/30 min |
| BBG-Pr | 60B2O3-30BaO-9.9Ga2O3-0.1Pr2O3 | 1250 °C/45 min |
| Glass Code | Chemical Composition [mol%] | Melting Conditions |
|---|---|---|
| PPG-0.1Pr | 45PbO-45P2O5-9.9Ga2O3-0.1Pr2O3 45PbO-45P2O5-9.5Ga2O3-0.5Pr2O3 45PbO-45P2O5-9.0Ga2O3-1.0Pr2O3 45PbO-45P2O5-7.5Ga2O3-2.5Pr2O3 | 1100 °C/30 min |
| PPG-0.5Pr | 1100 °C/30 min | |
| PPG-1.0Pr | 1100 °C/30 min | |
| PPG-2.5Pr | 1100 °C/30 min |
| Parameters | Glass-Host | |||
|---|---|---|---|---|
| IZSBGL-Pr | GBG-Pr | PPG-Pr | BBG-Pr | |
| Average molecular weight (M g mol−1) | 140.94 | 127.63 | 183.22 | 106.63 |
| Density (d g cm−3) | 4.38 | 4.58 | 4.11 | 3.19 |
| Pr3+ content (molar %) | 0.1 | 0.1 | 0.1 | 0.1 |
| Pr3+ concentration (Nx1019 ions cm−3) | 1.87 | 4.31 | 2.70 | 3.59 |
| Average interionic separation (R Å) | 18.5 | 14.0 | 16.3 | 14.9 |
| Critical transfer distance (R0 Å) | 11.3 | 6.5 | 8.5 | 7.8 |
| Refractive index (n) | 1.48 | 1.73 | 1.75 | 1.61 |
| Glass transition temperature (Tg °C) | 295 | 620 | 437 | 566 |
| Phonon energy of the host (hω cm−1) | 510 | 790 | 1120 | 1400 |
| Judd–Ofelt parameters Ωt (10−20 cm2) | ||||
| Ω2 | 2.01 | 6.93 | 1.81 | 2.17 |
| Ω4 | 5.25 | 19.68 | 18.33 | 9.75 |
| Ω6 | 5.10 | 8.95 | 15.51 | 2.62 |
| Radiative transition rate (AJ s−1) | ||||
| from 3P0 state (Pr3+) | 30,200 | 123,050 | 95,250 | 60,450 |
| from 1D2 state (Pr3+) | 2440 | 8930 | 8330 | 3370 |
| Quantum efficiency 1D2 Pr3+ (η %) | 88 | 98 | 50 | 5 |
| Glass-Host Composition [mol%] | 3P0 [µs] | 1D2 [µs] | Ref. |
|---|---|---|---|
| 57ZrF4-34BaF2-4AlF3-4.5LaF3-0.5PrF3 | 37 | - | [8] |
| 50SiO2-10Al2O3-2MgO-20CaO-15SrO-3BaO-0.1Pr2O3 | 115 | - | [11] |
| 74.8TeO2-15Sb2O3-10WO3-0.2Pr6O11 | 11.73 | - | [12] |
| 60P2O5-4B2O3-7Al2O3-10K2O-17.95BaO-0.05Pr2O3 | - | 173 | [13] |
| 49.5P2O5-10AlF3-10BaF2-10SrF2-10PbO-10MxOy-0.5Pr6O11 | 10–11 | 14–17 | [15] |
| M = Li, Na, K, Zn, Bi | |||
| 60P2O5-4B2O3-7Al2O3-10K2O-17.9BaO-0.1Pr2O3 | - | 137 | [16] |
| 55SiO2-8B2O3-5Al2O3-14Li2O-2Na2O-10GeO2-5.9Y2O3-0.1Pr2O3 | - | 73 | [16] |
| 75TeO2-20ZnO-5Na2O-0.1Pr2O3 | - | 51 | [16] |
| 69H3BO3-20Li2CO3-10LiF-1Pr2O3 | 25.1 | 30 | [17] |
| 60PbO-40GeO2-0.05Pr2O3 | 6 | 145 | [23] |
| 5ZnO-15PbO-20WO3-59TeO2-1Pr6O11 | 4.5 | - | [27] |
| 44P2O5-17K2O-9Al2O3-23.9PbO-6Na2O-0.1Pr6O11 | - | 66 | [28] |
| 30PbO-5Bi2O3-64SiO2-1Pr2O3 | 69 | - | [31] |
| 25Na2O-5LaF3-10CaF2-10AlF3-49.9B2O3-0.1Pr6O11 | - | 51 | [59] |
| 30Li2CO3-20Al2O3-10B2O3-39.9SiO2-0.1Pr2O3 | 85.5 | 108.2 | [61] |
| 60TeO2-25ZnO-10BaO-4.5La2O3-0.5Pr2O3 | 21 | 39 | [64] |
| 29.95B2O3-30TeO2-16ZnO-10ZnF2-7CaF2-7BaF2-0.05Pr2O3 | 45 | 76 | [65] |
| 10Li2O-10PbO-9.95Al2O3-70B2O3-0.05Pr6O11 | - | 165 | [66] |
| Glass Code | CIE Chromaticity Coordinates | |
|---|---|---|
| (A) | IZSBGL-Pr | x = 0.380; y = 0.327 |
| (B) | GBFG-Pr | x = 0.433; y = 0.370 |
| (C) | GBG-Pr | x = 0.523; y = 0.353 |
| (D) | PPG-Pr | x = 0.582; y = 0.374 |
| (E) | BBG-Pr | x = 0.622; y = 0.366 |
| Glass Code | Spectroscopic Parameters | ||
|---|---|---|---|
| σem [10−20 cm2] | σem × τm [10−26 cm2s] | σem × FWHM [10−27 cm3] | |
| IZSBGL-Pr | 0.50 | 154 | 65 |
| GBFG-Pr | 0.98 | 108 | 206 |
| GBG-Pr | 0.97 | 107 | 201 |
| PPG-Pr | 1.28 | 77 | 165 |
| BBG-Pr | 0.37 | 6 | 81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisarska, J.; Kuwik, M.; Pisarski, W.A. Spectroscopic Properties of Inorganic Glasses Doped with Pr3+: A Comparative Study. Materials 2022, 15, 767. https://doi.org/10.3390/ma15030767
Pisarska J, Kuwik M, Pisarski WA. Spectroscopic Properties of Inorganic Glasses Doped with Pr3+: A Comparative Study. Materials. 2022; 15(3):767. https://doi.org/10.3390/ma15030767
Chicago/Turabian StylePisarska, Joanna, Marta Kuwik, and Wojciech A. Pisarski. 2022. "Spectroscopic Properties of Inorganic Glasses Doped with Pr3+: A Comparative Study" Materials 15, no. 3: 767. https://doi.org/10.3390/ma15030767






