Indigo—A New Tribological Substance Class for Non-Toxic and Ecological Gliding Surfaces on Ice, Snow, and Water
Abstract
1. Introduction
2. Synthesis and Specific Properties of Indigo
2.1. Synthesis of Indigo
2.2. Individual and Collective Properties of Indigo
3. Indigo as a Gliding Surface
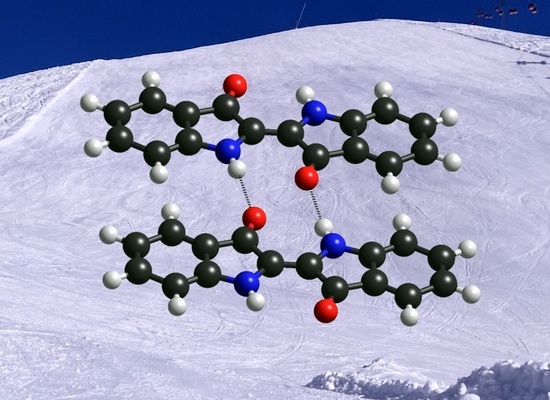
3.1. Self-Assembly of Indigo Molecules
3.2. Gliding Properties of Indigo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Splitstoser, J.C.; Dillehay, T.D.; Wouters, J.; Claro, A. Early Pre-Hispanic Use of Indigo Blue in Peru. Sci. Adv. 2016, 2, e1501623. [Google Scholar] [CrossRef] [PubMed]
- Doménech, A.; Doménech-Carbó, M.T.; del Río, M.S.; Vázquez de Agredos Pascual, M.L.; Lima, E. Maya Blue as a Nanostructured Polyfunctional Hybrid Organic–Inorganic Material: The Need to Change Paradigms. New J. Chem. 2009, 33, 2371–2379. [Google Scholar] [CrossRef]
- Woodtli, P.; Giger, S.; Müller, P.; Sägesser, L.; Zucchetto, N.; Reber, M.J.; Ecker, A.; Brühwiler, D. Indigo in the Nanochannels of Zeolite L: Towards a New Type of Colorant. Dye. Pigment. 2018, 149, 456–461. [Google Scholar] [CrossRef]
- Hawley, G.G. The Condensed Chemical Dictionary, 9th ed.; Van Nostrand Reinhold Co.: New York, NY, USA, 1977; ISBN 978-0-442-23240-5. [Google Scholar]
- Villagomez, C.J.; Guillermet, O.; Goudeau, S.; Ample, F.; Xu, H.; Coudret, C.; Bouju, X.; Zambelli, T.; Gauthier, S. Self-Assembly of Enantiopure Domains: The Case of Indigo on Cu(111). J. Chem. Phys. 2010, 132, 074705. [Google Scholar] [CrossRef] [PubMed]
- Seligman, T.K.; Loughran, K.; Bernus, E. Art of Being Tuareg: Sahara Nomads in a Modern World; UCLA Fowler Museum of Cultural History: Los Angeles, CA, USA, 2006; ISBN 0-9748729-6-2. [Google Scholar]
- Zhang, L.; Wang, L.; Cunningham, A.B.; Shi, Y.; Wang, Y. Island Blues: Indigenous Knowledge of Indigo-Yielding Plant Species Used by Hainan Miao and Li Dyers on Hainan Island, China. J. Ethnobiol. Ethnomed. 2019, 15, 31. [Google Scholar] [CrossRef]
- Anokhin, D.V.; Leshanskaya, L.I.; Piryazev, A.A.; Susarova, D.K.; Dremova, N.N.; Shcheglov, E.V.; Ivanov, D.A.; Razumov, V.F.; Troshin, P.A. Towards Understanding the Behavior of Indigo Thin Films in Organic Field-Effect Transistors: A Template Effect of the Aliphatic Hydrocarbon Dielectric on the Crystal Structure and Electrical Performance of the Semiconductor. Chem. Commun. 2014, 50, 7639–7641. [Google Scholar] [CrossRef][Green Version]
- Klimovich, I.V.; Leshanskaya, L.I.; Troyanov, S.I.; Anokhin, D.V.; Novikov, D.V.; Piryazev, A.A.; Ivanov, D.A.; Dremova, N.N.; Troshin, P.A. Design of Indigo Derivatives as Environment-Friendly Organic Semiconductors for Sustainable Organic Electronics. J. Mater. Chem. C 2014, 2, 7621–7631. [Google Scholar] [CrossRef]
- OECD SIDS Indigo Blue, 3H-Indol-3-one, 2-(1,3-Dihydro-3-oxo-2H-indol-2-ylidene)-1,2-dihydro, CAS N°: 482-89-3, SIDS Initial Assessment Report for SIAM 2. 1994. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.006.898 (accessed on 14 December 2021).
- Miranda, M.D. Forensic Analysis of Tattoos and Tattoo Inks; CRC Press: Boca Raton, FL, USA, 2015; ISBN 1-4822-2146-2. [Google Scholar]
- Herrero, M.; Rovira, J.; Nadal, M.; Domingo, J.L. Risk Assessment Due to Dermal Exposure of Trace Elements and Indigo Dye in Jeans: Migration to Artificial Sweat. Environ. Res. 2019, 172, 310–318. [Google Scholar] [CrossRef]
- John, P. Extraction. In Handbook of Natural Colorants; Bechtold, T., Mussak, R., Eds.; Wiley: Hoboken, NJ, USA, 2009; ISBN 0-470-51199-0. [Google Scholar]
- Chia, M.A.; Musa, R.I. Effect of Indigo Dye Effluent on the Growth, Biomass Production and Phenotypic Plasticity of Scenedesmus Quadricauda (Chlorococcales). An. Acad. Bras. Cienc. 2014, 86, 419–428. [Google Scholar] [CrossRef]
- Bützer, M.; Bützer, P. Gleitmittel für Wintersportgeräte auf Basis Indigoider Moleküle. Patent WO2019145282A1, 1 August 2019. Available online: https://patents.google.com/patent/WO2019145282A1/de (accessed on 10 January 2022).
- Scheffer, J. The History of Lapland. Available online: https://www.gutenberg.org/ebooks/59695 (accessed on 6 January 2022).
- Gambaretto, G.P. Solid Lubricant and Process for Preparing It. Patent EP0132879A2, 3 February 1985. Available online: https://patents.google.com/patent/EP0132879A2/en (accessed on 10 January 2022).
- ECHA: Scientific Committees Support Further Restrictions of PFAS. Available online: https://echa.europa.eu/-/scientific-committees-support-further-restrictions-of-pfas (accessed on 6 January 2022).
- Brennan, N.M.; Evans, A.T.; Fritz, M.K.; Peak, S.A.; von Holst, H.E. Trends in the Regulation of Per- and Polyfluoroalkyl Substances (PFAS): A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 10900. [Google Scholar] [CrossRef]
- Update on FIS Fluorinated Ski Wax Ban. Available online: https://www.fis-ski.com/en/international-ski-federation/news-multimedia/news/update-on-fis-fluorinated-ski-wax-ban (accessed on 6 January 2022).
- Saling, P.; Kicherer, A.; Dittrich-Krämer, B.; Wittlinger, R.; Zombik, W.; Schmidt, I.; Schrott, W.; Schmidt, S. Eco-Efficiency Analysis by BASF: The Method. Int. J. Life Cycle Assess. 2002, 7, 203–218. [Google Scholar] [CrossRef]
- Plitzko, I.; Mohn, T.; Sedlacek, N.; Hamburger, M. Composition of Indigo Naturalis. Planta Med. 2009, 75, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Fabara, A.; Fraaije, M. An Overview of Microbial Indigo-Forming Enzymes. Appl. Microbiol. Biotechnol. 2020, 104, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Aerts, O.; Duchateau, N.; Lambert, J.; Bechtold, T. Sodium Metabisulfite in Blue Jeans: An Unexpected Cause of Textile Contact Dermatitis. Contact Dermat. 2014, 70, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Bektaş, İ.; Karaman, Ş.; Dıraz, E.; Çelik, M. The Role of Natural Indigo Dye in Alleviation of Genotoxicity of Sodium Dithionite as a Reducing Agent. Cytotechnology 2016, 68, 2245–2255. [Google Scholar] [CrossRef]
- Cordin, M.; Bechtold, T.; Pham, T. Quantification of Aniline and N-Methylaniline in Indigo. Sci. Rep. 2021, 11, 21135. [Google Scholar] [CrossRef]
- Fritsche, J. Ueber Das Anilin, Ein Neues Zersetzungsproduct Des Indigo. J. Prakt. Chem. 1840, 20, 453–459. [Google Scholar] [CrossRef]
- Rannug, U.; Bramstedt, H.; Nilsson, U. The Presence of Genotoxic and Bioactive Components in Indigo Dyed Fabrics—A Possible Health Risk? Mutat. Res. 1992, 282, 219–225. [Google Scholar] [CrossRef]
- PubChem CID 10215. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/10215 (accessed on 20 October 2021).
- ChemIDplus. Available online: https://chem.nlm.nih.gov/chemidplus/rn/482-89-3 (accessed on 23 November 2021).
- Vetter, J. Toxins of Amanita Phalloides. Toxicon 1998, 36, 13–24. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Aconite Poisoning. Clin. Toxicol. 2009, 47, 279–285. [Google Scholar] [CrossRef]
- ECHA. 2-(1,3-Dihydro-3-oxo-2H-indol-2-ylidene)-1,2-dihydro-3H-indol-3-one. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/14413/4/3 (accessed on 23 November 2021).
- Ferber, K.H. Toxicology of Indigo. A Review. J. Environ. Pathol. Toxicol. Oncol. 1987, 7, 73–83. [Google Scholar] [PubMed]
- Williams, A.J.; Grulke, C.M.; Edwards, J.; McEachran, A.D.; Mansouri, K.; Baker, N.C.; Patlewicz, G.; Shah, I.; Wambaugh, J.F.; Judson, R.S.; et al. The CompTox Chemistry Dashboard: A Community Data Resource for Environmental Chemistry. J. Cheminform. 2017, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Bützer, P. QSAR Data of Indigo, Indirubin, and Isoindigotin, Including Tautomers [Data Set]. Zenodo 2021. [Google Scholar] [CrossRef]
- Vazirisereshk, M.R.; Martini, A.; Strubbe, D.A.; Baykara, M.Z. Solid Lubrication with MoS2: A Review. Lubricants 2019, 7, 57. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, D.-H.; Park, B.H.; Choi, J.S. Nanotribology of 2D Materials and Their Macroscopic Applications. J. Phys. Appl. Phys. 2020, 53, 393001. [Google Scholar] [CrossRef]
- Luan, B.; Ruhong, Z. Wettability and Friction of Water on a MoS2 Nanosheet. Appl. Phys. Lett. 2016, 108, 131601. [Google Scholar] [CrossRef]
- Süsse, P.; Steins, M.; Kupcik, V. Indigo: Crystal structure refinement based on synchrotron data. Z. Krist. Cryst. Mater. 1988, 184, 269–274. [Google Scholar] [CrossRef]
- Klessinger, M.; Lüttke, W. Theoretische und spektroskopische Untersuchungen an Indigofarbstoffen, III. Der Einfluß zwischenmolekularer Wasserstoffbrücken auf die Spektren von Indigo im festen Zustand. Chem. Ber. 1966, 99, 2136–2145. [Google Scholar] [CrossRef]
- Raffay, L.J. Abhandlung über Indigo: Inaugural-Dissertation; Typis Leopoldi Grund; Österreichische Nationalbibliothek: Vienna, Austria, 1835. [Google Scholar]
- Głowacki, E.D.; Voss, G.; Leonat, L.; Irimia-Vladu, M.; Bauer, S.; Sariciftci, N.S. Indigo and Tyrian Purple—From Ancient Natural Dyes to Modern Organic Semiconductors. Isr. J. Chem. 2012, 52, 540–551. [Google Scholar] [CrossRef]
- Beilby, G.T.; Neville, F.H. Surface Flow in Crystalline Solids under Mechanical Disturbance. Proc. R. Soc. Lond. 1904, 72, 218–225. [Google Scholar] [CrossRef]
- Beilby, G.T.; Neville, F.H. The Effect of Heat and of Solvents on Thin Films of Metal. Proc. R. Soc. Lond. 1904, 72, 226–235. [Google Scholar] [CrossRef]
- Cadalbert, M. Analyse der Zusammensetzung und der Morphologie von Indigo-Partikeln im Hochleistungsgleitmittel Isantin; Zürich University of Applied Sciences: Wädenswil, Switzerland, 2021. [Google Scholar]
- PubChem CID 5354391. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5354391 (accessed on 20 October 2021).
- Vautier, M.; Guillard, C.; Herrmann, J.-M. Photocatalytic Degradation of Dyes in Water: Case Study of Indigo and of Indigo Carmine. J. Catal. 2001, 201, 46–59. [Google Scholar] [CrossRef]
- GESTIS-Stoffdatenbank. Available online: https://gestis.dguv.de/data?name=491987 (accessed on 20 October 2021).
- Japan National Institute of Technology and Evaluation (NITE). Chemical Risk Information Platform (CHRIP), CAS-Nr. 482-89-3. Available online: http://www.safe.nite.go.jp/japan/db.html (accessed on 23 November 2021).
- Toxicity Estimation Software Tool (T.E.S.T.) v5.1.1; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2021.
- Exposure Assessment Tools and Models. Estimation Program Interface (EPI) v4.1.1; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2019.
- National Toxicology Program (NTP), OPERA: Open Structure Activity Relationship App; U.S. Department of Health and Human Services: Washington, DC, USA, 2021.
- Virtual Models for Property Evaluation of Chemicals within a Global Architecture (VEGA); Istituto di Ricerche Farmacologiche Mario Negri, Istituto di Ricovero e Cura a Carattere Ccientifico (IRCCS): Milan, Italy, 2020.
- Gospodinova, N.; Tomsik, E. Hydrogen-Bonding versus π-π Stacking in the Design of Organic Semiconductors: From Dyes to Oligomers. Prog. Polym. Sci. 2014, 43, 33–47. [Google Scholar] [CrossRef]
- Zhao, X.-J.; Hou, H.; Fan, X.-T.; Wang, Y.; Liu, Y.-M.; Tang, C.; Liu, S.-H.; Ding, P.-P.; Cheng, J.; Lin, D.-H.; et al. Molecular Bilayer Graphene. Nat. Commun. 2019, 10, 3057. [Google Scholar] [CrossRef]
- Głowacki, E.D.; Voss, G.; Demirak, K.; Havlicek, M.; Sünger, N.; Okur, A.C.; Monkowius, U.; Gąsiorowski, J.; Leonat, L.; Sariciftci, N.S. A Facile Protection–Deprotection Route for Obtaining Indigo Pigments as Thin Films and Their Applications in Organic Bulk Heterojunctions. Chem. Commun. 2013, 49, 6063–6065. [Google Scholar] [CrossRef]
- Mayrhofer, L.; Moras, G.; Mulakaluri, N.; Rajagopalan, S.; Stevens, P.A.; Moseler, M. Fluorine-Terminated Diamond Surfaces as Dense Dipole Lattices: The Electrostatic Origin of Polar Hydrophobicity. J. Am. Chem. Soc. 2016, 138, 4018–4028. [Google Scholar] [CrossRef]
- Dellamatrice, P.M.; Silva-Stenico, M.E.; de Moraes, L.A.B.; Fiore, M.F.; Monteiro, R.T.R. Degradation of Textile Dyes by Cyanobacteria. Braz. J. Microbiol. 2017, 48, 25–31. [Google Scholar] [CrossRef]
- Faraday, M.I. Note on Regelation. Proc. R. Soc. Lond. 1860, 10, 440–450. [Google Scholar] [CrossRef]
- McConnel, J.C.; Glazebrook, R.T. VI. On the Plasticity of an Ice Crystal. (Preliminary Note). Proc. R. Soc. Lond. 1891, 48, 259–260. [Google Scholar] [CrossRef]
- Bowden, F.P.; Hughes, T.P.; Desch, C.H. The Mechanism of Sliding on Ice and Snow. Proc. R. Soc. Lond. Ser. Math. Phys. Sci. 1939, 172, 280–298. [Google Scholar] [CrossRef]
- Michaelides, A.; Slater, B. Melting the Ice One Layer at a Time. Proc. Natl. Acad. Sci. USA 2017, 114, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Canale, L.; Comtet, J.; Niguès, A.; Cohen, C.; Clanet, C.; Siria, A.; Bocquet, L. Nanorheology of Interfacial Water during Ice Gliding. Phys. Rev. X 2019, 9, 041025. [Google Scholar] [CrossRef]
- Chen, L.; Qian, L. Role of Interfacial Water in Adhesion, Friction, and Wear—A Critical Review. Friction 2021, 9, 1–28. [Google Scholar] [CrossRef]
- Swarén, M.; Karlöf, L.; Holmberg, H.-C.; Eriksson, A. Validation of Test Setup to Evaluate Glide Performance in Skis. Sports Technol. 2014, 7, 89–97. [Google Scholar] [CrossRef]
- Breitschädel, F. A New Approach for the Grinding of Nordic Skis. Procedia Eng. 2015, 112, 385–390. [Google Scholar] [CrossRef][Green Version]
- Al-Godari, N. Charakterisierung Indigoider Gleitschichten auf UHMWPE; Zürich University of Applied Sciences: Wädenswil, Switzerland, 2020. [Google Scholar]
- Rhyner, H.; Zeilinger, F.; Duelli, A. Isantin B3 als Gleitmittel für Ski-Beläge; Technical Report; WSL Institute for Snow and Avalanche Research SLF: Davos, Switzerland, 2019. [Google Scholar]
- Bruhin, B.; Neff, A. Internal Test Report on Isantin; Swiss-Ski: Muri bei Bern, Switzerland, 2021. [Google Scholar]






 |  |  | |
|---|---|---|---|
| water solubility (mg/L, 25 °C) | exp.: 0.05–2 mg/L pred.: 7.19 a, 13.44 b, 1.0 c | pred.: 59.92 a, 6.36 b, 1.8 c | pred.: 35.68 a, 0.411 b, 8.2 c |
| log(Kow) | exp.: 2.7–3.72 pred.: 3.11 a,1.99 ± 0.63 d | pred.: 4.10 a, 2.31 ± 1.50 d | pred.: 5.49 a, 2.53 ± 2.58 d |
| log(BCF) | exp.: 2.5–4.5 pred.: 5.6 a, 1.5 b, 0.8 ± 0.2 c | pred.: 4.1 a, 1.8 b, 1.6 ± 1.0 c | pred.: 3.8 a, 3.3 b, 2.7 ± 1.7 c |
| pKa | pred.: 9.00 c | pred.: 8.16 c | pred.: 5.17 c |
| Snow Temperature | ||||
|---|---|---|---|---|
| Cold <−8 °C | Medium −8 °C to −1 °C | Warm >−1 °C | ||
| reference ski (HF) | ski with HF | 0.008 (4) | −0.003 (2) | −0.033 (5) |
| ski with indigo | 0.022 (4) | −0.005 (2) | 0.024 (5) | |
| reference ski (NF) | ski with HF | (0) | −0.008 (1) | −0.089 (3) |
| ski with indigo | (0) | 0.024 (1) | 0.057 (3) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bützer, P.; Brühwiler, D.; Bützer, M.R.; Al-Godari, N.; Cadalbert, M.; Giger, M.; Schär, S. Indigo—A New Tribological Substance Class for Non-Toxic and Ecological Gliding Surfaces on Ice, Snow, and Water. Materials 2022, 15, 883. https://doi.org/10.3390/ma15030883
Bützer P, Brühwiler D, Bützer MR, Al-Godari N, Cadalbert M, Giger M, Schär S. Indigo—A New Tribological Substance Class for Non-Toxic and Ecological Gliding Surfaces on Ice, Snow, and Water. Materials. 2022; 15(3):883. https://doi.org/10.3390/ma15030883
Chicago/Turabian StyleBützer, Peter, Dominik Brühwiler, Marcel Roland Bützer, Nassim Al-Godari, Michelle Cadalbert, Mathias Giger, and Sandro Schär. 2022. "Indigo—A New Tribological Substance Class for Non-Toxic and Ecological Gliding Surfaces on Ice, Snow, and Water" Materials 15, no. 3: 883. https://doi.org/10.3390/ma15030883
APA StyleBützer, P., Brühwiler, D., Bützer, M. R., Al-Godari, N., Cadalbert, M., Giger, M., & Schär, S. (2022). Indigo—A New Tribological Substance Class for Non-Toxic and Ecological Gliding Surfaces on Ice, Snow, and Water. Materials, 15(3), 883. https://doi.org/10.3390/ma15030883