Vacuum Brazing of Metallized YSZ and Crofer Alloy Using 72Ag-28Cu Filler Foil
Abstract
:1. Introduction
2. Materials and Experimental Procedures
3. Results and Discussion
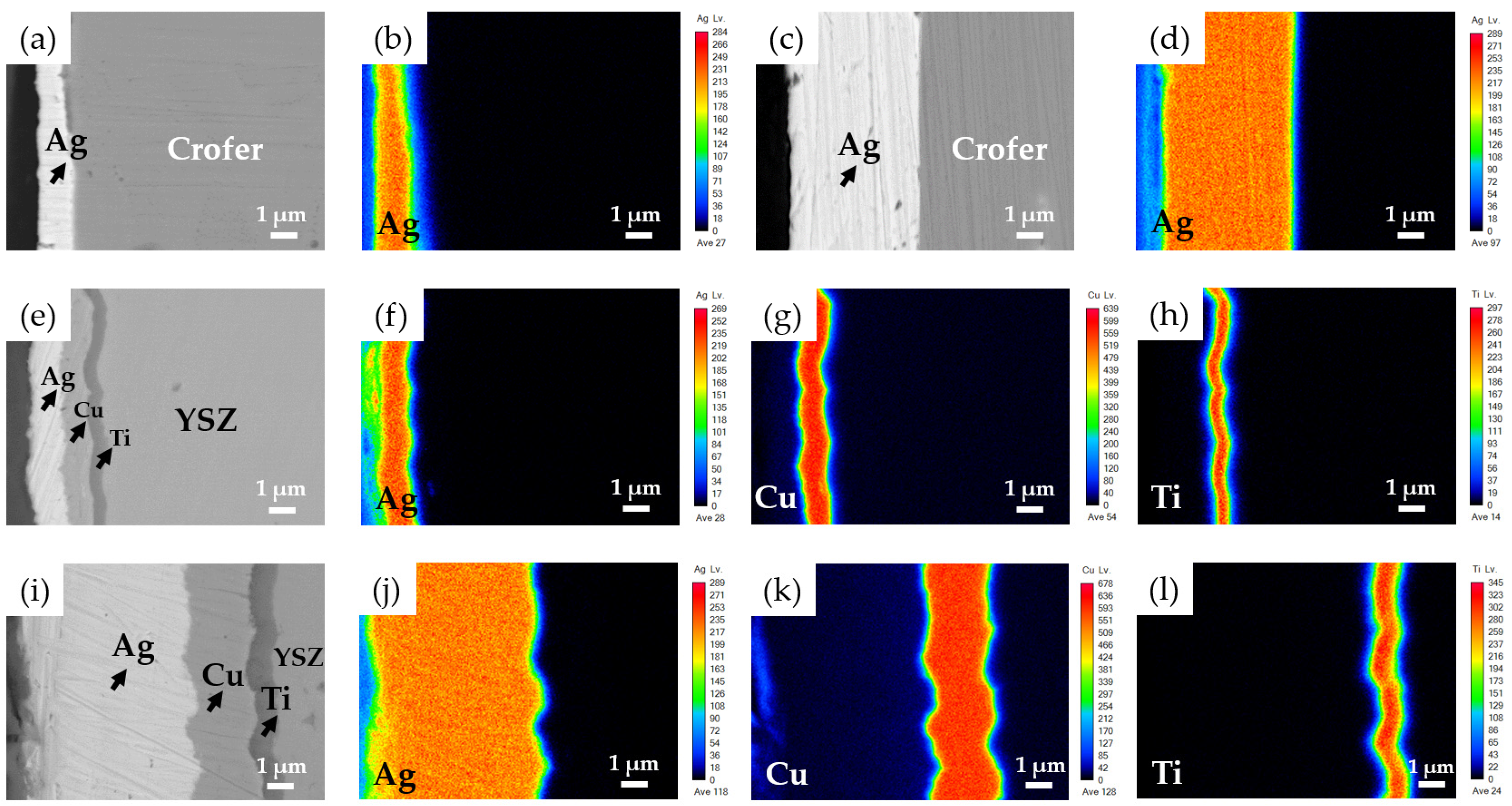
3.1. Wetting of the BAg-8 Filler on Metallized Crofer and YSZ Substrates
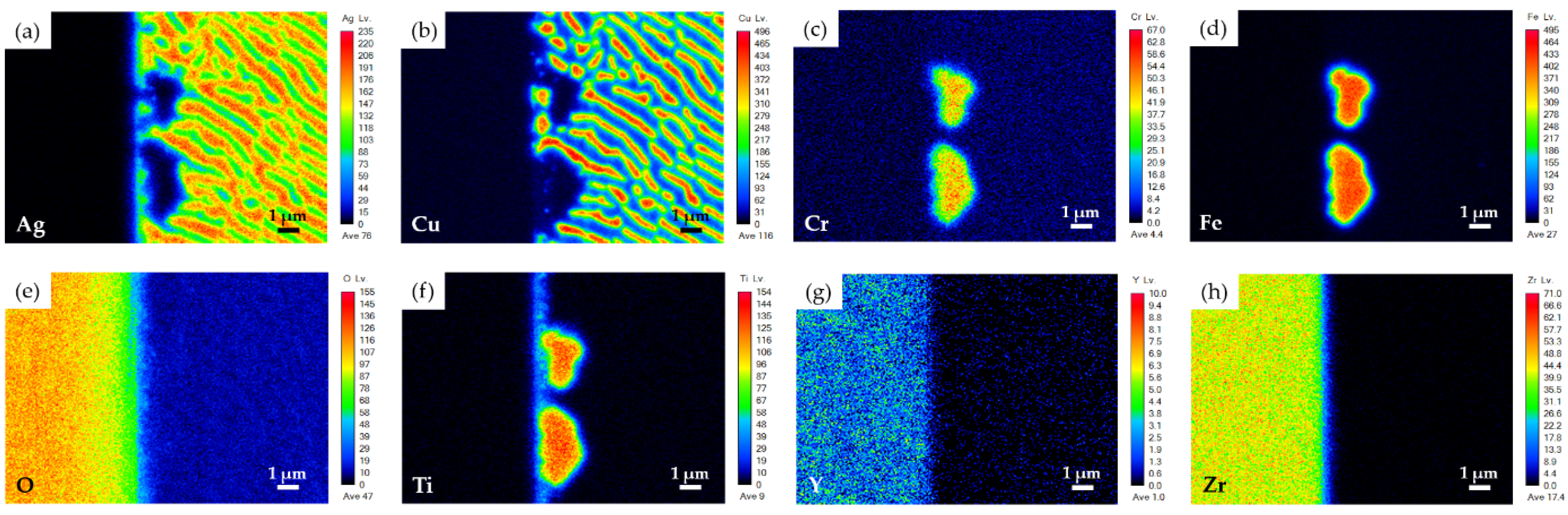
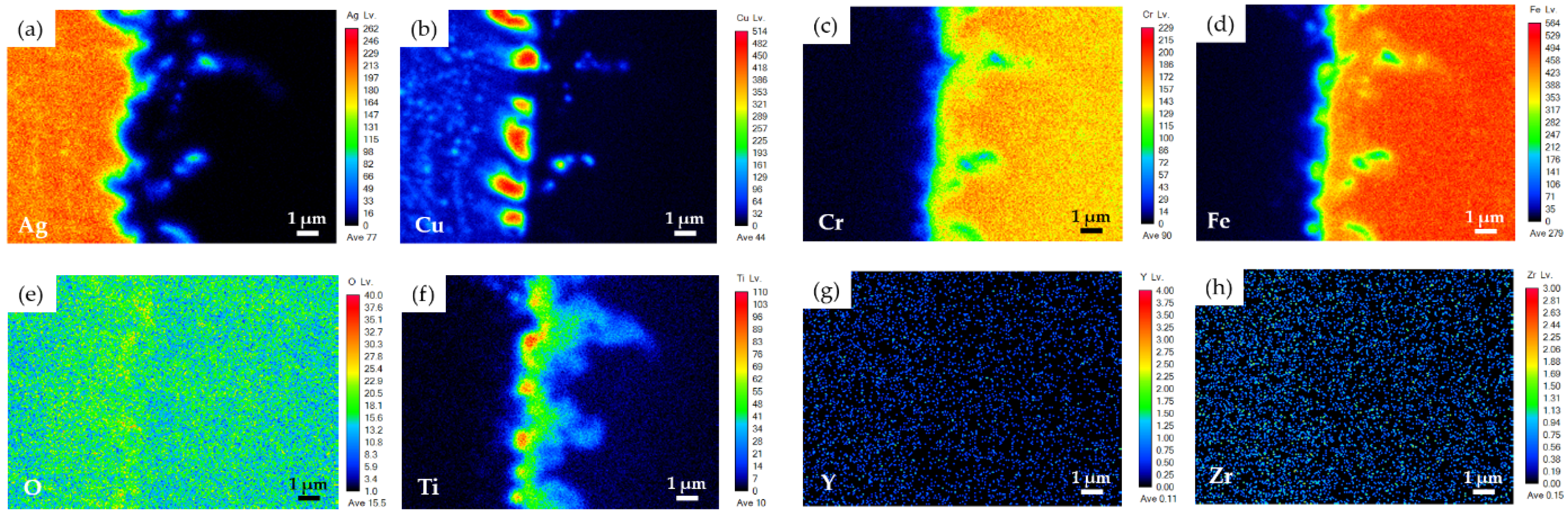
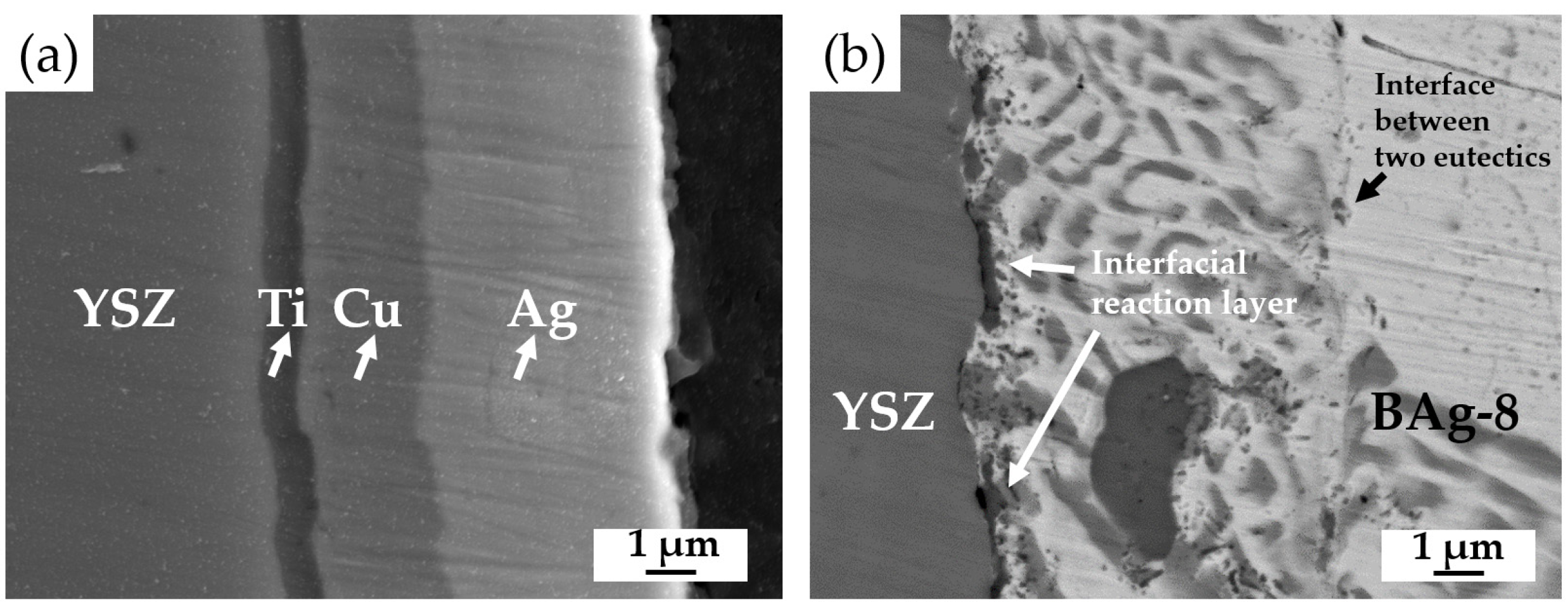
3.2. YSZ/Ti(0.5μ)/Cu(1μ)/Ag(1.5μ)/Bag-8(50μ)/Ag(1.5μ)/Crofer Joint
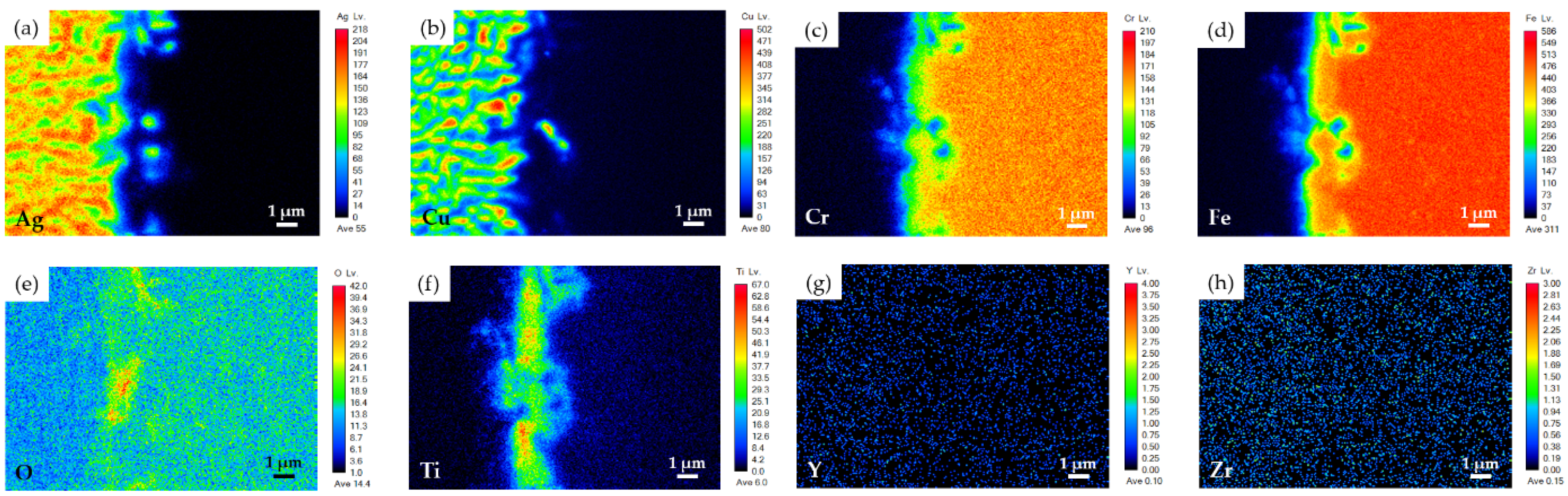
3.3. YSZ/Ti(1μ)/Cu(3μ)/Ag(5μ)/BAg-8(50μ)/Ag(5μ)/Crofer Joint
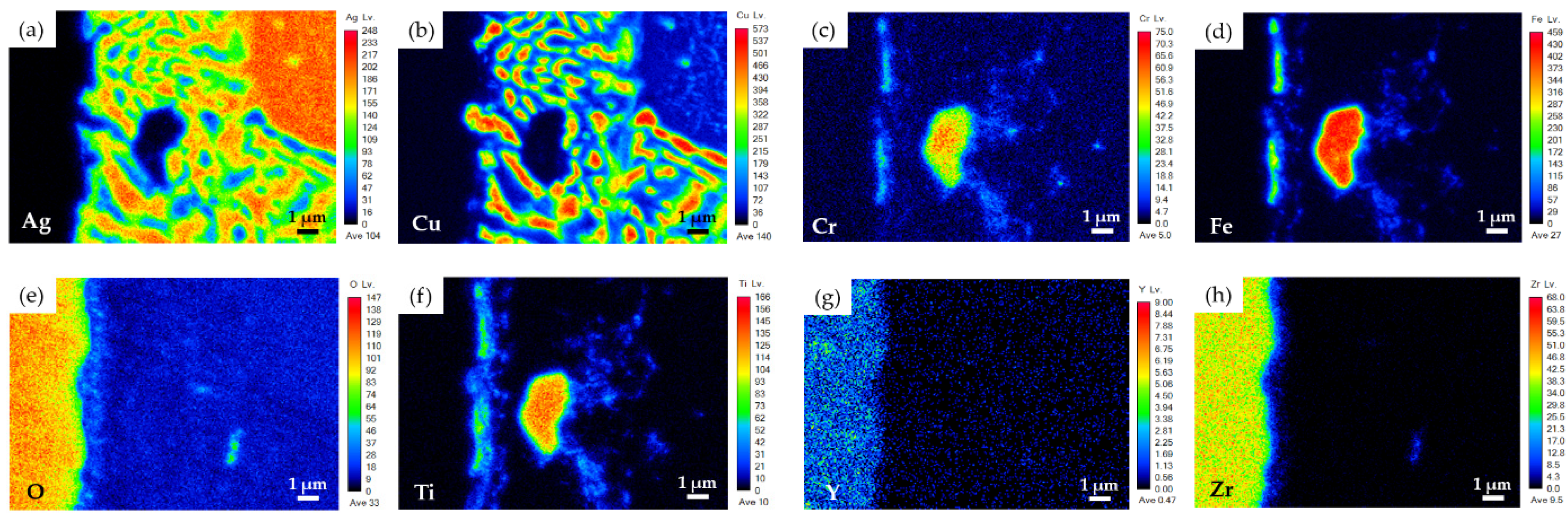
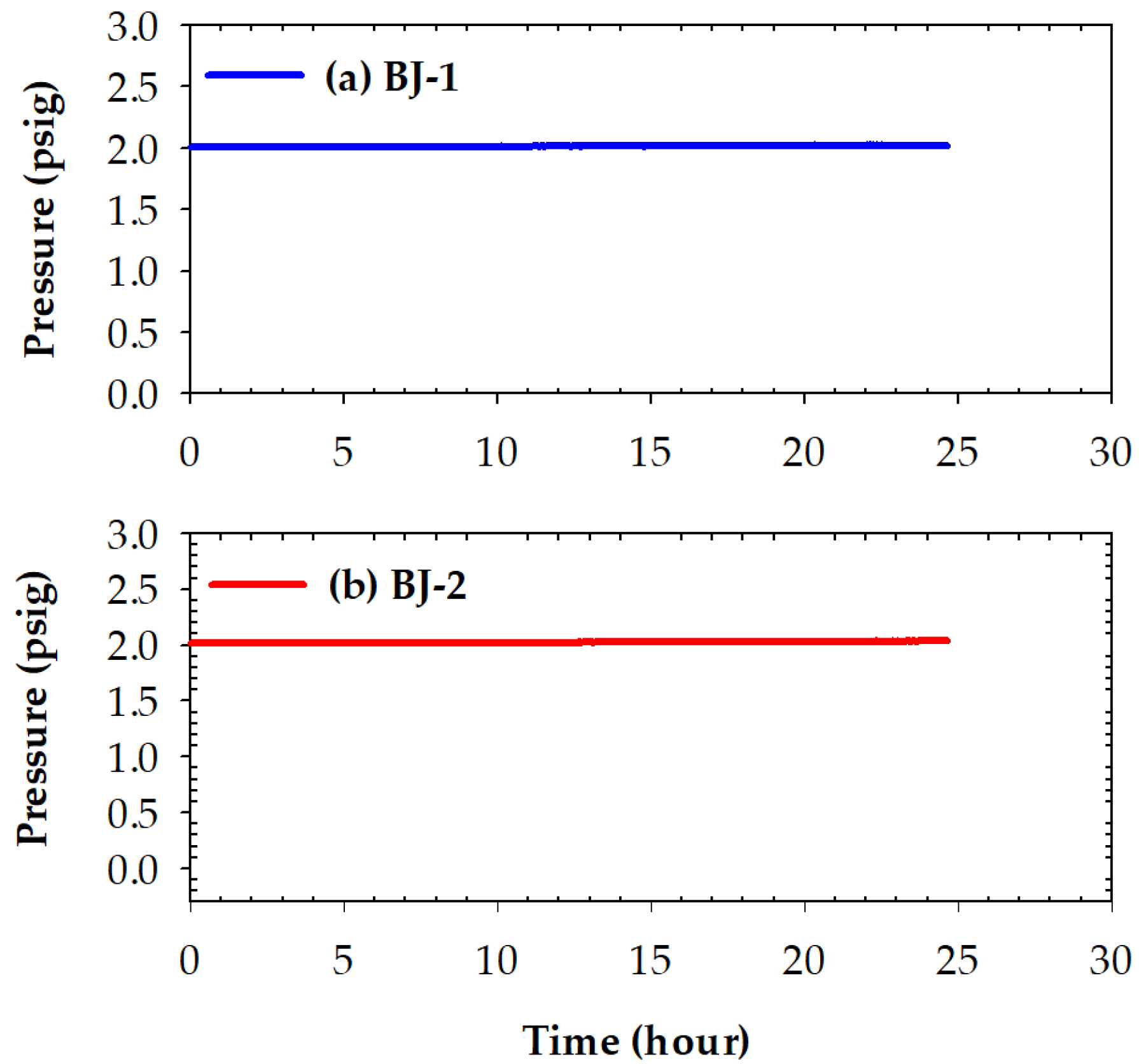
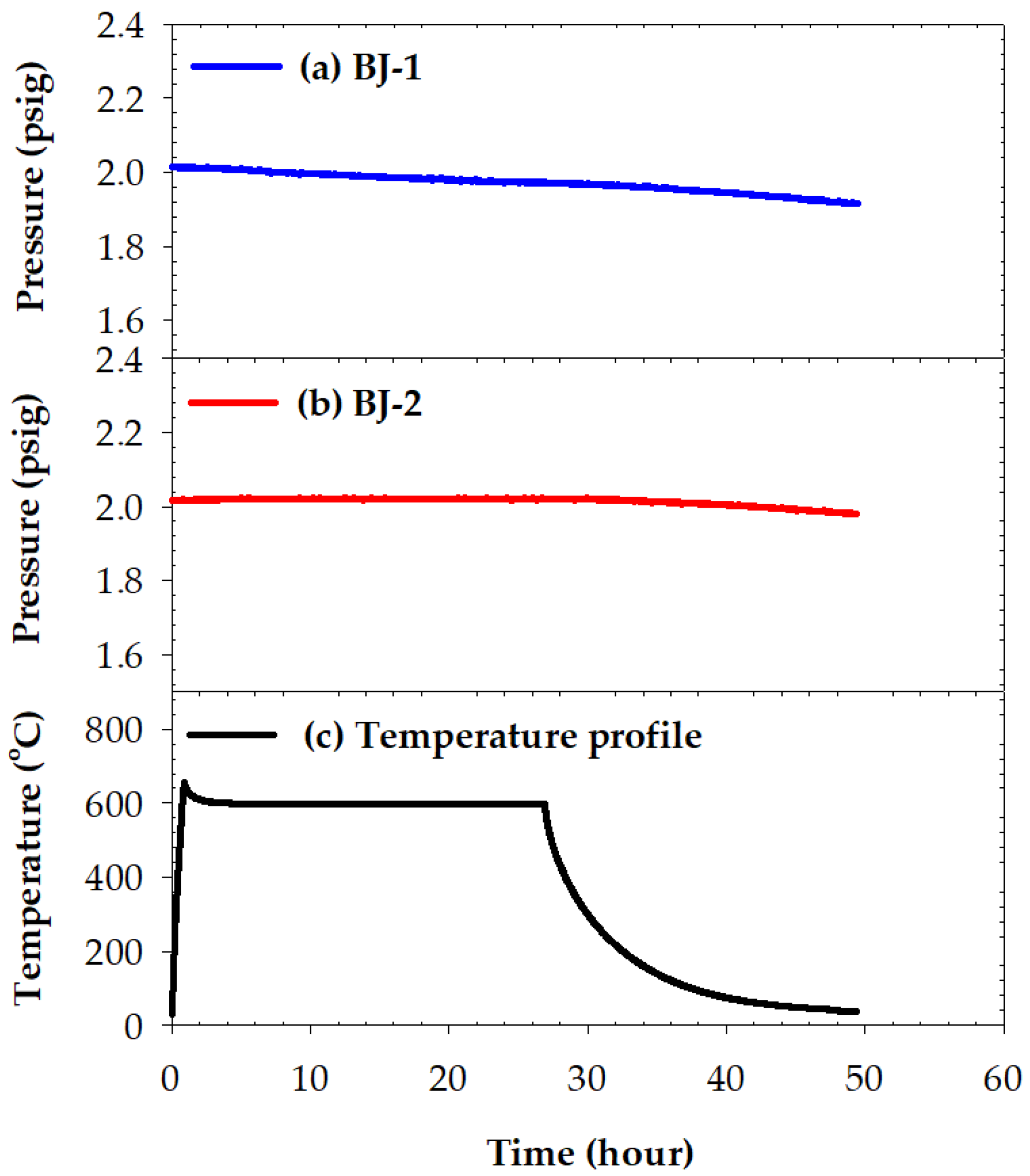
3.4. Pressure Drops Tests and Microstructures of Two Brazed Joints after Tests
4. Conclusions
- (1)
- The BAg-8 filler well wets both metallized YSZ and Crofer substrates. The wetting angle of Crofer with 1.5-μm Ag was 30 degrees, and it was decreased to approximately 0 degrees for the Crofer with 5-μm Ag. For the Ag/Cu/Ti-coated YSZ substrate, strong interfacial reactions occurred among the Bag-8 filler, Ag/Cu/Ti metallized layers, and YSZ substrate. The wetting angles in both cases were close to 0 degrees due to reactive wetting;
- (2)
- The brazed zone primarily consists of Ag-Cu eutectic. The metallized Ti layer dissolves into the braze melt, and the Ti preferentially reacts with YSZ and Fe from the Crofer substrate. Globular Fe2Ti intermetallic compounds form on the YSZ side of the joint. The interfacial reaction among Ti and the two substrates increases when the thickness of the metallized Ti layer is increased from 0.5 to 1 μm;
- (3)
- No pressure drop was detected for the two BAg-8 brazed metallized YSZ and Crofer joints during testing at room temperature for 24 h. For the YSZ/Ti(0.5μ)/Cu(1μ)/Ag(1.5μ)/BAg-8(50μ)/Ag(1.5μ)/Crofer joint tested at 600 °C for 24 h, the pressure of helium gradually decreased from 2.01 to 1.91 psig. In contrast, the helium pressure drop test of YSZ/Ti(1μ)/Cu(3μ)/Ag(5μ)/BAg-8(50μ)/Ag(5μ)/Crofer joint showed a slight decrease from 2.02 to 1.98 psig during the cooling cycle of the test. The greater interfacial reaction between the metallized YSZ and BAg-8 filler due to the thicker metallized Ti layer on the YSZ substrate was responsible for the improved gas-tight performance of the joint;
- (4)
- The combination of the BAg-8 filler foil and metallized YSZ and Crofer substrates demonstrates a potential approach to create joints for gas-tight applications.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Zhang, K.; Mao, X.J.; Si, X.Q.; Lan, B.; Liu, Z.G.; Huang, Y.X.; Qi, J.L.; Feng, J.C.; Cao, J. Microstructure and mechanical properties of the AlON/Ti6Al4V active element brazing joint. Mater. Sci. Eng. A 2020, 793, 139859. [Google Scholar] [CrossRef]
- Akselsen, O.M. Advances in brazing of ceramics. J. Mater. Sci. 1992, 27, 1989–2000. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Z.Z.; Chen, G.; Jiang, Q.Y.; Dong, W.; Lei, K. Effects of brazing temperature on microstructure and properties of interface between CBN and Co-based active filler metals. Vacuum 2017, 145, 30–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.K.; Zhou, J.P.; Sun, D.Q.; Li, H.M. Laser welding-brazing of alumina to 304 stainless steel with an Ag-based filler material. Metall. Res. Technol. 2020, 118, 104. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.K.; Yu, D.S.; Sun, D.Q.; Li, H.M. A review paper on effect of the welding process of ceramics and metals. J. Mater. Res. Technol. 2020, 9, 16214–16236. [Google Scholar] [CrossRef]
- Mishra, S.; Sharma, A.; Jung, D.H.; Jung, J.P. Recent advances in active metal brazing of ceramics and process. Met. Mater. Int. 2020, 26, 1087–1098. [Google Scholar] [CrossRef]
- Chen, B.; Zou, W.J.; Li, W.W.; Wu, S.B.; Xiong, H.P.; Wu, X. Joining of SiO2f/SiO2 composite to Nb using Ag-Cu-In-Ti brazing alloys. J. Mater. Sci. Technol. 2020, 50, 13–20. [Google Scholar] [CrossRef]
- Heo, H.; Joung, H.; Jung, K.; Kang, C.Y. Formation of interfacial reaction layers in Al2O3/SS 430 brazed joints using Cu-7Al-3.5Zr alloys. Metals 2018, 8, 990. [Google Scholar] [CrossRef] [Green Version]
- Simoes, S. Recent progress in the joining of titanium alloys to ceramics. Metals 2018, 8, 876. [Google Scholar] [CrossRef] [Green Version]
- Heo, H.; Kim, G.; Park, Y.C.; Jung, K.; Kang, C.Y. Effect of bonding temperature on crack occurrences in Al2O3/SS 430 joints using Cu-based brazing alloys. Metals 2018, 8, 752. [Google Scholar] [CrossRef] [Green Version]
- Vila, M.; Prieto, C.; Zahr, J.; Perez-Castellanos, J.L.; Bruno, G.; Jimenez-Ruiz, M.; Miranzo, P.; Osendi, M.I. Residual stresses in ceramic-to-metal joints: Diffraction measurements and finite element method analysis. Philos. Mag. 2007, 87, 5551–5563. [Google Scholar] [CrossRef]
- Park, J.W.; Mendez, P.F.; Eagar, T.W. Strain energy release in ceramic-to-metal joints by ductile metal interlayers. Scr. Mater. 2005, 53, 857–861. [Google Scholar] [CrossRef]
- Hafez, K.M.; El-Sayed, M.H.; Naka, M. Joining of alumina ceramics to metals. Sci. Technol. Weld. Join. 2005, 10, 125–130. [Google Scholar] [CrossRef]
- Hu, G.; Zhou, Q.; Bhatlawande, A.; Park, J.; Termuhlen, R.; Ma, Y.X.; Bieler, T.R.; Yu, H.C.; Qi, Y.; Hogan, T.; et al. Patterned nickel interlayers for enhanced silver wetting, spreading and adhesion on ceramic substrates. Scr. Mater. 2021, 196, 113767. [Google Scholar] [CrossRef]
- Yeon, J.; Yamamoto, M.; Ni, P.Y.; Nakamoto, M.; Tanaka, T. Joining of metal to ceramic plate using super-spread wetting. Metals 2020, 10, 1377. [Google Scholar] [CrossRef]
- Tillmann, W.; Anar, N.B.; Wojarski, L.; Lassner, J.J. Microstructure and mechanical properties of reactive-air-brazed 3YSZ/Crofer 22 APU joints at ambient temperature. Metall. Microstruct. Anal. 2020, 9, 529–540. [Google Scholar] [CrossRef]
- Pereira, J.C.; Nascimento, R.M.; Martinelli, A.E.; Acchar, W.; Rocha, L.A. Wetting behavior of silver braze alloys onto metallized zirconia inserts. Weld. J. 2015, 94, 211–218. [Google Scholar]
- Eustathopoulos, N. Wetting by liquid metals-application in materials processing: The contribution of the grenoble group. Metals 2015, 5, 350–370. [Google Scholar] [CrossRef] [Green Version]
- Timakul, P.; Jinawath, S.; Aungkavattana, P. Fabrication of electrolyte materials for solid oxide fuel cells by tape-casting. Ceram. Int. 2008, 34, 867–871. [Google Scholar] [CrossRef]
- Brazing Handbook, 5th ed.; American Welding Society: Miami, FL, USA, 2007.
- Humpston, G.; Jacobson, D.M. Principles of Soldering and Brazing; ASM International: Materials Park, OH, USA, 1993. [Google Scholar]
- Huang, L.W.; Wu, Y.Y.; Shiue, R.K. The effect of oxygen pressure in active brazing 8YSZ and Crofer 22H alloy. J. Mater. Res. Technol. 2021, 10, 1382–1388. [Google Scholar] [CrossRef]
- Xin, C.L.; Li, N.; Yan, J.Z.; Cao, Y.T. Effects of Ti activity on mechanical properties and microstructures of Al2O3/Ag-Cu-Ti/Fe-Ni-Co brazed joints. Rare Met. Mater. Eng. 2018, 47, 1031–1036. [Google Scholar]
- Ali, M.; Knowles, K.M.; Mallinson, P.M.; Fernie, J.A. Evolution of the interfacial phases in Al2O3-Kovar joints brazed using a Ag-Cu-Ti-based alloy. Philos. Mag. 2017, 97, 718–742. [Google Scholar] [CrossRef] [Green Version]
- Shiue, R.K.; Wu, S.K.; O, J.M.; Wang, J.Y. Microstructural evolution at the bonding interface during the early-stage infrared active brazing of alumina. Metall. Mater. Trans. 2000, 31, 2527–2536. [Google Scholar] [CrossRef]
- Weil, K.S.; Hardy, J.S.; Rice, J.P.; Kim, J.Y. Brazing as a means of sealing ceramic membranes for use in advanced coal gasification processes. Fuel 2006, 85, 156–162. [Google Scholar] [CrossRef]
- Cao, J.; Si, X.Q.; Li, W.J.; Song, X.G.; Feng, J.C. Reactive air brazing of YSZ-electrolyte and Al2O3 substrate for gas sensor sealing: Interfacial microstructure and mechanical properties. Int. J. Hydrogen Energy 2017, 42, 10683–10694. [Google Scholar] [CrossRef]
- Waetzig, K.; Schilm, J.; Mosch, S.; Tillmann, W.; Eilers, A.; Wojarski, L. Influence of the brazing paste composition on the wetting behavior of reactive air brazed metal-ceramic joints. Adv. Eng. Mater. 2021, 23, 2000711. [Google Scholar] [CrossRef]
- Bram, M.; Reckers, S.; Drinovac, P.; Monch, J.; Steinbrech, R.W.; Buchkremer, H.P.; Stover, D. Deformation behavior and leakage tests of alternate sealing materials for SOFC stacks. J. Power Sources 2004, 138, 111–119. [Google Scholar] [CrossRef]
- Sang, S.B.; Li, W.; Pu, J.; Li, J. Novel Al2O3-based compressive seals for IT-SOFC applications. J. Power Sources 2008, 177, 77–82. [Google Scholar] [CrossRef]
- Li, R.Z.; Liang, X.P.; Wang, X.C.; Zeng, W.L.; Yang, J.J.; Yan, D.; Pu, J.; Chi, B.; Li, J. Improvement of sealing performance for Al2O3 fiber-reinforced compressive seals for intermediate temperature solid oxide fuel cell. Ceram. Int. 2019, 45, 21953–21959. [Google Scholar] [CrossRef]
- Massalski, T.B. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1992. [Google Scholar]
- Mannan, S. Chapter 19, Plant Commissioning and Inspection, In Lees’ Loss Prevention in the Process Industries, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2012; p. 1794. [Google Scholar]
- Zapfe, K. Leak Detection. CAS-CERN Accelerator School and ALBA Synchrotron Light Facility: Course on Vacuum in Accelerators. Platja d’Aro, Spain, 16–24 May 2006; pp. 227–240. [Google Scholar]



| Substrate | Sputtering Power (600 s) 1 | Thickness (μm) 1 |
|---|---|---|
| YSZ-1 | Ti: 175 W, Cu: 57 W, Ag: 65 W | Ti: 0.5, Cu: 1.0, Ag: 1.5 |
| YSZ-2 | Ti: 357 W, Cu: 172 W, Ag: 217 W | Ti: 1.0, Cu: 3.0, Ag: 5.0 |
| Crofer-1 | Ag: 65 W | Ag: 1.5 |
| Crofer-2 | Ag: 217 W | Ag: 5.0 |
| Element 1/at% | Ag | Cu | Cr | Fe | O | Ti | Y | Zr | Phase/Alloy |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 0.0 | 0.2 | 0.0 | 0.0 | 61.1 | 0.0 | 2.2 | 36.4 | YSZ 3 (substrate) |
| B1 1 | 0.1 | 0.1 | 24.9 | 73.0 | 0.0 | 0.2 | 0.0 | 0.0 | Crofer 4 (substrate) |
| C1 5 | 72.0 | 27.8 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | Ag-Cu eutectic 5 |
| D1 | 1.1 | 1.1 | 7.1 | 58.3 | 0.3 | 31.7 | 0.0 | 0.1 | Fe2Ti |
| E1 2 | 10.0 | 60.7 | 1.1 | 8.2 | 5.5 | 11.5 | 0.1 | 2.5 | --- |
| F1 2 | 27.7 | 49.3 | 4.8 | 11.1 | 0.4 | 6.4 | 0.0 | 0.0 | --- |
| G1 2 | 10.5 | 2.3 | 16.9 | 54.0 | 5.8 | 9.0 | 0.0 | 0.0 | --- |
| H1 1 | 5.3 | 36.4 | 13.9 | 37.9 | 0.0 | 5.0 | 0.0 | 0.0 | --- |
| Element 1 /at% | Ag | Cu | Cr | Fe | O | Ti | Y | Zr | Phase/Alloy |
|---|---|---|---|---|---|---|---|---|---|
| A2 | 0.0 | 0.2 | 0.0 | 0.1 | 63.5 | 0.0 | 1.5 | 34.6 | YSZ 3 (substrate) |
| B2 1 | 0.1 | 0.1 | 25.0 | 73.0 | 0.1 | 0.3 | 0.0 | 0.0 | Crofer 4 (substrate) |
| C2 5 | 71.6 | 28.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | Ag-Cu eutectic 5 |
| D2 | 90.7 | 8.4 | 0.1 | 0.2 | 0.3 | 0.0 | 0.0 | 0.0 | Primary Ag |
| E2 | 3.2 | 94.7 | 0.2 | 0.9 | 0.1 | 0.8 | 0.0 | 0.0 | Cu-rich |
| F2 | 0.9 | 1.3 | 9.0 | 56.4 | 0.2 | 31.5 | 0.0 | 0.2 | Fe2Ti |
| G2 2 | 13.4 | 1.9 | 3.9 | 33.6 | 10.1 | 20.1 | 0.7 | 15.6 | --- |
| H2 2 | 14.4 | 4.2 | 5.8 | 47.8 | 0.5 | 26.6 | 0.0 | 0.0 | Fe2Ti |
| I2 1 | 0.5 | 0.8 | 24.0 | 62.2 | 0.1 | 10.4 | 0.0 | 0.0 | Crofer alloyed with Ti |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.-W.; Shiue, R.-K.; Liu, C.-K.; Cheng, Y.-N.; Lee, R.-Y.; Tsay, L.-W. Vacuum Brazing of Metallized YSZ and Crofer Alloy Using 72Ag-28Cu Filler Foil. Materials 2022, 15, 939. https://doi.org/10.3390/ma15030939
Huang L-W, Shiue R-K, Liu C-K, Cheng Y-N, Lee R-Y, Tsay L-W. Vacuum Brazing of Metallized YSZ and Crofer Alloy Using 72Ag-28Cu Filler Foil. Materials. 2022; 15(3):939. https://doi.org/10.3390/ma15030939
Chicago/Turabian StyleHuang, Liang-Wei, Ren-Kae Shiue, Chien-Kuo Liu, Yung-Neng Cheng, Ruey-Yi Lee, and Leu-Wen Tsay. 2022. "Vacuum Brazing of Metallized YSZ and Crofer Alloy Using 72Ag-28Cu Filler Foil" Materials 15, no. 3: 939. https://doi.org/10.3390/ma15030939






