The Influence of Low-Temperature Food Waste Biochars on Anaerobic Digestion of Food Waste
Abstract
:1. Introduction
1.1. Background
1.2. Bio-Waste Processing Methods
1.3. Problems with AD of Bio-Waste
1.4. Study Aim
2. Materials and Methods
2.1. Materials
2.1.1. Inoculum Preparation
2.1.2. Food Waste Preparation
2.1.3. Low-Temperature Biochar Preparation and Analyses
- —mass yield, %;
- —dry mass of biochar after the process, g,
- —dry mass of material before process, g.
2.2. Methods
2.2.1. Biochemical Methane Potential Test
2.2.2. Materials and Process Residue Analysis
- —oxygen % share in dry mass, %;
- —carbon % share in dry mass, %;
- —hydrohen % share in dry mass, %;—sulfur % share in dry mass, %;
- —ash % share in dry mass, %.
- —elemental composition of the substrate, C—carbon, H—hydrogen, O—oxygen, N—nitrogen, S—sulphury, and a, b, c, d, e stands for molar % share of specific elements of the volatile solids of biomass [29].
- —water needed for substrate decomposition, mol;
- —methane, mol;
- —carbon dioxide, mol;
- —ammonia, mol;
- —hydrogen sulfide, mol.
- —biodegradability of FW obtained in the methane fermentation process, %;
- —experimental biochemical methane potential, ml × gVS−1;
- —theoretical biochemical methane potential, ml × gVS−1;
- —change of CH4 produced after biochar addition to the process, %;
- —CH4 produced from a sample without biochar added, ml;
- —CH4 produced from a sample with biochar added, ml.
2.2.3. Methane Production Kinetics
- —the cumulative methane production obtained from a substrate after time t, mlCH4 × gVS−1;
- —the estimated value of experimental maximum methane production obtains from a substrate, mlCH4 × gVS−1;
- —constant reaction rate, d−1;
- —process time, d;
- —methane production rate, mlCH4 × (gVS × d)−1.
2.2.4. Statistical Analysis of Biochar Effect
3. Results and Discussion
3.1. Substrate and Biochar Properties
3.2. Biochemical Methane Potential—Theoretical and Experimental
3.3. Biomethane Production Kinetics
4. Conclusions
- not all low-temperature biochars at the presented dose can improve biomethane production yield;
- the biomethane yield changes are visible for extreme cases. The worst biochar led to an average 4.5% CH4 decrease, while two of the best biochars increased CH4 production on average by 3.5%;
- biomethane production was improved on average by 3.5% by biochar made at 400 °C in 60 min at atmospheric pressure, and by low-pressure hydrochar produced at 280 °C, while the biodegradability of FW was higher than 81% in those variants;
- the theoretical CH4 potential of food waste was 460 mlCH4 × gVS−1, while the first-order constant reaction rate was k = 0.24 d−1;
- the FW thermal treatment pressure may influence the EC of biochar.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
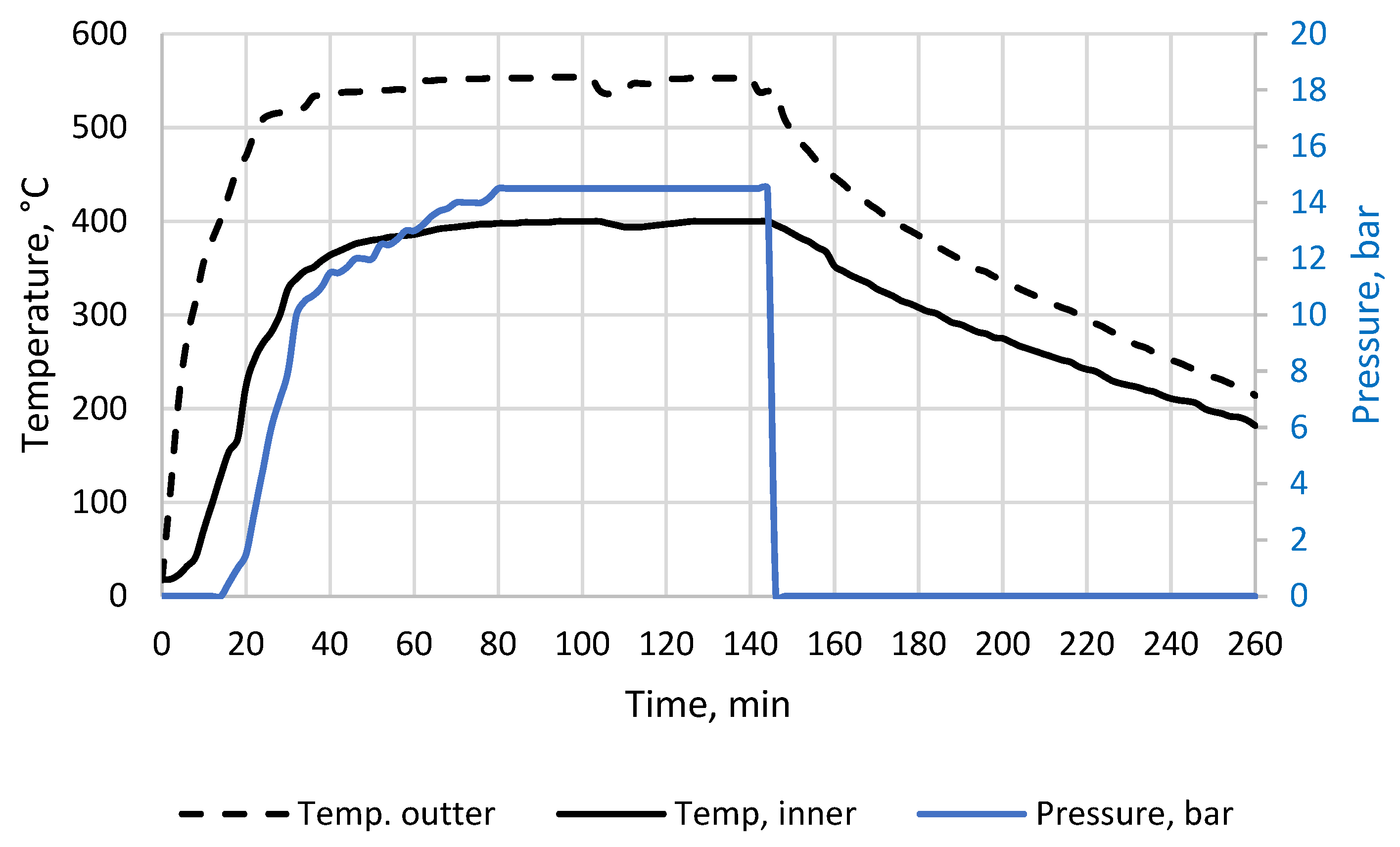
References
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.; et al. Planetary boundaries: Exploring the safe operating space for humanity. Ecol. Soc. 2009, 14, 1–32. Available online: https://www.ecologyandsociety.org/vol14/iss2/art32/ (accessed on 29 November 2021). [CrossRef]
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0098 (accessed on 29 November 2021).
- Store, J. Waste Management and Recycling: Council Adopts New Rules. Available online: https://www.consilium.europa.eu/en/press/press-releases/2018/05/22/waste-management-and-recycling-council-adopts-new-rules/# (accessed on 29 November 2021).
- European Environment Agency Bio-Waste in Europe-Turning Challenges into Opportunities. Available online: https://www.eea.europa.eu/publications/bio-waste-in-europe (accessed on 29 November 2021).
- Połomka, J.; Jedrczak, A. Potential of mineral fraction in compost-like-output, methods of its obtaining and the possibility of using it in the context of circular economy. Materials 2020, 13, 3023. [Google Scholar] [CrossRef] [PubMed]
- Zamri, M.F.M.A.; Hasmady, S.; Akhiar, A.; Ideris, F.; Shamsuddin, A.H.; Mofijur, M.; Fattah, I.M.R.; Mahlia, T.M.I. A comprehensive review on anaerobic digestion of organic fraction of municipal solid waste. Renew. Sustain. Energy Rev. 2021, 137, 110637. [Google Scholar] [CrossRef]
- Sarkar, S.; Pal, S.; Chanda, S. Optimization of a vegetable waste composting process with a significant thermophilic phase. Procedia Environ. Sci. 2016, 35, 435–440. [Google Scholar] [CrossRef]
- Sobieraj, K.; Stegenta-Dąbrowska, S.; Koziel, J.A.; Białowiec, A. Modeling of Co accumulation in the headspace of the bioreactor during organic waste composting. Energies 2021, 14, 1367. [Google Scholar] [CrossRef]
- Stegenta-Dabrowska, S.; Sobieraj, K.; Koziel, J.A.; Bieniek, J.; Bialowiec, A. Kinetics of biotic and abiotic CO production during the initial phase of biowaste composting. Energies 2020, 13, 5451. [Google Scholar] [CrossRef]
- Bouallagui, H.; Cheikh, R.B.; Marouani, L.; Hamdi, M. Mesophilic biogas production from fruit and vegetable waste in a tubular digester. Bioresour. Technol. 2003, 86, 85–89. [Google Scholar] [CrossRef]
- Drosg, B. Process Monitoring in Biogas Plants; IEA Bioenergy. 2013. Available online: https://www.ieabioenergy.com/blog/publications/process-monitoring-in-biogas-plants/ (accessed on 29 November 2021).
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food Waste–Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- Issah, A.A.; Kabera, T.; Kemausuor, F. Biogas optimisation processes and effluent quality: A review. Biomass Bioenergy 2020, 133. [Google Scholar] [CrossRef]
- Qiu, L.; Deng, Y.F.; Wang, F.; Davaritouchaee, M.; Yao, Y.Q. A Review on biochar-mediated anaerobic digestion with enhanced methane recovery. Renew. Sustain. Energy Rev. 2019, 115, 109373. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, C.W.; Ong, H.C.; Show, P.L.; Hsieh, T.H. Torrefaction, pyrolysis and two-stage thermodegradation of hemicellulose, cellulose and lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An overview of effect of process parameters on hydrothermal carbonization of biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Morales, V.L.; Pérez-Reche, F.J.; Hapca, S.M.; Hanley, K.L.; Lehmann, J.; Zhang, W. Reverse engineering of biochar. Bioresour. Technol. 2015, 183, 163–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudek, M.; Świechowski, K.; Manczarski, P.; Koziel, J.A.; Białowiec, A. The effect of biochar addition on the biogas production kinetics from the anaerobic digestion of brewers’ spent grain. Energies 2019, 12, 1518. [Google Scholar] [CrossRef] [Green Version]
- Valta, K.; Sotiropoulos, A.; Malamis, D.; Kosanovic, T.; Antonopoulou, G.; Alexandropoulou, M.; Jonuzay, S.; Lyberatos, G.; Loizidou, M. Assessment of the effect of drying temperature and composition on the biochemical methane potential of in-house dried household food waste. Waste Manag. Res. 2019, 37, 461–468. [Google Scholar] [CrossRef]
- Matyjewicz, B.; Świechowski, K.; Koziel, J.A.; Białowiec, A. Proof-of-Concept of high-pressure torrefaction for improvement of pelletized biomass fuel properties and process cost reduction. Energies 2020, 13, 4790. [Google Scholar] [CrossRef]
- Cai, J.; He, P.; Wang, Y.; Shao, L.; Lü, F. Effects and Optimization of the Use of Biochar in Anaerobic Digestion of Food Wastes. Waste Manag. Res. J. A Sustain. Circ. Econ. 2016, 34, 409–416. [Google Scholar] [CrossRef]
- Sunyoto, N.M.S.; Zhu, M.; Zhang, Z.; Zhang, D. Effect of biochar addition on hydrogen and methane production in two-phase anaerobic digestion of aqueous carbohydrates food waste. Bioresour. Technol. 2016, 219, 29–36. [Google Scholar] [CrossRef]
- de Blasio, C. Fundamentals of Biofuels Engineering and Technology; Green Energy and Technology; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-11598-2. [Google Scholar]
- PN-EN 14346:2011 Standard. Waste Characteristics. Calculation of Dry Mass on the Basis of Dry Residue or Water Content. Available online: https://sklep.pkn.pl/pn-en-14346-2011p.html?options=cart (accessed on 29 November 2021).
- PN-EN 15169:2011 Standard. Waste Characteristics. Determination of Organic Matter Content for Waste, Slurry and Sludge. Available online: https://sklep.pkn.pl/pn-en-15169-2011p.html (accessed on 29 November 2021).
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- PKN ISO/TS 12902:2007 Solid Mineral Fuel-Determination of Total Carbon, Hydrogen and Nitorgen-Instrumental Methods. Available online: https://sklep.pkn.pl/pkn-iso-ts-12902-2007p.html?options=cart (accessed on 29 November 2021).
- Achinas, S.; Euverink, G.J.W. Theoretical analysis of biogas potential prediction from agricultural waste. Resour.-Effic. Technol. 2016, 2, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y. Anaerobic Digestion Fundamentals II Thermodynamics; Jyväskylä. 2013. Available online: http://www.valorgas.soton.ac.uk/Pub_docs/JyU%20SS%202013/VALORGAS_JyU_2013_Lecture%202.pdf (accessed on 29 November 2021).
- Orangun, A.; Kaur, H.; Kommalapati, R.R. Batch anaerobic co-digestion and biochemical methane potential analysis of goat manure and food waste. Energies 2021, 14, 1952. [Google Scholar] [CrossRef]
- Stępień, P.; Serownik, M.; Koziel, J.A.; Bialowiec, A. Waste to carbon: Estimating the energy demand for production of carbonized refuse-derived fuel. Sustainability 2019, 11, 5685. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Dutta, S.; You, S.; Luo, G.; Zhang, S.; Show, P.L.; Sawarkar, A.D.; Singh, L.; Tsang, D.C.W. A Critical Review on Biochar for Enhancing Biogas Production from Anaerobic Digestion of Food Waste and Sludge. J. Clean. Prod. 2021, 305, 127143. [Google Scholar] [CrossRef]
- Codignole Luz, F.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Biochar characteristics and early applications in anaerobic digestion-a review. J. Environ. Chem. Eng. 2018, 6, 2892–2909. [Google Scholar] [CrossRef]
- Zatorska, J. Badania Porowatych Materiałów Węglowych Otrzymywanych Poprzez Karbonizację Poli (Tereftalanu Etylenu) w Mieszaninie z Wybranymi Związkami Magnezu, PhD Thesis, Szczecin. 2013. Available online: https://zbc.ksiaznica.szczecin.pl/dlibra/publication/31080/edition/29484?language=en (accessed on 29 November 2021).
- Zdravkov, B.D.; Čermák, J.J.; Šefara, M.; Janků, J. Pore Classification in the characterization of porous materials: A perspective. Cent. Eur. J. Chem. 2007, 5, 385–395. [Google Scholar] [CrossRef]
- Xu, M.; Li, D.; Yan, Y.; Guo, T.; Pang, H.; Xue, H. Porous high specific surface area-activated carbon with co-doping N, S and P for high-performance supercapacitors. RSC Adv. 2017, 7, 43780–43788. [Google Scholar] [CrossRef] [Green Version]
- Gaffar, S.; Dattamudi, S.; Baboukani, A.R.; Chanda, S.; Novak, J.M.; Watts, D.W.; Wang, C.; Jayachandran, K. Physiochemical characterization of biochars from six feedstocks and their effects on the sorption of atrazine in an organic soil. Agronomy 2021, 11, 716. [Google Scholar] [CrossRef]
- Cruz Viggi, C.; Simonetti, S.; Palma, E.; Pagliaccia, P.; Braguglia, C.; Fazi, S.; Baronti, S.; Navarra, M.A.; Pettiti, I.; Koch, C.; et al. Enhancing methane production from food waste Fermentate using biochar: The added value of electrochemical testing in pre-selecting the most effective type of biochar. Biotechnol. Biofuels 2017, 10, 303. [Google Scholar] [CrossRef]
- Chen, S.; Rotaru, A.-E.; Liu, F.; Philips, J.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Carbon cloth stimulates direct interspecies electron transfer in syntrophic Co-Cultures. Bioresour. Technol. 2014, 173, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Gabhi, R.S.; Kirk, D.W.; Jia, C.Q. Preliminary investigation of electrical conductivity of monolithic biochar. Carbon 2017, 116, 435–442. [Google Scholar] [CrossRef]
- Hoffmann, V.; Rodriguez Correa, C.; Sautter, D.; Maringolo, E.; Kruse, A. Study of the electrical conductivity of biobased carbonaceous powder materials under moderate pressure for the application as electrode materials in energy storage technologies. GCB Bioenergy 2019, 11, 230–248. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Dolk, M.M.; Shen, Q.; Arbestain, M.C. Biochar PH, Electrical Conductivity and Liming Potential. In Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 23–38. Available online: https://www.researchgate.net/publication/319206365 (accessed on 29 November 2021).
- Banks, C.; Heaven, S.; Zhang, Y.; Baier, U. Food Waste Digestion: Anaerobic Digestion of Food Waste for a Circular Economy; IEA Bioenergy: Göteborg, Sweden, 2018; ISBN 9781910154588. Available online: https://www.ieabioenergy.com/blog/publications/food-waste-digestion-anaerobic-digestion-of-food-waste-for-a-circular-economy/ (accessed on 29 November 2021).
- Pan, X.; Lv, N.; Cai, G.; Zhou, M.; Wang, R.; Li, C.; Ning, J.; Li, J.; Li, Y.; Ye, Z.; et al. Carbon- and metal-based mediators modulate anaerobic methanogenesis and phenol removal: Focusing on stimulatory and inhibitory mechanism. J. Hazard. Mater. 2021, 420, 126615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lim, E.Y.; Loh, K.C.; Ok, Y.S.; Lee, J.T.E.; Shen, Y.; Wang, C.H.; Dai, Y.; Tong, Y.W. Biochar enhanced thermophilic anaerobic digestion of food waste: Focusing on biochar particle size, microbial community analysis and pilot-scale application. Energy Convers. Manag. 2020, 209, 112654. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, H.; He, S.; Zhao, Q.; Wei, L. A review of biochar in anaerobic digestion to improve biogas production: Performances, mechanisms and economic assessments. Bioresour. Technol. 2021, 341, 125797. [Google Scholar] [CrossRef]
- Kaur, G.; Johnravindar, D.; Wong, J.W.C. Enhanced volatile fatty acid degradation and methane production efficiency by biochar addition in food waste-sludge Co-Digestion: A step towards increased organic loading efficiency in Co-Digestion. Bioresour. Technol. 2020, 308, 123250. [Google Scholar] [CrossRef]
- Chen, T.-H.; Hashimoto, A.G. Effects of PH and substrate: Inoculum ratio on batch methane fermentation. Bioresour. Technol. 1996, 56, 179–186. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Kim, J.; Park, C.; Kim, T.-H.; Lee, M.; Kim, S.; Kim, S.-W.; Lee, J. Effects of various pretreatments for enhanced anaerobic digestion with waste activated sludge. J. Biosci. Bioeng. 2003, 95, 271–275. [Google Scholar] [CrossRef]
- Lee, D.H.; Behera, S.K.; Kim, J.W.; Park, H.-S. Methane production potential of leachate generated from korean food waste recycling facilities: A lab-scale study. Waste Manag. 2009, 29, 876–882. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y.; Zhou, Q. Waste activated sludge hydrolysis and short-chain fatty acids accumulation under mesophilic and thermophilic conditions: Effect of PH. Water Res. 2009, 43, 3735–3742. [Google Scholar] [CrossRef]
- Dinamarca, S.; Aroca, G.; Chamy, R.; Guerrero, L. The influence of PH in the hydrolytic stage of anaerobic digestion of the organic fraction of urban solid waste. Water Sci. Technol. 2003, 48, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.H.P.; Liu, H. Effect of PH on hydrogen production from glucose by a mixed culture. Bioresour. Technol. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Klein, R.; Slaný, V.; Krčálová, E. Conductivity measurement for control of a biogas plant. Acta Univ. Agric. Et Silvic. Mendel. Brun. 2018, 66, 1151–1156. [Google Scholar] [CrossRef] [Green Version]
- Xue, B.; Zifu, L.; Xuemei, W.; Xi, H.; Shikun, C.; Xiaofeng, B.; Ruiling, G. Online measurement of alkalinity in anaerobic Co-Digestion using linear regression method. Int. J. Agric. Biol. Eng. 2017, 10, 176–183. [Google Scholar] [CrossRef]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical Methane Potential (BMP) assay method for anaerobic digestion research. Water 2019, 11, 921. [Google Scholar] [CrossRef] [Green Version]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Experimental and kinetic study on anaerobic digestion of food waste: The effect of total solids and PH. J. Renew. Sustain. Energy 2015, 7, 063104. [Google Scholar] [CrossRef]



| Material | Basic Properties | Share in Mixture | |||||
|---|---|---|---|---|---|---|---|
| MC, % * | TS, % * | VS, % ** | AC, % ** | By Fresh Mass, % | by Dry Mass, % | by VS, % | |
| Mixture | 64.2 | 35.8 | 95.8 | 4.2 | - | - | - |
| Orange | 86.2 | 13.8 | 95.3 | 4.7 | 3.67 | 1.42 | 1.43 |
| Banana | 81.4 | 18.6 | 87.8 | 12.2 | 8.67 | 4.51 | 4.19 |
| Apple | 87.4 | 12.6 | 95.4 | 4.6 | 7.33 | 2.58 | 2.60 |
| Lemon | 85.4 | 14.6 | 93.5 | 6.5 | 1.33 | 0.55 | 0.54 |
| Potatoes | 61.6 | 38.4 | 93.1 | 6.9 | 24.33 | 26.11 | 25.73 |
| Onion | 89.2 | 10.8 | 93.4 | 6.6 | 4.67 | 1.41 | 1.40 |
| Salad | 94.9 | 5.1 | 85.7 | 14.3 | 3.33 | 0.48 | 0.43 |
| Cabbage | 92.2 | 7.8 | 91.6 | 8.4 | 3.33 | 0.72 | 0.70 |
| Tomatoes | 95.1 | 4.9 | 82.1 | 17.9 | 2.33 | 0.32 | 0.32 |
| Rice | 13.2 | 86.8 | 99.4 | 0.6 | 6.00 | 14.55 | 15.31 |
| Pasta | 11.6 | 88.4 | 95.5 | 4.5 | 6.00 | 14.84 | 15.00 |
| Bread | 22.5 | 77.5 | 95.2 | 4.8 | 3.00 | 6.50 | 6.54 |
| Meat | 69.8 | 30.2 | 96.0 | 4.0 | 3.00 | 2.53 | 2.57 |
| Fish meat | 81.7 | 18.3 | 95.5 | 4.5 | 12.00 | 6.12 | 6.19 |
| Cheese | 43.5 | 56.5 | 92.8 | 7.2 | 11.00 | 17.37 | 17.06 |
| Sample | Digestate | Food Waste Mixture | Biochar |
|---|---|---|---|
| D | + | - | - |
| D | + | - | - |
| D + FW | + | + | - |
| D + FW | + | + | - |
| D + FW + BC_300/60/0 | + | + | + |
| D + FW + BC_300/60/0 | + | + | + |
| D + FW + BC_300/60/15 | + | + | + |
| D + FW + BC_300/60/15 | + | + | + |
| D + FW + BC_400/60/0 | + | + | + |
| D + FW + BC_400/60/0 | + | + | + |
| D + FW + BC_400/60/15 | + | + | + |
| D + FW + BC_400/60/15 | + | + | + |
| D + FW + BC_ HTC280 | + | + | + |
| D + FW + BC_ HTC280 | + | + | + |
| Material | MY, % ** | MC, % * | TS, % * | VS, % ** | AC, % ** | SSA, m2 × g−1 | Vt, cm3 × g−1 | L, nm | pH *** | EC, mS × cm−1 *** |
|---|---|---|---|---|---|---|---|---|---|---|
| 300/60/0 | 42.6 | 4.5 | 95.5 | 79.5 | 20.5 | 0.62 | 8.2 × 10−4 | 5.2 | 8.61 | 3.04 |
| 300/60/15 | 45.9 | 3.3 | 96.7 | 89.6 | 10.4 | 0.26 | 3.3 × 10−4 | 5.0 | 8.04 | 3.57 |
| 400/60/0 | 37.4 | 4.4 | 95.6 | 77.3 | 22.7 | 0.61 | 7.6 × 10−4 | 5.0 | 10.19 | 4.53 |
| 400/60/15 | 34.3 | 4.0 | 96.0 | 60.9 | 39.1 | 0.64 | 11.3 × 10−4 | 7.1 | 10.75 | 7.69 |
| HTC280 | 56.4 | 18.4 | 81.6 | 88.1 | 11.9 | 0.38 | 5.6 × 10−4 | 5.9 | 5.59 | 4.71 |
| Biochar | No. | Initial | End | Process Residues’ Properties | Mass Reduction, % | BD, % | CH4 Production Effect, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | EC, µS × cm−1 | pH | EC, µS × cm−1 | MC, % | TS, % | VS, % | AC, % | |||||
| D + FW | 1 | 7.91 | 61.4 | 7.92 | 76.1 | 95.8 | 4.2 | 61.0 | 39.0 | 3.6 | 79.6 | - |
| 2 | 7.85 | 63.6 | 7.92 | 72.7 | 95.6 | 4.4 | 58.9 | 41.1 | 3.7 | 78.2 | - | |
| 3 | 7.69 | 65.7 | 8.02 | 73.5 | 95.8 | 4.2 | 59.6 | 40.4 | 2.1 | 73.3 | - | |
| 4 | 7.68 | 65.1 | 7.99 | 73.5 | 95.8 | 4.2 | 60.3 | 39.7 | 2.5 | 71.7 | - | |
| Mean | 7.78 | 64.0 | 7.96 | 74.0 | 95.7 | 4.3 | 59.9 | 40.1 | 3.0 | 75.5 | - | |
| 300/60/0 | 1 | 7.82 | 56.1 | 7.97 | 75.6 | 95.6 | 4.4 | 60.7 | 39.3 | 3.0 | 78.7 | −0.2 |
| 2 | 7.85 | 58.6 | 7.92 | 74.4 | 95.6 | 4.4 | 60.5 | 39.5 | 3.0 | 78.4 | −0.7 | |
| 3 | 7.62 | 66.1 | 7.96 | 74.1 | 95.7 | 4.3 | 63.1 | 36.9 | 2.5 | 75.5 | 4.1 | |
| 4 | 7.67 | 66.3 | 7.96 | 74.7 | 95.6 | 4.4 | 61.0 | 39.0 | 2.3 | 72.3 | −0.3 | |
| Mean | 7.74 | 61.8 | 7.95 | 74.7 | 95.7 | 4.3 | 61.3 | 38.7 | 2.7 | 76.2 | 0.7 | |
| 300/60/15 | 1 | 7.85 | 66.1 | 7.93 | 74.1 | 95.6 | 4.4 | 59.5 | 40.5 | 3.1 | 81.9 | 3.8 |
| 2 | 7.84 | 63.8 | 7.93 | 73.5 | 95.6 | 4.4 | 61.5 | 38.5 | 3.1 | 81.2 | 2.9 | |
| 3 | 7.67 | 66.6 | 8.02 | 75.6 | 95.7 | 4.3 | 59.6 | 40.4 | 2.2 | 72.4 | −0.2 | |
| 4 | 7.65 | 65.1 | 8.02 | 74.4 | 95.6 | 4.4 | 62.2 | 37.8 | 2.4 | 72.7 | 0.3 | |
| Mean | 7.75 | 65.4 | 7.98 | 74.4 | 95.6 | 4.4 | 60.7 | 39.3 | 2.7 | 77.0 | 1.7 | |
| 400/60/0 | 1 | 7.86 | 57.9 | 7.92 | 75.1 | 95.6 | 4.4 | 58.5 | 41.5 | 3.1 | 82.6 | 4.7 |
| 2 | 7.84 | 65.1 | 7.92 | 75.9 | 95.6 | 4.4 | 59.6 | 40.4 | 3.1 | 81.6 | 3.4 | |
| 3 | 7.65 | 65.7 | 7.95 | 74.3 | 95.7 | 4.3 | 61.1 | 38.9 | 2.2 | 75.4 | 3.9 | |
| 4 | 7.64 | 64.5 | 8.01 | 74.5 | 95.5 | 4.5 | 61.0 | 39.0 | 2.3 | 73.9 | 1.9 | |
| Mean | 7.75 | 63.3 | 7.95 | 75.0 | 95.6 | 4.4 | 60.0 | 40.0 | 2.7 | 78.4 | 3.5 | |
| 400/60/15 | 1 | 7.83 | 65.2 | 7.93 | 77.7 | 95.7 | 4.3 | 60.1 | 39.9 | 2.7 | 72.4 | −8.2 |
| 2 | 7.85 | 65.8 | 7.92 | 76.5 | 95.7 | 4.3 | 59.9 | 40.1 | 3.4 | 72.0 | −0.7 | |
| 3 | 7.68 | 67.9 | 8.03 | 73.9 | 95.8 | 4.2 | 64.4 | 35.6 | 2.4 | - | - | |
| 4 | 7.67 | 61.6 | 8.00 | 72.5 | 95.6 | 4.4 | 61.8 | 38.2 | 2.3 | - | - | |
| Mean | 7.76 | 65.1 | 7.97 | 75.2 | 95.7 | 4.3 | 61.6 | 38.4 | 2.7 | 72.7 | −4.5 | |
| HTC280 | 1 | 7.78 | 64.7 | 7.95 | 75.9 | 95.7 | 4.3 | 61.0 | 39.0 | 3.0 | 81.6 | 3.4 |
| 2 | 7.82 | 63.2 | 7.93 | 76.8 | 95.6 | 4.4 | 60.3 | 39.7 | 3.9 | 81.5 | 3.3 | |
| 3 | 7.64 | 66.0 | 7.99 | 72.0 | 95.7 | 4.3 | 69.6 | 30.4 | 2.3 | 75.4 | 4.0 | |
| 4 | 7.64 | 67.4 | 8.02 | 68.7 | 95.7 | 4.3 | 61.4 | 38.6 | 3.0 | - | - | |
| Mean | 7.72 | 65.3 | 7.97 | 73.4 | 95.7 | 4.3 | 63.1 | 36.9 | 3.1 | 79.5 | 3.6 | |
| Variant | No. | k, d−1 | r, mlCH4 × (gVS × d)−1 | R2, - | |
|---|---|---|---|---|---|
| Control | 1 | 0.265 | 362.13 | 95.89 | 0.997 |
| 2 | 0.270 | 354.13 | 95.48 | 0.996 | |
| 3 | 0.217 | 348.40 | 75.46 | 0.993 | |
| 4 | 0.208 | 340.94 | 70.88 | 0.992 | |
| Mean | 0.240 | 351.40 | 84.43 | 0.995 | |
| 300/60/0 | 1 | 0.266 | 357.43 | 95.25 | 0.996 |
| 2 | 0.264 | 357.42 | 94.29 | 0.996 | |
| 3 | 0.205 | 357.16 | 73.31 | 0.995 | |
| 4 | 0.202 | 343.32 | 69.23 | 0.993 | |
| Mean | 0.234 | 353.83 | 83.02 | 0.995 | |
| 300/60/15 | 1 | 0.281 | 371.93 | 104.62 | 0.997 |
| 2 | 0.273 | 371.08 | 101.45 | 0.997 | |
| 3 | 0.212 | 342.20 | 72.62 | 0.993 | |
| 4 | 0.217 | 344.88 | 74.90 | 0.993 | |
| Mean | 0.246 | 357.52 | 88.40 | 0.995 | |
| 400/60/0 | 1 | 0.249 | 377.05 | 93.77 | 0.996 |
| 2 | 0.268 | 368.88 | 98.99 | 0.996 | |
| 3 | 0.200 | 356.88 | 71.20 | 0.994 | |
| 4 | 0.222 | 347.68 | 77.29 | 0.994 | |
| Mean | 0.235 | 362.62 | 85.31 | 0.995 | |
| 400/60/15 | 1 | 0.250 | 326.62 | 81.75 | 0.995 |
| 2 | 0.208 | 341.82 | 70.96 | 0.994 | |
| 3 | - | - | - | - | |
| 4 | - | - | - | - | |
| Mean | 0.229 | 334.22 | 76.36 | 0.995 | |
| HTC280 | 1 | 0.254 | 361.80 | 91.93 | 0.992 |
| 2 | 0.238 | 364.77 | 86.82 | 0.992 | |
| 3 | 0.210 | 356.53 | 74.69 | 0.995 | |
| 4 | - | - | - | - | |
| Mean | 0.234 | 361.04 | 84.48 | 0.993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świechowski, K.; Matyjewicz, B.; Telega, P.; Białowiec, A. The Influence of Low-Temperature Food Waste Biochars on Anaerobic Digestion of Food Waste. Materials 2022, 15, 945. https://doi.org/10.3390/ma15030945
Świechowski K, Matyjewicz B, Telega P, Białowiec A. The Influence of Low-Temperature Food Waste Biochars on Anaerobic Digestion of Food Waste. Materials. 2022; 15(3):945. https://doi.org/10.3390/ma15030945
Chicago/Turabian StyleŚwiechowski, Kacper, Bartosz Matyjewicz, Paweł Telega, and Andrzej Białowiec. 2022. "The Influence of Low-Temperature Food Waste Biochars on Anaerobic Digestion of Food Waste" Materials 15, no. 3: 945. https://doi.org/10.3390/ma15030945
APA StyleŚwiechowski, K., Matyjewicz, B., Telega, P., & Białowiec, A. (2022). The Influence of Low-Temperature Food Waste Biochars on Anaerobic Digestion of Food Waste. Materials, 15(3), 945. https://doi.org/10.3390/ma15030945








