Green Silver Nanoparticles Promote Inflammation Shutdown in Human Leukemic Monocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ag NPs
2.1.1. Conventional Approach
2.1.2. Green Approach
Preparation of Leaves Extracts
Synthetic Procedure
2.2. Characterization of Conventional and Green Ag NPs
2.2.1. Transmission Electron Microscopy (TEM) Analysis
2.2.2. Dynamic Light Scattering (DLS) and ζ-Potential Analyses
2.2.3. UV-Vis Analysis
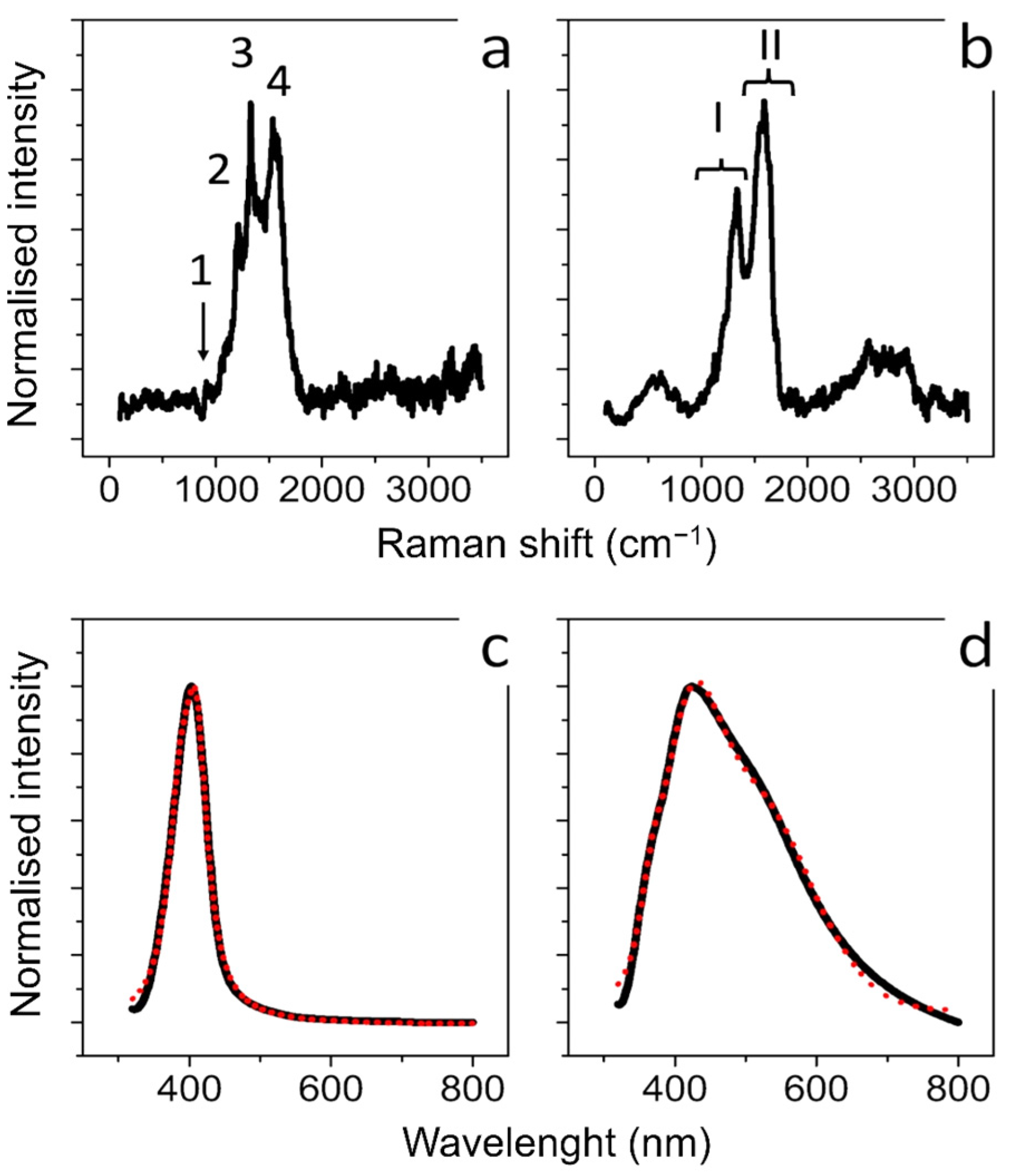
2.2.4. Raman Scattering Analysis
2.2.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.3. THP-1 Culture and Differentiation
2.4. MCF-7 and MCF-10 Cell Culture
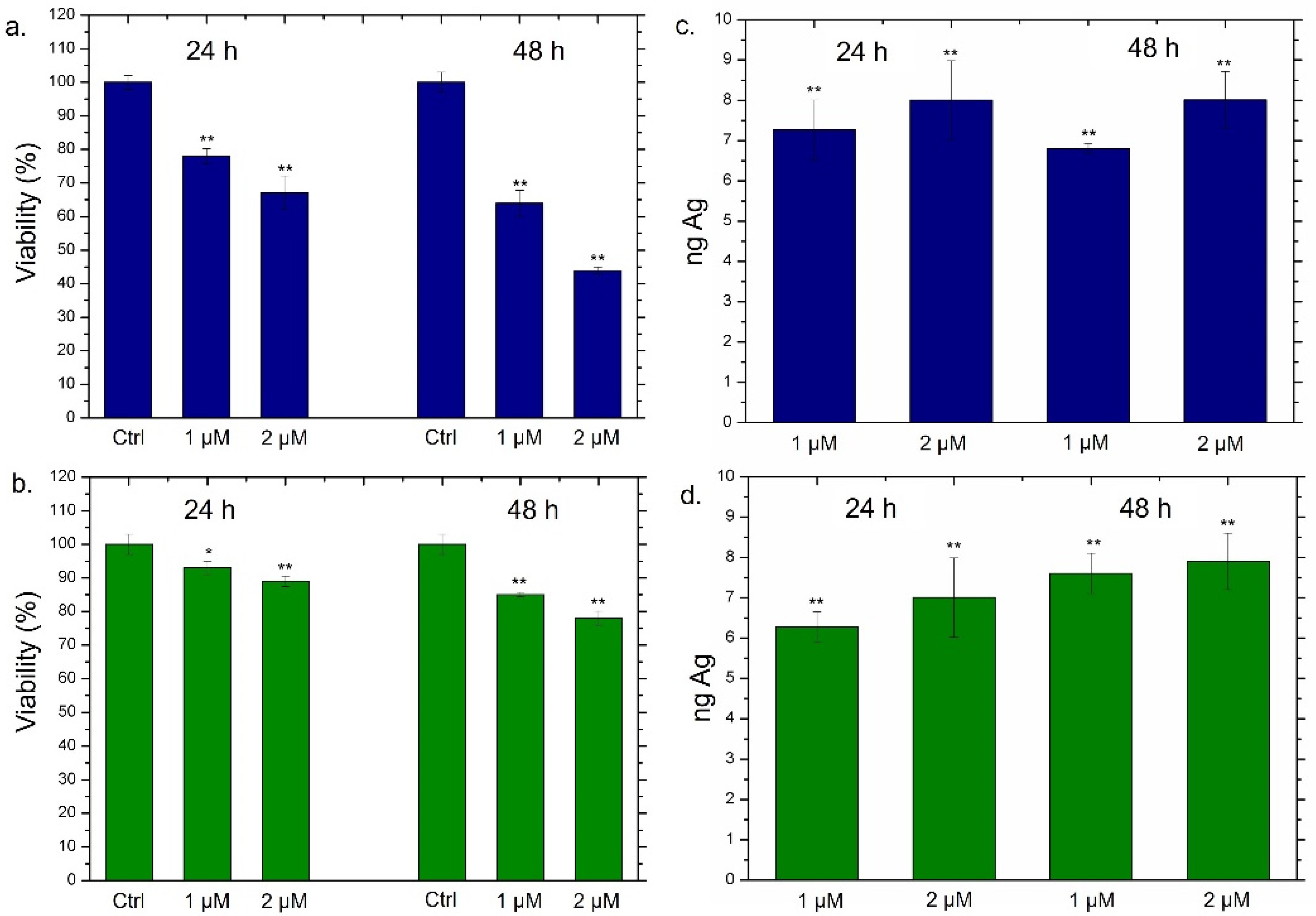
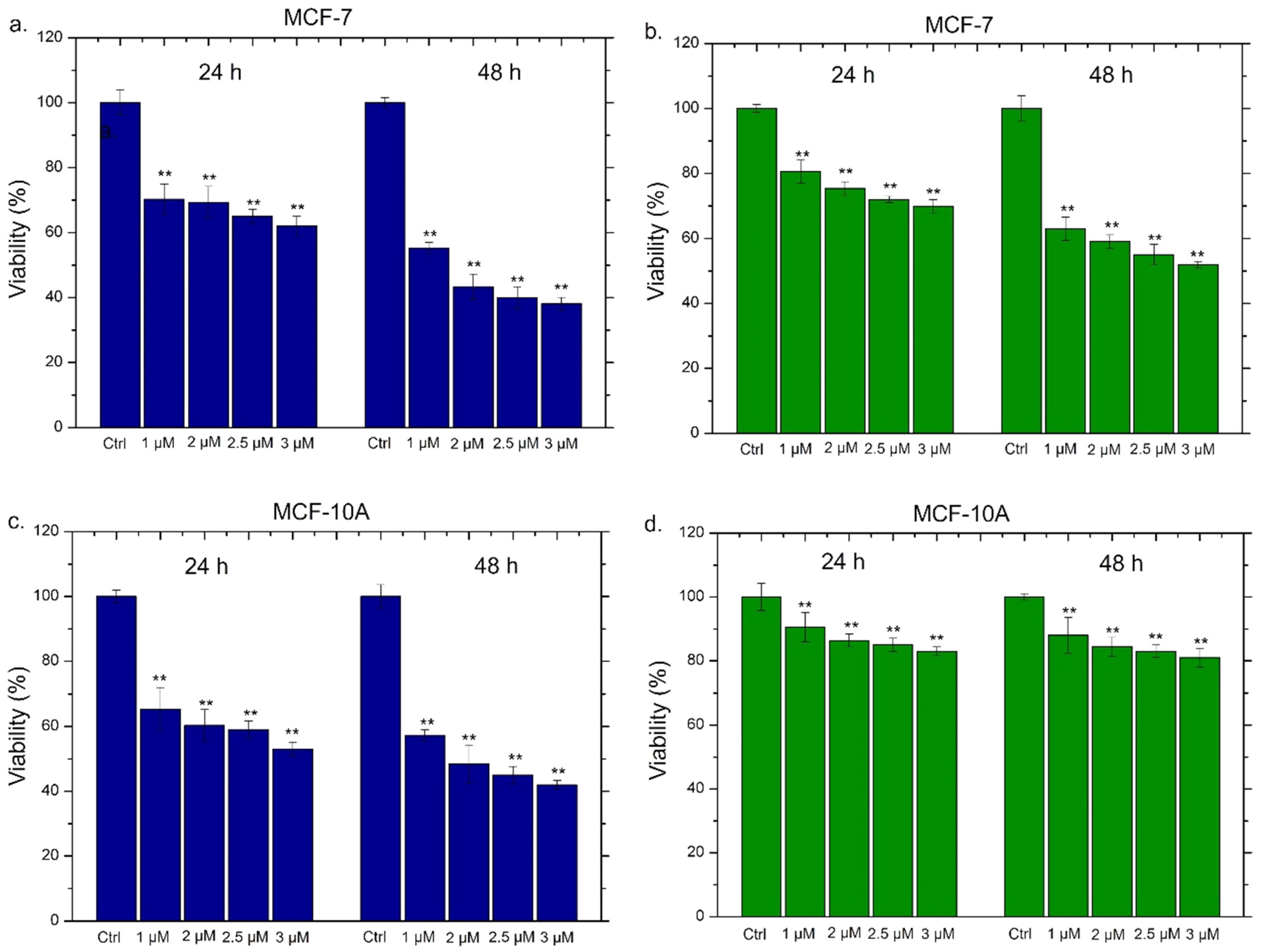
2.5. Viability Assay of THP-1, MCF-7 and MCF-10
2.6. NPs Concentration and Uptake Determination by Elemental Analysis
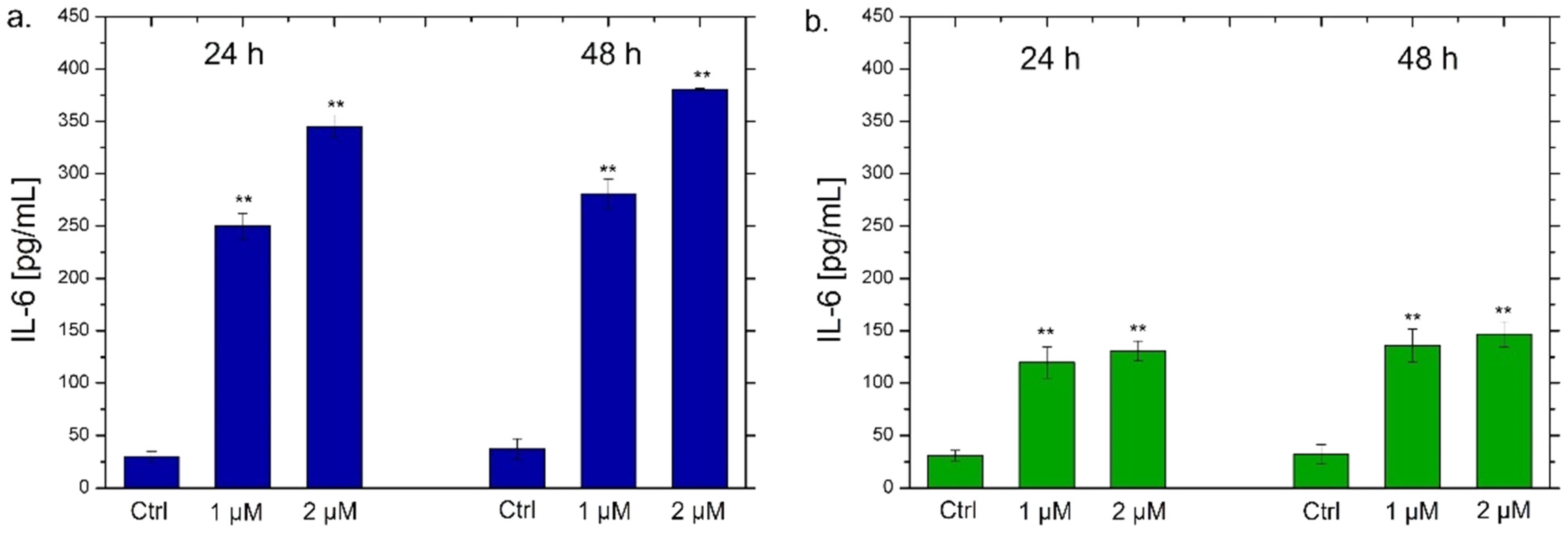
2.7. Interleukins 6, 8 (IL-6, IL-8) Quantification by ELISA Assay
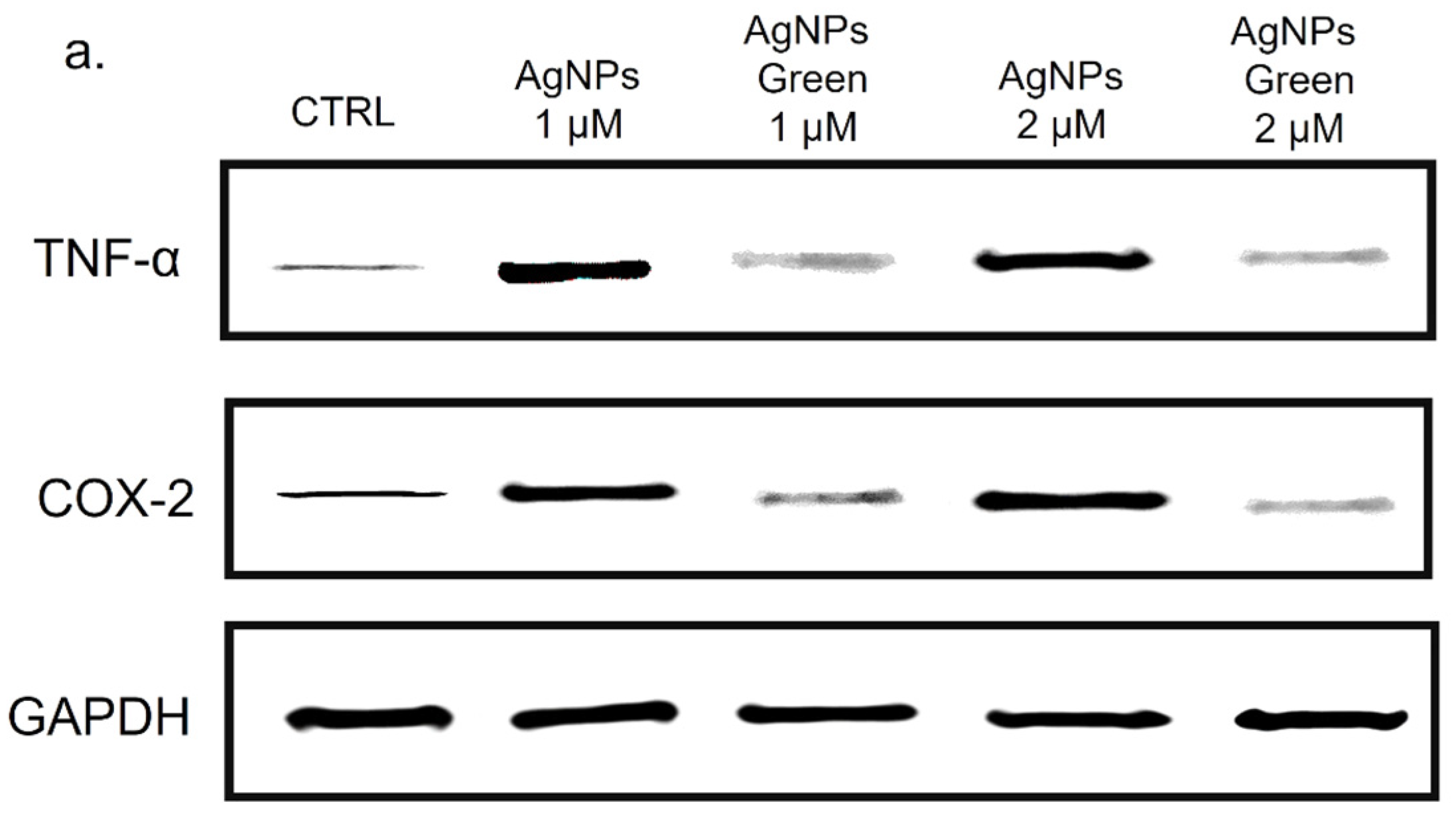
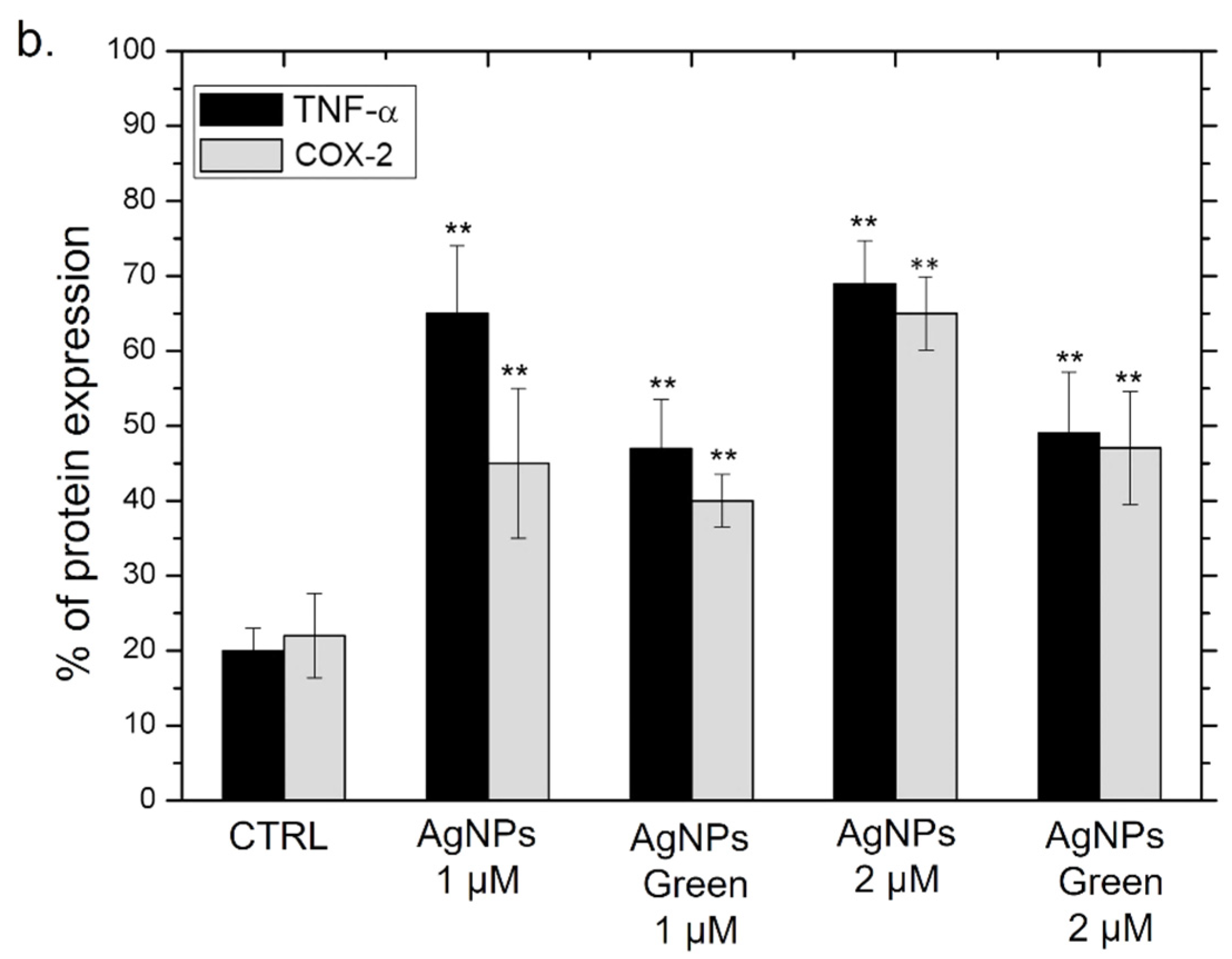
2.8. TNF-α and COX-2 Expression Levels by Western Blot Analysis
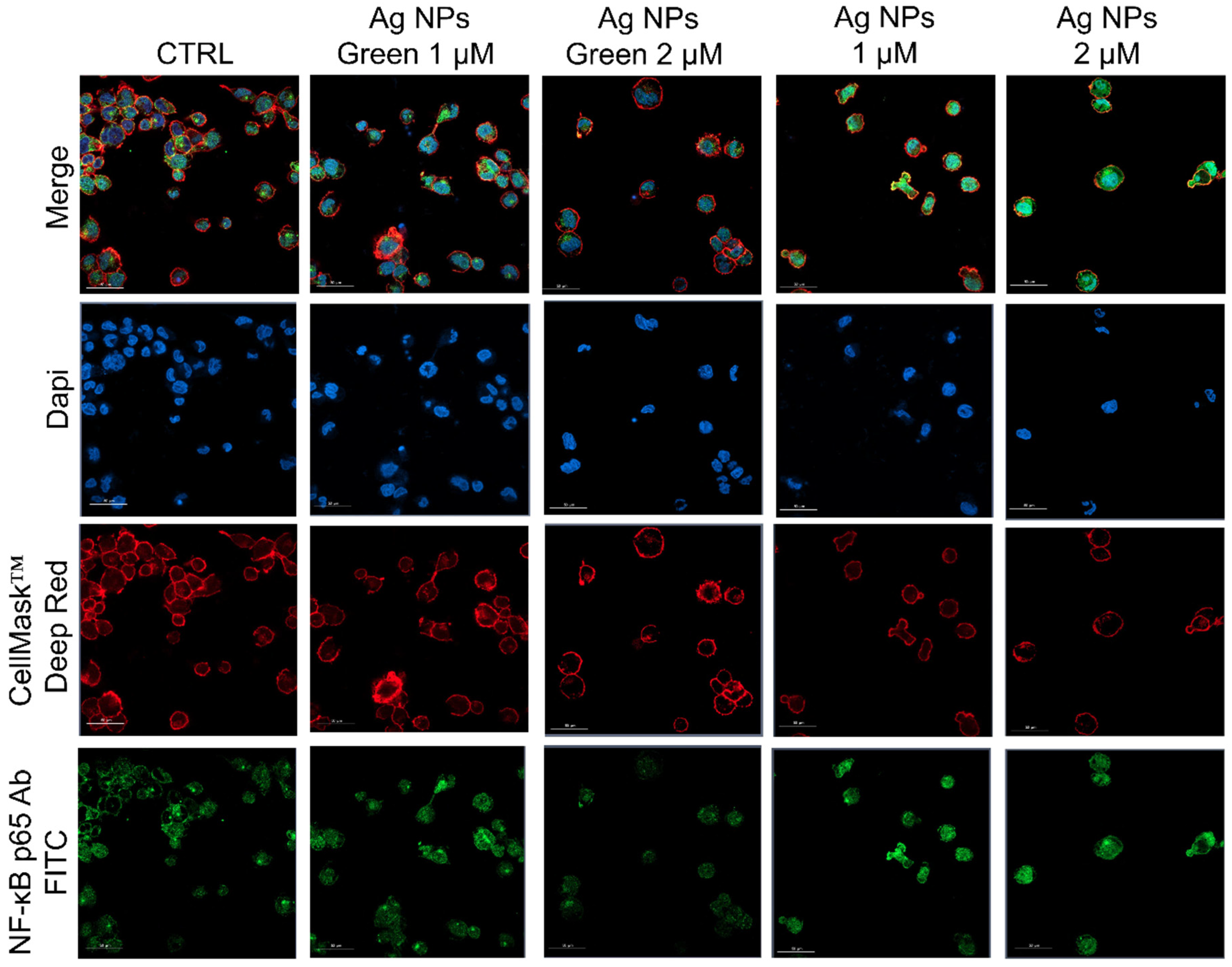
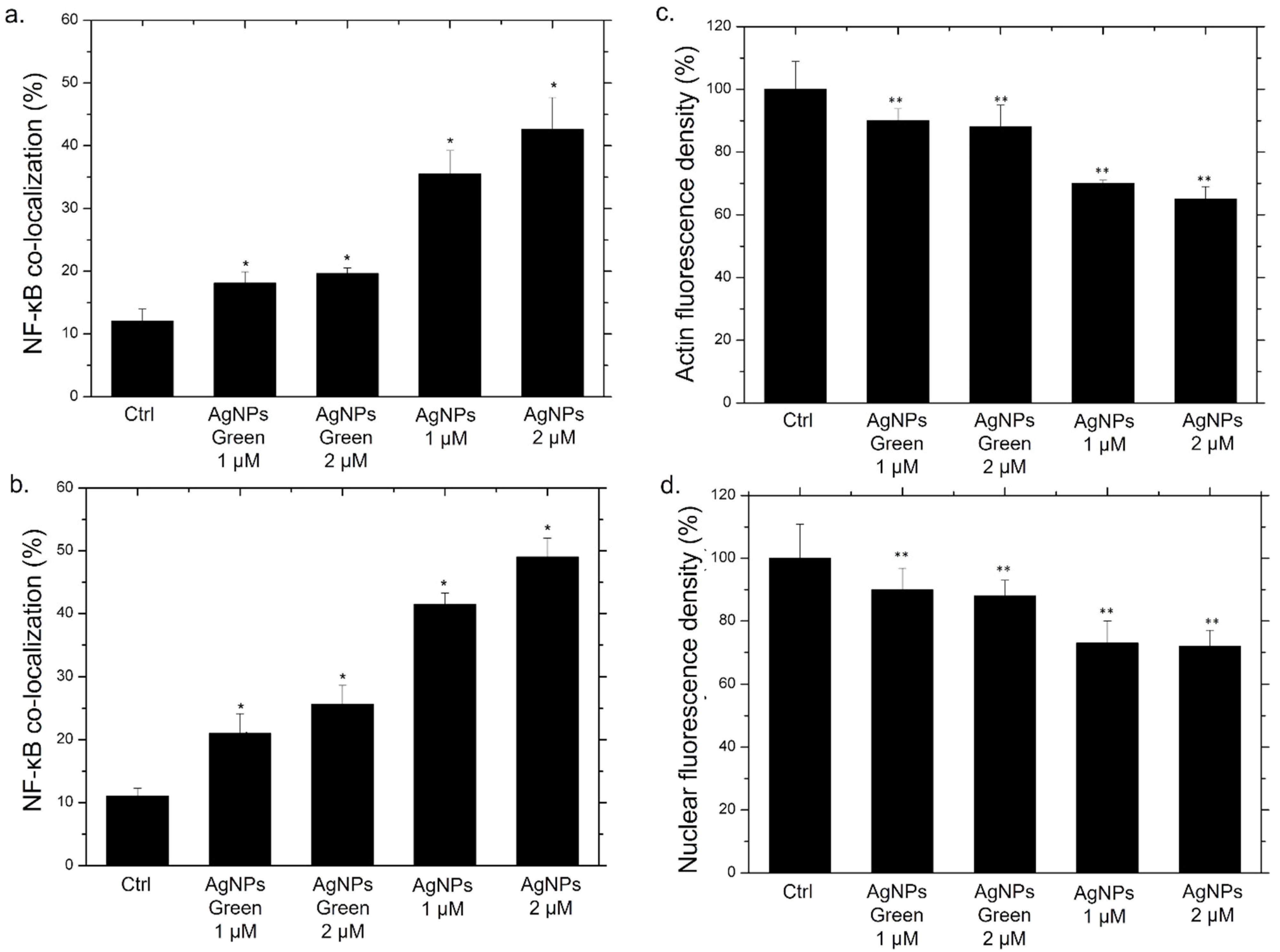
2.9. NF-κB Signaling Imaging, Quantification Assay, and Morphometric Parameters
2.10. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Contado, C. Nanomaterials in consumer products: A challenging analytical problem. Front. Chem. 2015, 3, 48. [Google Scholar] [CrossRef] [Green Version]
- Rai, M.; Birla, S.; Ingle, A.P.; Gupta, I.; Gade, A.; Abd-Elsalam, K.; Marcato, P.D.; Durán, N. Nanosilver: An inorganic nanoparticle with myriad potential applications. Nanotechnol. Rev. 2014, 3, 281–309. [Google Scholar] [CrossRef]
- De Matteis, V.; Rizzello, L.; Di Bello, M.P.; Rinaldi, R. One-step synthesis, toxicity assessment and degradation in tumoral pH environment of SiO2@Ag core/shell nanoparticles. J. Nanopart. Res. 2017, 19, 14. [Google Scholar] [CrossRef]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A Study of the Surface Plasmon Resonance of Silver Nanoparticles by the Discrete Dipole Approximation Method: Effect of Shape, Size, Structure, and Assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Govindappa, M.; Tejashree, S.; Thanuja, V.; Hemashekhar, B.; Srinivas, C.; Nasif, O.; Pugazhendhi, A.; Raghavendra, V.B. Pomegranate fruit fleshy pericarp mediated silver nanoparticles possessing antimicrobial, antibiofilm formation, antioxidant, biocompatibility and anticancer activity. J. Drug Deliv. Sci. Technol. 2020, 61, 102289. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Peng, Q.-L.; Gurunathan, S. Silver nanoparticles enhance the apoptotic potential of gemcitabine in human ovarian cancer cells: Combination therapy for effective cancer treatment. Int. J. Nanomed. 2017, 12, 6487–6502. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, N.E.-A.; Hussein, M.H.; El-Sawah, A.A. Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Sci. Rep. 2017, 7, 10844. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.C. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2525–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Albanese, G.; De Giorgi, M.L.; Corsalini, M.; Rinaldi, R. Silver Nanoparticles Addition in Poly(Methyl Methacrylate) Dental Matrix: Topographic and Antimycotic Studies. Int. J. Mol. Sci. 2019, 20, 4691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartucci, R.; van der Meer, A.Z.; Boersma, Y.L.; Olinga, P.; Salvati, A. Nanoparticle-induced inflammation and fibrosis in ex vivo murine precision-cut liver slices and effects of nanoparticle exposure conditions. Arch. Toxicol. 2021, 95, 1267–1285. [Google Scholar] [CrossRef]
- Zelikoff, J.; Willis, D.; Degheidy, H.; Zhang, Q.; Umbreit, T.; Goering, P. Immune cell profiles in response to silver nanoparticles associated with medical devices (P3357). J. Immunol. 2013, 190, 202.1. [Google Scholar]
- Luo, Y.-H.; Chang, L.W.; Lin, P. Metal-Based Nanoparticles and the Immune System: Activation, Inflammation, and Potential Applications. BioMed Res. Int. 2015, 2015, 143720. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Fahmi, A.; Mahltig, B. Impact of Silver Nanoparticles and Silver Ions on Innate Immune Cells. J. Biomed. Nanotechnol. 2014, 10, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Vuković, B.; Cvetić, Ž.; Bendelja, K.; Barbir, R.; Milić, M.; Dobrošević, B.; Šerić, V.; Vrček, I.V. In vitro study on the immunomodulatory effects of differently functionalized silver nanoparticles on human peripheral blood mononuclear cells. J. Biol. Inorg. Chem. 2021, 26, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Verdon, R.; Gillies, S.L.; Brown, D.M.; Henry, T.; Tran, L.; Tyler, C.R.; Rossi, A.G.; Stone, V.; Johnston, H.J. Neutrophil activation by nanomaterials in vitro: Comparing strengths and limitations of primary human cells with those of an immortalized (HL-60) cell line. Nanotoxicology 2020, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Chen, j.; Cai, S.; Xie, J.; Yang, F.; Liu, T. Blockade of Cycloxygenase-2 ameliorates sepsis induced immune-suppression by regulating myeloid-derived suppressor cells. Int. Immunopharmacol. 2022, 7, 108506. [Google Scholar] [CrossRef]
- Gouveia, V.M.; Rizzello, L.; Nunes, C.; Poma, A.; Ruiz-Perez, L.; Oliveira, A.; Reis, S.; Battaglia, G. Macrophage Targeting pH Responsive Polymersomes for Glucocorticoid Therapy. Pharmaceutics 2019, 11, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.S.; Mann, M.; Dubois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Multhoff, G.; Molls, M.; Radons, J. Chronic Inflammation in Cancer Development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Robertson, A.K.L. Inflammation and atherosclerosis. Annu. Rev. Pathol. 2006, 1, 297–329. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Hardie, J.; Zhang, X.; Rotello, V.M. Effects of engineered nanoparticles on the innate immune system. Semin. Immunol. 2017, 34, 25–32. [Google Scholar] [CrossRef]
- Cronin, J.G.; Jones, N.; Thornton, C.A.; Jenkins, G.J.S.; Doak, S.H.; Clift, M.J.D. Nanomaterials and Innate Immunity: A Perspective of the Current Status in Nanosafety. Chem. Res. Toxicol. 2020, 33, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2015, 9, 385–406. [Google Scholar]
- Alhamid, M.Z.; Hadi, B.S.; Khumaeni, A. Synthesis of silver nanoparticles using laser ablation method utilizing Nd:YAG laser. AIP Conf. Proc. 2019, 2202, 020013. [Google Scholar] [CrossRef]
- Shahid, M. Water soluble gold nanoparticles based high relaxivity MRI contrast agents. Mater. Res. Express 2019, 6, 1250h1. [Google Scholar] [CrossRef]
- Bhardwaj, B.; Singh, P.; Kumar, A.; Kumar, S.; Budhwar, V. Eco-Friendly Greener Synthesis of Nanoparticles. Adv. Pharm. Bull. 2020, 10, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Al-Rajhi, A.M.H.; Al Abboud, M.A.; AlAwlaqi, M.M.; Magdah, A.G.; Helmy, E.A.M.; Mabrouk, A.S. Recent Advances in Green Synthesis of Silver Nanoparticles and Their Applications: About Future Directions. A Review. BioNanoScience 2017, 8, 5–16. [Google Scholar] [CrossRef]
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Nath, D.; Banerjee, P. Green Nanotechnology—A new hope for medical biology. Environ. Toxicol. Pharmacol. 2013, 36, 997–1014. [Google Scholar] [CrossRef]
- Zheng, B.; Kong, T.; Jing, X.; Wubah, T.O.; Li, X.; Sun, D.; Lu, F.; Zheng, Y.; Huang, J.; Li, Q. Plant-mediated synthesis of platinum nanoparticles and its bioreductive mechanism. J. Colloid. Interface Sci. 2013, 396, 138–145. [Google Scholar] [CrossRef] [PubMed]
- GreMarslin, g.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikshit, P.; Kumar, J.; Das, A.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.; Kim, B. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Mohamed, A.; Shafey, E. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.-Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent Developments in the Facile Bio-Synthesis of Gold Nanoparticles (AuNPs) and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef]
- Tyavambiza, C.; Elbagory, A.; Madiehe, A.; Meyer, M.; Meyer, S. The Antimicrobial and Anti-Inflammatory Effects of Silver Nanoparticles Synthesised from Cotyledon orbiculata Aqueous Extract. Nanomaterials 2021, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Ivanova, K.; Hoyo, J.; Perelshtein, I.; Owen, G.; Haegert, A.; Lin, Y.-Y.; LeBihan, S.; Gedanken, A.; Häfeli, U.O.; et al. Novel Lignin-Capped Silver Nanoparticles against Multidrug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2021, 13, 22098–22109. [Google Scholar] [CrossRef]
- Singh, M.; Manikandan, S.; Yadav, M.; Kumar, S.; Sehrawat, N.; Meashi, V.; Diksha, D.; Sharma, P.; Sharma, A.K. Bio-functionalized Gold Nanoparticles: A Potent Probe for Profound Antibacterial Efficiency through Drug Delivery System. Asian J. Biol. Life Sci. 2020, 9, 139–144. [Google Scholar] [CrossRef]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef]
- Kashkouli, S.; Jamzad, M.; Nouri, A. Total Phenolic and Flavonoids Contents, Radical Scavenging Activity and Green Synthesis of Silver Nanoparticles by Laurus nobilis L. Leaves Aqueous Extract. J. Med. Plants -Prod. 2018, 7, 25–32. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Zhou, J.; Creyer, M.N.; Yim, W.; Chen, Z.; Messersmith, P.B.; Jokerst, J.V. Phenolic-enabled nanotechnology: Versatile particle engineering for biomedicine. Chem. Soc. Rev. 2021, 50, 4432–4483. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sun, Q.; Wu, F.; Dai, Y.; Chen, X. Polyphenol-Containing Nanoparticles: Synthesis, Properties, and Therapeutic Delivery. Adv. Mater. 2021, 33, 2007356. [Google Scholar] [CrossRef]
- De Matteis, V.V.; Rizzello, L.; Ingrosso, C.; Liatsi-Douvitsa, E.; De Giorgi, M.L.; De Matteis, G.; Rinaldi, R. Cultivar-Dependent Anticancer and Antibacterial Properties of Silver Nanoparticles Synthesized Using Leaves of Different Olea Europaea Trees. Nanomaterials 2019, 9, 1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- ImageJ-Image Processing and Analysis in Java. Available online: https://imagej.nih.gov/ij/ (accessed on 18 November 2019).
- Munro, C.H.; Smith, W.E.; Garner, M.; Clarkson, J.; White, P.C. Characterization of the Surface of a Citrate-Reduced Colloid Optimized for Use as a Substrate for Surface-Enhanced Resonance Raman Scattering. Langmuir 1995, 11, 3712–3720. [Google Scholar] [CrossRef]
- Bell, S.E.; Sirimuthu, N.M. Surface-enhanced Raman pectroscopy as a probe of competitive binding by anions to citrate-reduced silver colloids. J. Phys. Chem. A 2005, 109, 33. [Google Scholar] [CrossRef]
- Bokobza, J.L.; Bruneel, M.C. Raman spectroscopy as a tool for the analysis of carbon-basedmaterials (highly oriented pyrolitic graphite, multilayer grapheneand multiwall carbon nanotubes) and of some of theirelastomeric composites. Vib. Spectrosc. 2014, 74, 57–63. [Google Scholar] [CrossRef]
- Al-Zahrani, S.; Astudillo-Calderón, S.; Pintos, B.; Pérez-Urria, E.; Manzanera, J.A.; Martín, L.; Gomez-Garay, A. Role of Synthetic Plant Extracts on the Production of Silver-Derived Nanoparticles. Plants 2021, 10, 1671. [Google Scholar] [CrossRef]
- Mie, G. Contributions to the Optics of Turbid Media, Especially Colloidal Metal Solutions. Ann. Phys. 1908, 25, 377–445. [Google Scholar] [CrossRef]
- Hale, G.M.; Querry, M.R. Optical Constants of Water in the 200-nm to 200-μm Wavelength Region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef]
- Manno, D.; Filippo, E.; Di Giulio, M.; Serra, A. Synthesis and characterization of starch-stabilized Ag nanostructures for sensors applications. J. Non-Cryst. Solids 2008, 354, 5515–5520. [Google Scholar] [CrossRef]
- Wang, B.; Ding, C.; Ding, X.; Tesch, G.; Zheng, J.; Tian, P.; Li, Y.; Ricardo, S.; Shen, H.; Xue, W. WNT1-inducible signaling pathway protein 1 regulates kidney inflammation through the NF-κB pathway. Clin. Sci. 2022, 136, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Sig. Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Gilmore, T.D. The Rel/NF-kB signal transduction pathway: Introduction. Oncogene 1999, 18, 6842–6844. [Google Scholar] [CrossRef] [Green Version]
- Kustermans, G.; Benna, J.E.; Piette, J.; Legrand-Poels, S. Perturbation of actin dynamics induces NF-kappaB activation in myelomonocytic cells through an NADPH oxidase-dependent pathway. Biochem. J. 2005, 87, 5. [Google Scholar]
- Banan, A.; Keshavarzian, A.; Zhang, L.; Shaikh, M.; Forsyth, C.B.; Tang, Y.; Fields, J.Z. NF-κB activation as a key mechanism in ethanol-induced disruption of the F-actin cytoskeleton and monolayer barrier. Alcohol 2007, 41, 447–460. [Google Scholar] [CrossRef]
- Wang, W.; Mani, A.M.; Wu, Z.-H. DNA damage-induced nuclear factor-kappa B activation and its roles in cancer progression. J. Cancer Metastasis Treat. 2017, 3, 45–59. [Google Scholar] [CrossRef]
- Janssens, S.; Tschopp, J. Signals from within: The DNA-damage-induced NF-κB response. Cell Death Differ. 2006, 13, 773–784. [Google Scholar] [CrossRef] [Green Version]
- Martins, G.R.; Gelaleti, G.B.; Moschetta, M.G.; Maschio-Signorini, L.B.; Zuccari, D.A.; de Campos, P. Proinflammatory and Anti-Inflammatory Cytokines Mediated by NF-κB Factor as Prognostic Markers in Mammary Tumors. Mediators Inflamm. 2016, 2016, 9512743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 27–148. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Kishimoto, T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol. Rev. 1992, 127, 25–50. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016. [Google Scholar] [CrossRef]
- Kuhns, D.B.; Young, H.A.; Gallin, E.K.; I Gallin, J. Ca2+-dependent production and release of IL-8 in human neutrophils. J. Immunol. 1998, 161, 4332–4339. [Google Scholar]
- Bernharda, S.; Huga, S.; Stratmanna, A.E.P.; Erbera, M.; Vidonia, L.; Knappa, C.L.; Thomaßa, B.D.; Nilssonc, M.F.B.; Kristina Nilsson Ekdahlc, K.; Föhr, K.; et al. Interleukin 8 Elicits Rapid Physiological Changes in Neutrophils That Are Altered by Inflammatory Conditions. J. Innate. Immun. 2021, 13, 225–241. [Google Scholar] [CrossRef]
- Brat, D.J.; Bellail, A.C.; Van Meir, E.G. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-Oncol. 2005, 7, 122–133. [Google Scholar] [CrossRef]
- Murphy, A.; Casey, A.; Byrne, G.; Chambers, G.; Howe, O. Silver nanoparticles induce pro-inflammatory gene expression and inflammasome activation in human monocytes. J. Appl. Toxicol. 2016, 36, 1311–1320. [Google Scholar] [CrossRef]
- Pool, E.J.; Lategan, K.L.; Walters, C.R. The effects of silver nanoparticles on RAW 264 7 Macrophages and human whole blood cell cultures. Front. Biosci. 2019, 24, 347–365. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Rizzello, L.; Manno, D.; Di Guglielmo, C.; Rinaldi, R. Synergistic Effect Induced by Gold Nanoparticles with Polyphenols Shell during Thermal Therapy: Macrophage Inflammatory Response and Cancer Cell Death Assessment. Cancers 2021, 13, 3610. [Google Scholar] [CrossRef] [PubMed]
- Chanda, D.; Dudefoi, W.; Anadu, J.; Minghetti, M. Evaluation of the effect of silver and silver nanoparticles on the function of selenoproteins using an in-vitro model of the fish intestine: The cell line RTgutGC. Ecotoxicol. Environ. Saf. 2021, 211, 111930. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.; Kushwaha, P.; Bhutia, Y.C.; Flora, S. Oxidative stress following exposure to silver and gold nanoparticles in mice. Toxicol. Ind. Health 2014, 32, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Broggi, F.; Ponti, J.; Giudetti, G.; Franchini, F.; Stone, V.; Garcia, C.P.; Rossi, F. Silver nanoparticles induce cytotoxicity, but not cell transformation or genotoxicity on Balb3T3 mouse fibroblasts. BioNanoMaterials 2013, 14, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.-J.; Huang, C.-C.; Pranata, R.; Lee, Y.-H.; Chen, Y.-Y.; Wu, Y.-H.; Wang, Y.-J. Modulation of Innate Immune Toxicity by Silver Nanoparticle Exposure and the Preventive Effects of Pterostilbene. Int. J. Mol. Sci. 2021, 22, 2536. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, G.; Sundaramoorthy, A.; Shanmugam, N. Biosynthesis of Silver Nanoparticles from Leaf Extract of Salvia coccinea and Its Effects of Anti-inflammatory Potential in Human Monocytic THP-1 Cells. ACS Appl. Bio Mater. 2021, 4, 8433–8442. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2021, 188, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonavita, E.; Bromley, C.P.; Jonsson, G.; Pelly, V.S.; Sahoo, S.; Walwyn-Brown, K.; Mensurado, S.; Moeini, A.; Flanagan, E.; Bell, C.R.; et al. Antagonistic Inflammatory Phenotypes Dictate Tumor Fate and Response to Immune Checkpoint Blockade. Immunity 2020, 53, 1215–1229.e8. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar] [CrossRef]
- Shao, Y.; Cheng, Z.; Li, X.; Chernaya, V.; Wang, H.; Yang, X.-F. Immunosuppressive/anti-inflammatory cytokines directly and indirectly inhibit endothelial dysfunction- a novel mechanism for maintaining vascular function. J. Hematol. Oncol. 2014, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Pathan, J.; Kazzam, E.R.; Ali, B.H. Oxidative stress, inflammation, and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles. Int. J. Nanomed. 2016, 11, 919–928. [Google Scholar] [CrossRef] [Green Version]
- Strieter, R.M.; Kunkel, S.L.; Bone, R.C. Role of tumor necrosis factor-α in disease states and inflammation. Crit. Care Med. 1993, 21, S447–S463. [Google Scholar] [CrossRef]
- Skomorokhova, E.A.; Sankova, T.P.; Orlov, I.A.; Savelev, A.N.; Magazenkova, D.N.; Pliss, M.G.; Skvortsov, A.N.; Sosnin, I.M.; Kirilenko, D.A.; Grishchuk, I.V.; et al. Size-Dependent Bioactivity of Silver Nanoparticles: Antibacterial Properties, Influence on Copper Status in Mice, and Whole-Body Turnover. Nanotechnol. Sci. Appl. 2020, 13, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Hakeem, K.R.; Rehman, R.U. Synergistic effect of plant extract coupled silver nanoparticles in various therapeutic applications- present insights and bottlenecks. Chemosphere 2022, 288, 132527. [Google Scholar] [CrossRef] [PubMed]




| Samples in Water | DLS (nm) | Zeta Potential (mV) |
| Conventional Ag NPs | 20 ± 3 | −30 ± 3 |
| Green Ag NPs | 32 ± 6 | −35 ± 4 |
| Samples in DMEM | DLS (nm) | Zeta Potential (mV) |
| Conventional Ag NPs | 29 ± 2 | −38 ± 2 |
| Green Ag NPs | 36 ± 4 | −41 ± 3 |
| Samples in RPMI-1640 | DLS (nm) | Zeta Potential (mV) |
| Conventional Ag NPs | 31 ± 5 | −40 ± 5 |
| Green Ag NPs | 38 ± 3 | −43 ± 2 |
| Conventional Ag NPs | MCF-7 (24 h) | MCF-7 (48 h) | MCF-10A (24 h) | MCF-10A (48 h) |
| IC50 | 2 µM | 2.9 µM | 2.05 µM | 2.4 µM |
| Green Ag NPs | MCF-7 (24 h) | MCF-7 (48 h) | MCF-10A (24 h) | MCF-10A (48 h) |
| IC50 | 1.6 µM | 2 µM | 1.3 µM | 1.4 µM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascione, M.; Rizzello, L.; Manno, D.; Serra, A.; De Matteis, V. Green Silver Nanoparticles Promote Inflammation Shutdown in Human Leukemic Monocytes. Materials 2022, 15, 775. https://doi.org/10.3390/ma15030775
Cascione M, Rizzello L, Manno D, Serra A, De Matteis V. Green Silver Nanoparticles Promote Inflammation Shutdown in Human Leukemic Monocytes. Materials. 2022; 15(3):775. https://doi.org/10.3390/ma15030775
Chicago/Turabian StyleCascione, Mariafrancesca, Loris Rizzello, Daniela Manno, Antonio Serra, and Valeria De Matteis. 2022. "Green Silver Nanoparticles Promote Inflammation Shutdown in Human Leukemic Monocytes" Materials 15, no. 3: 775. https://doi.org/10.3390/ma15030775
APA StyleCascione, M., Rizzello, L., Manno, D., Serra, A., & De Matteis, V. (2022). Green Silver Nanoparticles Promote Inflammation Shutdown in Human Leukemic Monocytes. Materials, 15(3), 775. https://doi.org/10.3390/ma15030775








